Abstract
Atomoxetine (ATM) is a potent norepinephrine (NE) uptake inhibitor and increases both NE and dopamine synaptic levels in prefrontal cortex, where it is thought to exert its beneficial effects on attention and impulsivity. At the behavioral level, ATM has been shown to cause improvements on measures of executive functions, such as response inhibition, working memory and attentional set shifting across different species. However, the exact mechanism of action for ATM’s effects on cognition is still not clear. One possible target for the cognitive enhancing effects of ATM is the noradrenergic locus coeruleus (LC), the only source of NE to key forebrain areas such as cerebral cortex and hippocampus. Although it is known that ATM increases NE availability overall by blocking reuptake of NE, the effects of this agent on impulse activity of LC neurons have not been reported. Here, the effect of ATM (0.1 – 1 mg/kg, ip) on NE-LC neurons was investigated by recording extracellular activity of LC neurons in isoflurane-anesthetized rats. ATM caused a significant decrease of the tonic activity of LC single-units, although leaving intact the sensory-evoked excitatory component of LC phasic response. Moreover, the magnitude of the inhibitory component of LC response to paw stimulation was increased after 1 mg/kg of ATM and its duration was prolonged at 0.3 mg/kg. Together, these effects of ATM produced an increase in the phasic-to-tonic ratio of LC phasic response to sensory stimulation. ATM also modulated the average sensory-evoked local field potential (LFP) and spike-field coherence in LC depending on the dose tested. The lower dose (0.1 mg/kg) significantly decreased early positive and negative components of the sensory-evoked LFP response. Higher doses (0.3–1 mg/kg) initially increased and then decreased the amplitude of components of the evoked fields, whereas the spike-field coherence was enhanced by 1 mg/kg ATM across frequency bands. Finally, coherence between LC fields and EEG signals was generally increased by 1 mg/kg ATM, whereas 0.1 and 0.3 mg/kg respectively decreased and increased coherence values in specific frequency bands. Taken together these results suggest that ATM effects on LC neuronal activity are dose-dependent, with different doses affecting different aspects of LC firing. This modulation of activity of LC-NE neurons may play a role in the cognitive effects of ATM.
Keywords: atomoxetine, norepinephrine, locus coeruleus, sensory response, local field potentials, spike-field coherence
1. Introduction
Noradrenergic transmission is implicated in waking and arousal (Berridge and Foote, 1996; De Sarro et al., 1987), vigilance and attention (Aston-Jones et al., 1997; Aston-Jones et al., 1994; Rajkowski et al., 1994), memory (Arnsten, 2001; McGaugh, 2000; Przybyslawski et al., 1999), decision-making (Clayton et al., 2004; Nieuwenhuis et al., 2005a; Usher et al., 1999) and many other cognitive, sensory and motor processes (see Aston-Jones and Cohen, 2005b; Berridge and Waterhouse, 2003; Ramos and Arnsten, 2007; and Robbins and Everitt, 1995 for reviews). A general role of norepinephrine (NE) seems to be the enhancement of neural activity in response to relevant stimuli and the suppression of interference from irrelevant ones, independently from their affective value (Aston-Jones and Bloom, 1981b; Foote et al., 1980). This is accomplished by modulating neural excitability producing an increase in gain (Servan-Schreiber et al., 1990) or ‘signal-to-noise’ ratios in target neurons (Foote et al., 1975; Waterhouse et al., 1980; Waterhouse et al., 1988; Woodward et al., 1991), where NE suppresses spontaneous neuronal firing rates and enhances stimulus-evoked cellular responses (Berridge and Waterhouse, 2003). The largest group of brain noradrenergic neurons is in the brainstem locus coeruleus (LC), which sends widespread projections to forebrain areas and is the only source of NE in the hippocampus and neocortex (Berridge and Waterhouse, 2003; Foote et al., 1983; Ungerstedt, 1971).
Psychostimulants are known to improve hyperactivity, impulsivity and inattentiveness in attention deficit/hyperactivity disorder (ADHD) by stimulating NE and dopamine (DA) neurotransmission (Biederman and Spencer, 1999; Zametkin and Rapoport, 1987), but they are also effective at improving attention and other cognitive processes in healthy individuals (Mohamed and Sahakian, 2011; Sahakian and Morein-Zamir, 2011). ADHD drugs such as methylphenidate and amphetamines have recently gained popularity as cognitive enhancers and their use by the “non-clinical” population has grown accordingly (Greely et al., 2008). Their effects on cognition are thought to be mediated in part by decreasing LC spontaneous activity (Pliszka et al., 1996; Solanto, 1998) and by fine modulation of prefrontal NE levels (Ramos and Arnsten, 2007). For instance, it has been demonstrated that methylphenidate administration decreases spontaneous discharge activity of LC neurons (Devilbiss and Berridge, 2006), while having an excitatory influence on prefrontal cortex (PFC) neuronal activity (Salek et al., 2012).
Moreover, methylphenidate decreases average sensory evoked field potentials recorded from several other brain regions (Yang et al., 2006a, b). Other non-stimulant medications that have been shown to improve cognitive processes in both healthy and clinical subjects include the wake-promoting agent modafinil and NE reuptake inhibitors (Ballon and Feifel, 2006; Bidwell et al., 2011; Minzenberg and Carter, 2008). A common effect of all these putative cognition-enhancing drugs is to modulate NE transmission in the forebrain, but their exact mechanism of action is still unclear.
Atomoxetine (ATM) is a selective NE reuptake inhibitor with high affinity for the NE transporter and much lower affinity for the DA and serotonin transporters (Bymaster et al., 2002). However, ATM also enhances prefrontal DA, as well as NE, levels in the rat PFC (Bymaster et al., 2002; Swanson et al., 2006). This is because DA transporters are sparse in PFC (Sesack et al., 1998; Soucy et al., 1997) and extracellular DA is taken up by the NET there (Carboni et al., 1990). ATM, which is the first non-stimulant medication indicated for ADHD treatment (Pliszka, 2003; Spencer et al., 1998), decreases omission errors in the continuous performance task (CPT) of sustained attention and normalizes altered electroencephalographic measures in children with ADHD (Barry et al., 2009a). ATM has also been shown to improve working memory, response inhibition and other executive functions in patients with ADHD (Brown et al., 2011; Faraone et al., 2005; Gau and Shang, 2010). Moreover, ATM decreases impulsivity in high impulsive rats (Fernando et al., 2012) and improves attentional set shifting in rats with a compromised noradrenergic system (Newman et al., 2008). Recently, ATM demonstrated better efficacy than placebo in preventing relapse to drug use in animal models (Economidou et al., 2011), but has not been fully evaluated yet in clinical trials for drug addiction (Sofuoglu, 2010).
ATM improves indices of prefrontal executive functions such as working memory (Gamo et al., 2010; Tzavara et al., 2006), inhibition (Chamberlain et al., 2006; Robinson et al., 2008) and attention (Jentsch et al., 2009) in normal subjects also. In the rodent version of the CPT task, the 5-choice serial reaction time task (5-CSRTT; Bari et al., 2008), ATM increased response accuracy and decreased premature responses, which is thought to reflect improved attention and impulse control, respectively (Baarendse and Vanderschuren, 2012; Blondeau and Dellu-Hagedorn, 2007; Navarra et al., 2008; Paterson et al., 2011; Robinson, 2012; Robinson et al., 2008). Using other translational tasks, researchers have shown improvements in response inhibition (Eagle et al., 2008), behavioural flexibility (Seu et al., 2009) and sustained attention (Jentsch et al., 2009) after ATM administration in animal models. Importantly, these effects seem to be mediated almost entirely by the modulation of the noradrenergic, rather than dopaminergic, system (Bari et al., 2009; Pattij et al., 2012; Pattij and Vanderschuren, 2008).
Clearly, there is much interest in the pro-cognitive effects of ATM, and a host of behavioral studies have demonstrated beneficial effects of this drug in various domains of cognitive functioning both in humans and non-human models, but little is known about the neural substrates responsible for such effects. Although the modulation of the NE-LC system by some putative cognitive-enhancing drugs such as methylphenidate (Devilbiss and Berridge, 2006; Lacroix and Ferron, 1988; Olpe et al., 1985) and modafinil (Akaoka et al., 1991) have been assessed by electrophysiological techniques, to our knowledge data are currently not available on ATM effects on the discharge activity of NE-LC neurons. Here, we investigated the modulatory effect of different doses of ATM on the spontaneous and sensory-evoked activity of LC neurons using single-unit extracellular recordings in anesthetized rats. Moreover, we recorded LC local field potentials (LFPs), and analyzed sensory-evoked LFP response (Katzner et al., 2009; Mitzdorf, 1985; Yang et al., 2006a), spike-field coherence in LC (Fries, 2005) and coherence between cortical EEG and LC LFPs before and after ATM administration. These measures may provide important information on modulation of afferent inputs to LC, and responsiveness of LC neurons to such afferents, by ATM.
1. Materials and methods
2.1 Animals
Male Long Evans rats (Charles River) were used in these experiments (350–600 g). Animals were singly housed under a 12:12 h reverse light:dark cycle with food (Harlan Teklad) and water available ad libitum. Every effort was made to minimize animal suffering and to use the minimum possible number of animals. All procedures followed National Institute of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
2.2 Surgical procedure
Rats were initially anesthetized with isoflurane in a plastic chamber and maintained at 2–2.5% throughout the experiment by nosecone administration. The animals were mounted in a stereotaxic frame (Kopf Instruments) equipped with atraumatic earbars and placed on an air table. Body temperature was maintained at ~37°C using a thermistor-controlled heating pad coupled with a rectal probe. The skull was exposed and two jeweler’s screws were implanted over frontal and contralateral parietal cortices where electroencephalogram (EEG) leads were attached. The head of the animal was tilted by 15° (nose down) and a hole was drilled for the insertion of a single barrel glass micropipette filled with 2% pontamine sky blue solution in 0.5M sodium acetate at the following coordinates: AP, −3.7 mm; ML, +1.2 mm (relative to lambda).
2.3 Locus coeruleus extracellular recordings
Recording micropipettes were pulled with a Narishige vertical puller and tips broken to 2–3 μm resulting in an impedance of 3–12MΩ. A manual hydraulic microdrive was used to slowly advance the recording pipette through the brain. LC neurons were identified according to well-established elecrophysiological and anatomical criteria (Aston-Jones and Bloom, 1981b; Aston-Jones et al., 1980; Cedarbaum and Aghajanian, 1976). These include their spontaneous discharge rate (1–3 Hz), biphasic response to contralateral foot pinch and proximity to ME5 neurons, which exhibit a characteristic response to jaw movement. The signal was pre-amplified by a neuroprobe amplifier (model 1600, A-M Systems Inc) and then split into two separate identical amplifiers (CWE, model BMA 200) for filtering at 100 – 3K Hz and 1–1K Hz bandpass for single-unit and local field potential (LFP) recordings, respectively. Both signals were digitized by a CED Micro 1401 at 5.5 KHz and stored on a computer running Spike 2 version 5 software (Cambridge Electronic Design, Cambridge, UK) for off-line spike sorting and signal processing. Single-unit traces were monitored on a dual beam storage oscilloscope and via a loudspeaker. The EEG trace was amplified by a BMA 831 (CWE), filtered (1–50 Hz), sampled at 925 Hz and monitored online on the computer screen to ensure a constant level of anesthesia (Fig. 1). Once an LC cell was isolated, data was collected for at least 2 minutes after the cell showed a stable firing pattern and before the beginning of sensory stimulation. Stimuli consisted of a train of 50 square electric pulses (0.5 ms, 0.5 Hz, 10 mA) delivered to the contralateral hind paw via two hypodermic needles implanted subcutaneously. Paw stimulation intensity was set on an ISO-flex system (AMPI, Alomone Labs), whereas the stimulation rate was software controlled by Spike 2. Several tracks at least 100 μm apart were explored throughout the mediolateral and rostrocaudal extension of the LC nucleus. For about half of the cells, spontaneous activity was recorded for at least two minutes after paw stimulation and no statistically significant differences from pre-stimulation activity were noted. At the end of the experiments a pontamine sky blue deposit was made iontophoretically at the site of the last cell recorded by passing a 7.5μ A cathodal current for 10 minutes. The animals were then killed by cervical dislocation and the brain quickly removed and immersed in ice-cold 2-methylbutane and stored at −20°C until histological assessment.
Fig. 1.
EEG traces were continuously monitored to ensure a stable level of anesthesia. EEG activity during extracellular recordings was characterized by slow, high amplitude oscillations and punctuated by regular spindle-like activity. a) The level of the anesthetic was regulated throughout the experiment so as to achieve reliable EEG responses to acoustic (finger snaps) sensory stimulation. At this level of anesthesia, LC tonic activity is comparable to that of awake animals (Aston-Jones and Bloom, 1981a, b) Comparison of the ‘spike’ trace (100–3K Hz bandpass) and LFP trace before (1–1K Hz bandpass; ‘raw’ LFP) and after digital filtering (1–30 Hz bandpass; ‘spike-free’ LFP). The digital filter applied off-line successfully removed spike contamination from the LFP trace. c) Representative coronal section through the LC from one of the experimental subjects. Pontamine blue deposit in ventral LC confirmed that all electrode tracks were in the intended area. Abbreviations: EEG, electroencephalogram; LFP, local field potential; ME5, mesencephalic nucleus 5; ventr, ventricle.
2.4 ATM administration
ATM was dissolved in sterile saline and diluted to 1mg/ml. Three doses (0.1, 0.3, and 1 mg/kg) were aliquoted and stored at −80° until the day of the experiment. The range of doses tested included dose levels effective in improving measures of executive functions in rodents (i.e., 0.3 and 1 mg/kg; Eagle et al., 2008; Floresco and Jentsch, 2011; Pattij and Vanderschuren, 2008). ATM was administered via intraperitoneal (ip) injection at least 2 hours from the induction of anesthesia. Each animal received one injection and the order of doses was randomized across experimental days.
2.5 Histological analysis
Frozen brains were mounted on a cryostat apparatus and coronal slices of 40μm thickness were obtained throughout the caudo-rostral extent of LC. Sections were mounted on glass slides, counterstained with neutral red and coverslipped. Anatomical specificity of the extracellular recordings was assessed by calculating recording sites from the pontamine sky blue deposits made in each animal at the final LC recording location.
2.6 Data analysis
Spike trains of LC neural activity were sorted using a combination of threshold, template and principal component scattergram analysis to obtain single-unit data uncontaminated by artifacts or neighboring cell activity. The majority of recordings included only one cell and this was confirmed by visual inspection of autocorrelograms for each recording file. Recordings including ambiguous spike shape or unresponsiveness to paw stimulation (as is characteristic of LC neurons; Aston-Jones et al., 1982) were not included in the analysis. Tonic activity was defined as the firing rate (spikes/s) during 2-minute epochs before paw stimulation. For phasic response analysis, peristimulus time histograms (PSTHs) computed over 50 trials of paw stimulation were created where possible for single cells in 2s epochs (1s pre- and 1s post-stimulus), representing spike-counts per 5ms bins. Spontaneous activity (background) and its standard deviation were measured over 1s pre-stimulus in the PSTH to control for any variation in basal firing rate due to paw stimulation (Devilbiss and Berridge, 2006). The excitatory component of the phasic response consisted of spike-counts during the epoch between a 5ms bin that exceeded the background average firing rate by at least 3 SD and the last bin that crossed this threshold followed by at least 5 bins below this threshold. The excitatory epoch also had to be comprised within 15 and 100ms post-stimulus. The magnitude of the inhibitory component was defined as the spike-count per 5ms bins during the epoch between 100 and 600ms post-stimulus. The duration of the inhibitory component was calculated as the longest epoch post-stimulus where no bins crossed the threshold of 2SD of the background firing rate (Fig. 3a). This method was chosen over alternative ones because it yielded reliable and consistent measurement of the different components of the phasic activity across cells and is similar to previous publications (Aston-Jones and Bloom, 1981b). Both excitatory and inhibitory magnitudes were adjusted for the background firing rate by subtracting background firing from activity during the response. The phasic-to-tonic (P:T) response ratio of the sensory-evoked excitatory and inhibitory responses were calculated according to the formulas: [(excitatory response − background)/background] and [(inhibitory response − background)/background], respectively. LC recordings from 8 animals before ATM administration (pre-drug; tonic: 27 cells; phasic: 7 animals, 21 cells, 1050 trials) were compared to each of the 3 ATM doses: 0.1 mg/kg (tonic: 26 cells; phasic: 21 cells, 1050 trials), 0.3 mg/kg (tonic: 29 cells: phasic: 26 cells, 1300 trials), 1 mg/kg (tonic: 44 cells; phasic: 33 cells, 1650 trials). Single-unit observations for each single dose were further divided into three 1h-periods according to the time elapsed since ATM injection.
Fig. 3.
a) Schematic representation of the method used to separate the different components of the LC sensory-evoked response in single-unit recordings (see text). b) Effects of ATM on LC evoked discharge. The single-unit PSTHs depict two representative neurons per dose. For most neurons, tonic activity was more sensitive to the inhibitory effects of ATM, while largely sparing the excitatory response with a consequent increase in the phasic-to-tonic ratio.
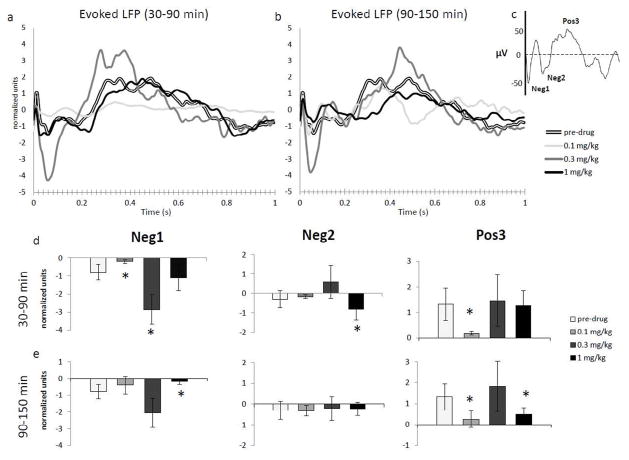
LFP recordings from LC were digitally filtered off-line at 30 Hz low pass (Aston-Jones and Bloom, 1981a). Average sensory-evoked LFPs were smoothed with a Boxcar filter (3 bin width) and consisted of 50 paw stimulation trials per LC site (pre-drug: 850 trials; 0.1 mg/kg: 750 trials; 0.3 mg/kg: 800 trials; 1 mg/kg: 700 trials). Results were normalized by dividing each data point for the absolute value of the average of two 5ms bins across the zero point (one above and one below zero) on the y axis so to obtain equal baseline levels. This adjustment was necessary to account for differences in electrode impedance or other factors causing amplitude discrepancies across experimental sessions. For statistical analysis, the magnitude of two early negative and one late positive components that were clearly recognizable on the majority of averaged LFPs were calculated. We arbitrarily called these components Neg1 (20–120 ms post-stimulus), Neg2 (125–280 ms) and Pos3 (285–640 ms) similar to previous reports (Dafny, 1975; Dafny and Burks, 1976; Yang et al., 2006a, b). It has been proposed that early negative components (i.e., Neg1) represent incoming presynaptic activity which, in normal conditions, result in LC phasic response (Aston-Jones and Bloom, 1981b). Later components may be related to postsynaptic activity around the recording site (Dafny, 1975). All LFP analyses included only traces free of artifacts or excessive noise and were restricted to the first two 1h-time periods after ATM administration (30–90 and 90–150min).
Spike-field and EEG-field coherence was calculated over 2-minute windows before stimulation using standard routines in Neuroexplorer (Nex Technologies, Lexington, MA, USA). Spike-field coherence was obtained for 256 frequency values with no window overlap and smoothed with a Boxcar filter (3 bin width). A coherence value of 1 indicates perfect phase relationship, whereas a value of 0 means that there is no phase relationship between the two signals considered (Fries et al., 2008). For statistical analysis of spike-field and EEG-field coherence, the mean coherence value of the following frequency bands was calculated for each ATM dose (n = pre-drug, 13; 0.1 mg/kg, 9; 0.3 mg/kg, 18; 1 mg/kg, 11): delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta (12–18 Hz) similar to previous reports (Khawaja et al., 2009; Manning et al., 2009; Ray et al., 2008). We restricted our analysis to low frequency ranges to avoid contamination of LFPs from spike data and possible artifacts produced by the digital filtering and also because these are the frequency bands most explored in the literature on cognition-enhancing drugs (e.g., Barry et al., 2009a; Barry et al., 2009b; Clarke et al., 2003; Solt et al., 2011).
Single-unit data for ATM were analyzed by ANOVA with two between-subjects independent factors of four levels each (dose: no-drug, 0.1, 0.3 and 1 mg/kg; and time: pre-drug, 30–90 min, 90–150 min, 150–210 min), whereas LFP analysis had only one independent variable (i.e., dose) and different time periods were analyzed separately. Significant results were further decomposed with appropriate post-hoc tests: ATM doses were compared to the pre-drug condition with Dunnett’s t-test (two-tailed), which does not require a significant main effect (Howell, 1997, p. 351), whereas Sidak’s test was used for the time variable. Levene’s test was used to control for departures from the ANOVA’s requirement of equality of variances. In case of violation of this assumption, variables were appropriately transformed (Log10 or square root). Sensory-evoked LFP and coherence data were not normally distributed, thus a one-way ANOVA non parametric equivalent for K independent samples Kruskal-Wallis H was used, followed by Dunn’s test for comparisons between dose levels and the pre-drug condition in case of the overall p < .05. For electrophysiological analysis of raw traces, Spike 2 (CED, Cambridge, UK) and Neuroexplorer (Nex Technologies, Lexington, MA, USA) were used, while statistical analysis was performed on SPSS (SPSS, Chicago) and Prism (Graph Pad Software Inc.).
2. Results
3.1 Tonic activity
Average spontaneous firing rates (spikes/s) were not significantly different between subjects at baseline (pre-drug: F(7,26) = 1.288, ns). Thus, they were grouped together (mean firing rate ± SEM = 1.74 ±0.13 Hz) and compared to the 3 ATM doses at each time point. ATM administration affected significantly LC spontaneous impulse activity (Fig. 2; dose: F(2,125) = 8.26, p < .005; time: F(2,125) = 4.83, p < .05), but there was no significant interaction between dose and time (dose x time, F(4,125) = 1.39, ns). Both 0.3 and 1 mg/kg of ATM decreased spontaneous LC activity to less than 50% of the pre-drug firing rate (both p < .005), whereas 0.1 mg/kg did not affect spontaneous firing rate. Moreover, the effect of ATM on LC firing rate was time dependent. Post-hoc analysis revealed a significantly lower spontaneous activity only during the first and the second 1h-time periods (30–90 min and 90–150 min) compared to the pre-drug condition (p < .05 and p < .005, respectively). The third 1h-period (150–210 min) was also significantly different from the second time period (90–150 min, p < .05), but not from the pre-drug condition indicating that LC firing rate returned to normal values after ~150 min.
Fig. 2.
Spontaneous firing activity of LC neurons recorded from 0 to 30 min after ATM injection. a) Upper panels depicts representative rate histograms (10s bins) from 3 different LC neurons recorded in animals that received 0.1, 0.3 or 1 mg/kg ATM. It can be seen that the two higher doses, but not 0.1 mg/kg, attenuated LC spontaneous activity and that 1 mg/kg caused a much faster onset of this effect. The lower panels depict the inter-spike intervals (ISI) of the above recordings (5ms bins). b) ATM at the 0.3 and 1 mg/kg doses decreased spontaneous LC firing activity and this variable was different from the pre-drug condition during the first two time windows considered (30–90 min and 90–150 min). Firing rates recovered between 150–210 min for both effective doses. c) Representative sampling of waveforms from one LC neuron during a 30 min period. (* = p < .05, for time).
3.2 Phasic activity
Mean background activity between paw stimuli was significantly affected by ATM dose (Fig. 4a; F(2,100) = 8.19, p < .005). There was also a significant difference in background activity between post-injection time windows (time: F(2,100) = 8.84, p < .005), but there was no interaction between dose and time (dose x time: F(4,100) = 1.67, ns). According to post-hoc tests this measure was significantly lower only at 0.3 and 1 mg/kg (both p < .005) compared to pre-drug condition, similar to the tonic measure. The difference between background activity at different time periods was significant only at the second 1h-time period (90–150 min; p < .005) compared to all other time windows. This measure of spontaneous tonic discharge has been used to normalize the components of the sensory-evoked response for each cell as described in the Methods section.
Fig. 4.
Effects of different doses of ATM on LC single-unit sensory-evoked response expressed as percentage change from the pre-drug condition. a) Baseline activity between trials was significantly decreased at 0.3 and 1 mg/kg of ATM (both p < .005) and between 90–150 min after ATM administration. The excitatory component (b) was not affected by ATM administration compared to pre-drug levels, whereas the magnitude of the inhibitory component (c) was increased during the second time-period (90–150 min) and at 1mg/kg (p < .05). The duration of the inhibitory component (d) was increased by 3 mg/kg (p < .05) only, whereas 1mg/kg of ATM increased the phasic-to-tonic ratio (e) of LC excitatory response to paw stimulation (p < .05). f) Both 0.3 and 1 mg/kg of ATM increased the phasic-to-tonic (P:T) ratio of the inhibitory response compared to pre-drug condition (p < .05 and p < .005, respectively) and the second time window (90–150 min) was different from all the other epoch considered. Increased variability was observed on some of the measures, but only during specific time-windows. This indicates that this variability is not due to noise in the data, but to the time course of ATM effects (* = p < .05 for time).
There was no significant effect of ATM on the absolute magnitude of the excitatory component of LC response to paw stimulation (Fig. 4b; dose: F(2,100) = .01, ns), no effect of time (time: F(2,100) = 1.93, ns) and no interaction between the independent variables (dose x time: F(4,100) = 1.27, ns).
There was also no significant main effect of ATM dose on the magnitude of the inhibitory LC response component (Fig. 4c; dose: F(2,100) = 1.18, ns). There was a significant difference for this LC response between time periods after ATM (time: F(2,100) = 8.21, p < .005), but no interaction between dose and time (dose x time: F(4,100) = 2.33, ns). For the dose variable, Dunnett’s test revealed that the magnitude of the inhibitory component was significantly increased by 1 mg/kg ATM compared to the pre-drug condition (p < .05). For the time variable, pairwise comparisons showed a significant effect only for the second 1h-time period (90–150 min) compared to all the other conditions (p < .005). The duration of the inhibitory component of the phasic response (Fig. 4d) was significantly affected by ATM (dose: F(2,100) = 8.65, p < .005), but there was no difference between different time windows (time: F(2,100) = .03, ns) and there was no interaction between dose and time (dose x time: F(4,100) = .8, ns). Post-hoc analysis showed that the inhibitory phase was longer only at the 0.3 mg/kg dose compared to pre-drug condition (p < .05).
ATM administration had a significant effect on the phasic-to-tonic ratio of the LC excitatory response (Fig. 4e) as a function of dose only (dose: F(2,100) = 5.11, p < .05; time: F(2,100) = 2.29, ns; dose x time: F(4,100) = 1.3, ns). Post-hoc tests showed that the dose of 1 mg/kg significantly increased this measure compared to the pre-drug condition (p < 0.5). Finally, the phasic-to-tonic ratio of the inhibitory response (Fig. 4f) was significantly affected by ATM dose (dose: F(2,100) = 6.73, p < .005) and time after injection (F(2,100) = 10.61, p < .005), but there was no interaction between the two variables (dose x time: F(4,100) = 2.03, ns). According to post-hoc tests, both the 0.3 and 1 mg/kg doses were significantly different from the pre-drug condition (p < .05 and p < .005, respectively) and the second 1h-time period (90–150 min) was different from all the other time windows (p < .05).
3.3 Local field potentials
LFPs were recorded simultaneously with spike traces. LFP waveforms generally were biphasic (negative-positive) similar to previous publications (Aston-Jones and Bloom, 1981a, b). The initial negative wave was divided into early (Neg1) and late (Neg2) components, as described in the Methods section. A third component was positive (Pos3) and was most prominent between 300 and 400 ms post-stimulus. It has been proposed that such field reflect spatiotemporal summation of postsynaptic dendritic responses to afferent activity (Nadasdy et al., 1998; Petsche et al., 1984).
For average sensory-evoked LFP response during the first 30–90 min post-administration (Fig. 5a and d), there was a significant effect of ATM dose on the early negative component of the LFP (Neg1; H(3) = 66.31, p < .005). The 0.3 mg/kg produced a stronger deflection in this LFP component compared to the pre-drug condition (p < .005), whereas the lower dose (0.1 mg/kg) significantly decreased, and 1 mg/kg did not affect, the amplitude of this component. There was a significant effect of ATM dose on the second negative component observed in these LFPs (Neg2; H(3) = 38.38, p < .005). The higher dose (1 mg/kg) increased the amplitude of this component (p < .05), while the other two doses did not have a significant effect. Finally, a late positive component of the sensory-evoked field potentials in LC (Pos3) was significantly affected by ATM (H(3) = 120.9, p < .005). According to post-hoc tests, the lower dose (0.1 mg/kg) decreased its amplitude compared to the pre-drug condition (p < .005).
Fig. 5.
Normalized average evoked LFPs in LC following paw stimulation before and after different doses of ATM. During the first time-period (a and d; 30–90 min), although having no effect on single-unit activity in the LC, the lower dose (0.1 mg/kg) tended to flatten the LFP response to paw stimulation and significantly decreased both the Neg1 and Pos3 components. Higher doses increased negative components only. In the second time window considered (b and e; 90–150 min), 1 mg/kg of ATM significantly decreased the early negative and the positive components, whereas 0.1 mg/kg decreased only the Pos3. c) Example of the average LFP response before normalization and relative components. (* = p < .05, compared to pre-drug condition).
During the second time period considered (90–150 min; Fig. 5b and e) ATM administration produce a significant effect on the Neg1 component (H(3) = 28.27, p < .005). The dose of 1 mg/kg significantly reduced this early negative component compared to the pre-drug condition (p < .05). There was no effect of ATM on the second negative component during this time window (Neg 2; H(3) = 5.9, ns). ATM administration significantly affected the Pos3 component of sensory-evoked LFP (H(3) = 141.5, p < .005). Both 0.1 and 1 mg/kg decreased the magnitude of the Pos3 compared to the pre-drug condition (both p < .005).
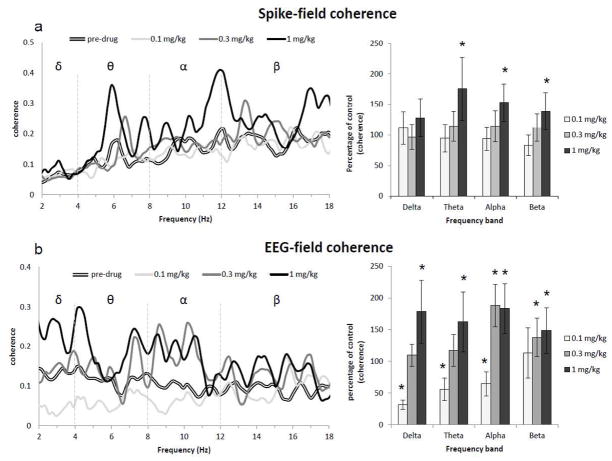
Spike-field coherence during the first time window post-administration (30–90 min; Fig. 6a) was affected by ATM across different frequency bands. There was a significant effect of dose in the delta band (2–4 Hz; H(3) = 13.29, p < .005), but none of the relevant comparisons reached statistical significance. In the theta (4–8 Hz) there was a significant effect of ATM dose (H(3) = 17.38, p < .005). Only 1 mg/kg increased spike-field coherence compared to the pre-drug condition (p < .05), an effect probably related to the increase in the LFPs power spectral density in the theta band that followed ATM administration (data not shown). ATM administration influenced coherence in the alpha (8–12 Hz; H(3) = 38.65, p < .005) and beta (12–18 Hz; H(3) = 46.65, p < .005) bands. In these frequency ranges, the 1 mg/kg dose increased coherence between spikes and LFPs (both p < .005). ATM did not affect coherence during the second time window analyzed (90–150 min; data not shown) in the delta (H(3) = 1.3, ns), theta (H(3) = 2.9, ns) or alpha bands (H(3) = 5.6, ns). Finally, there was a significant effect of ATM on spike-field coherence in the beta band (H(3) = 44.26, p < .005). In this frequency range, 0.3 mg/kg dose increased spike-field coherence during the second time window considered (p < .005).
Fig. 6.
Effects of ATM on spike-field and EEG-field coherence during the first 30–90 min post-administration for the four frequency bands analyzed: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta (12–18 Hz). a) ATM generally increased spike-field coherence at the high dose (1 mg/kg) across all frequency bands considered, with the exception of the delta frequency range. b) EEG-field coherence was affected by ATM across frequencies, with 1 mg/kg generally increasing coherence, whereas 0.3 mg/kg increased coherence only in the alpha and beta range. The low dose of ATM (0.1 mg/kg) decreased coherence across delta, theta and alpha frequency bands. (* = p < .05, compared to pre-drug condition).
Coherence between cortical EEG and LFPs in LC was also strongly affected by ATM administration during the first time window considered (30–90 min; Fig. 6b). There was a significant effect of ATM administration in all the frequency bands considered (delta: H(3) = 36.32, p < .005; theta: H(3) = 54.25, p < .005; alpha: H(3) = 60.67, p < .005; beta: H(3) = 31.88, p < .005). The dose of 1 mg/kg increased coherence values across frequency bands (p < .05 for delta and theta) with a stronger effect in the high-range frequencies (p < .005 for alpha and beta). 0.3 mg/kg of ATM only increased coherence in the alpha band (p < .005), whereas 0.1 mg/kg decreased coherence in the delta (p < .05), theta (p < .005) and alpha (p < .05) frequency bands. ATM had a significant effect on EEG-field coherence also during the second time period considered (90–150 min; data not shown; delta: H(3) = 30.61, p < .005; theta: H(3) = 43.29, p < .005; alpha: H(3) = 46.49, p < .005; beta: H(3) = 69.89, p < .005). 1 mg/kg of ATM increased coherence across frequency bands (p < .05 for delta; p < .005 for theta, alpha and beta), whereas the 0.3 mg/kg dose increased coherence only for alpha and beta frequencies (p < .005 for both). Finally, these effects were not dependent on LC neurons firing rate as inspection of EEG spectrograms did not reveal significant differences across ATM doses.
3. Discussion
The results of the present experiments generally confirm previous studies on the effects of different types of putative cognition-enhancing drugs on LC discharge activity in the anesthetized rat and, for the first time, extend these results to ATM. There has been no information about the effects of systemic ATM administration on phasic and tonic LC discharge activity, which has limited our understanding of the neural substrates involved in the cognition-enhancing effects of this drug in ADHD as well as in healthy, non-clinical subjects. Here we found that ATM decreases spontaneous (tonic) firing rate of LC neurons, but preserves the excitatory response and also increases the magnitude and duration of the inhibitory response to sensory stimuli. These effects combined produced an increase in the phasic-to-tonic (P:T) ratio of LC evoked responses, which may effectively enhance LC signaling temporally linked to sensory events in downstream target areas (Aston-Jones and Cohen, 2005b). Moreover, analysis of LFP in LC revealed a biphasic effect. The low ATM dose (0.1 mg/kg) generally decreased average sensory-evoked response. Higher ATM doses (0.3 and 1 mg/kg) initially increased the amplitude of negative components and then decreased it for both negative and positive components in the stimulus-evoked LFP response, while enhancing simultaneous neural activity in LC in the theta, alpha and beta frequency bands. Finally, analysis of coherence values between cortical EEG and LFP recorded in the LC showed that a low ATM dose decreased, whereas higher doses increased synchronized activity between these two brain regions.
The decrease in tonic LC activity after ATM is similar to what has been observed after administration of methylphenidate (Devilbiss and Berridge, 2006; Lacroix and Ferron, 1988; Olpe et al., 1985), amphetamine (Graham and Aghajanian, 1971; Ryan et al., 1985), cocaine (Curtis et al., 1993; Pitts and Marwah, 1987) and several NE reuptake inhibitors (Aghajanian, 1980; Nyback et al., 1975; Olpe et al., 1983; Scuvee-Moreau and Dresse, 1979; Valentino et al., 1990; Wong et al., 2000). This effect is mediated by indirect activation of inhibitory presynaptic α2 noradrenergic receptors and is blocked by co-administration of α2 antagonists (Fernandez-Pastor et al., 2005; Grandoso et al., 2004; Mateo et al., 1998; Miguelez et al., 2009; Starke and Montel, 1973; Svensson et al., 1975; Svensson and Usdin, 1978; Wong et al., 2000). In previous LC recordings in behaving animals, our lab found that animals displaying high levels of tonic LC discharge are disengaged from the task at hand and distractible (Aston-Jones and Cohen, 2005b). In fact, in physiological conditions, too high or too low tonic discharge rates are associated with low phasic response of LC neurons and impaired attentional task performance (Rajkowski et al., 1994). The decrease in spontaneous activity may be related to the mild sedative effects of ATM at higher doses (Bari et al., 2009; Fernando et al., 2012). Nonetheless, this effect together with the preservation of phasic response to sensory stimuli and increased in P:T ratio for LC neurons after ATM may contribute to the behavioural calming but simultaneous attention-enhancing effects of these drugs in ADHD individuals (Arnsten, 2011; Bidwell et al., 2011; Brown et al., 2011; Simpson and Perry, 2003; Solanto, 1998; Spencer et al., 1998).
It has been demonstrated that phasic burst-like firing of LC neurons causes a more efficient release of NE in target areas (Florin-Lechner et al., 1996), contributing to temporally specific regulation of forebrain NE levels and adaptively modulating vigilance and behavioural responses (Foote et al., 1983; Segal and Bloom, 1974; Waterhouse et al., 1980). The present results show that the excitatory component of the evoked response was not significantly affected by ATM, contrary to what has been reported for other drugs that decrease LC spontaneous activity such as methylphenidate (Devilbiss and Berridge, 2006), morphine (Korf et al., 1974) or desipramine (Valentino and Curtis, 1991). However, similar to methylphenidate and desipramine, ATM increased the magnitude and duration of the inhibitory component, which may be relevant for the improvements in sustained attention and response inhibition observed in animals administered with these drugs (Jentsch et al., 2009; Koffarnus and Katz, 2011; Navarra et al., 2008; Pattij et al., 2012; Robinson, 2012). ATM potentially increases the magnitude and duration of the inhibitory component either by indirectly increasing α2-mediated collateral or autoinhibition (Aghajanian et al., 1977; Ennis and Aston-Jones, 1986; Ivanov and Aston-Jones, 1995), or by potentiating inhibitory projections from the nucleus paragigantocelluaris (Aston-Jones et al., 1992). The post-activation pause of LC neurons may play a critical role in the efficacy of information transfer following events that phasically activate LC (Berridge and Waterhouse, 2003). Although the physiological significance of this component is still not known, it is possible that LC phasic inhibition enhances cortical responses to stimuli (Waterhouse et al., 1998) or makes LC neurons refractory to further stimulation for a longer period, thus increasing focused attention and inhibition of distracting stimuli. A similar result of LC post-activation inhibitory response on attentional processes (‘attentional blink’) has been proposed in previous neural modeling studies (Nieuwenhuis et al., 2005b).
The P:T ratio of the excitatory response was increased after the 1mg/kg ATM dose and in ~46% of neurons after 0.3 mg/kg, which indicates a borderline (though non-significant) effect at this intermediate dose. Moreover, both 0.3 and 1 mg/kg of ATM increased the P:T ratio of the inhibitory response. Similar increases in P:T ratios have been observed after desipramine (Valentino and Curtis, 1991) and methylphenidate (Devilbiss and Berridge, 2006) administration in anesthetized animals, although this latter drug also decreased the P:T ratio of the excitatory response. Importantly, the increase in P:T ratios after ATM was significant only after 90 min from its administration, which is relevant for behavioral studies involving the use of this drug (although absorption rates may be different in the anesthetized preparation) and may explain the discrepancy with the effects of other putative cognitive enhancers. There was also a consistent increase in the variability of the P:T ratio measures after both 0.3 and 1 mg/kg of ATM (Fig. 4). This is unlikely to be the consequence of non-specific ‘noise’ in the data because the increased variability was only present during the second 1h-time window (90–150 min) and not during the other time intervals considered. Instead, visual inspection of single data-points suggests that it reflects time-dependent effects of ATM that reached a peak approximately 130 min post-injection for both effective doses. Our ability to track ATM effects on LC activity for long time periods is important due to the long lasting effects of this drug, which has been shown to significantly increase extracellular NE in PFC of freely moving rats for at least 4 hours post-injection at the 1 mg/kg dose (Bymaster et al., 2002).
It is known that optimal levels of P:T discharge are required for successful performance during attentional tasks (Clayton et al., 2004; Rajkowski et al., 2004) and that ATM and methylphenidate are able to increase ‘signals’ and decrease ‘noise’ in prefrontal areas via effects on α2 and dopaminergic D1 receptors, respectively (Arnsten and Dudley, 2005; Gamo and Arnsten, 2011; Gamo et al., 2010; Robbins and Arnsten, 2009). Local infusions of ATM or methylphenidate in rat PFC improve performance on cognitive tasks (Bari et al., 2011; Devilbiss and Berridge, 2008), and these effects seem to be mediated at least in part by their action on the noradrenergic system (Berridge et al., 2006; Bymaster et al., 2002; Hannestad et al., 2010; Kuczenski and Segal, 2002). Here, we showed that another important site of action for the pro-cognitive effects of ATM is the LC, where it profoundly affects the firing rate characteristics of this nucleus that are known to be involved in higher order cognitive processes (Aston-Jones et al., 2000). Although the precise behavioral correlate of the increase in P:T ratio is still unknown, converging evidence has linked optimal levels of phasic and tonic discharge to improved behavioral flexibility and decision-making (Aston-Jones and Cohen, 2005a; Nieuwenhuis et al., 2005a), both of which are considered ‘prefrontal’ functions.
The LC receives inputs from a variety of brain regions, including the PFC. It is thus very important for future studies to investigate the effects of cognition-enhancing drugs on the activity of afferent connections to the LC, as well as its output activity. LFP recordings provide a measure of the simultaneous inputs of cellular ensembles to the neural population around the recording site (Creutzfeldt et al., 1966; Katzner et al., 2009; Klee et al., 1965; Mitzdorf, 1985). We recorded average evoked LFPs in LC and divided the observed response into 3 components: (Neg1, Neg2 and Pos3). During the first time period after ATM administration (30–90 min) we found that the lower dose of ATM (0.1 mg/kg) decreased both Neg1 and Pos3 components. Compared to pre-drug levels, higher doses of ATM caused an increase in the amplitude of both negative components, which are associated with phasic excitatory activity in LC single-unit recordings (Aston-Jones and Bloom, 1981a). The increase in negative components obtained after higher doses of ATM may thus potentially represent increased input to LC after sensory stimulation, although these results do not inform us about the brain site where this input originates. During the second time window considered (90–150 min) the 1 mg/kg decreased both Neg1 and Pos3 and 0.1 mg/kg also decreased this latter component. The effect of the lower dose of ATM (0.1 mg/kg) on average sensory-evoked field responses was surprising as this dose did not elicit any significant effect on single-unit LC activity. Since LFPs are thought to represent integrated synaptic potentials in local neurons, it is possible that low doses of ATM are able to selectively modulate sensory-evoked responses in other brain areas projecting to the LC, potentially through high affinity α2 postsynaptic receptors (Ramos and Arnsten, 2007). Accordingly, it has been shown that a low dose of the NE reuptake inhibitor desipramine causes a decrease of extracellular NE in PFC, whereas higher doses increase NE levels there and both high and low dose levels increase NE in the LC (Mateo et al., 1998). Further studies in behaving animals are warranted for a more exhaustive interpretation of the present results.
LC neurons are characterized by synchronous oscillatory activity (Ivanov and Aston-Jones, 1995; Williams and Marshall, 1987) which appears to be regulated by afferent projections and depends on electrotonic coupling (Aston-Jones et al., 1991; Ballantyne et al., 2004; Brown et al., 2004; Ishimatsu and Williams, 1996). Synchronous activity in the LC may arise from its distal dendrites located in the pericoerulear region (Ishimatsu and Williams, 1996), which is known to receive afferent inputs from distant brain areas (Aston-Jones et al., 1995; Ivanov and Aston-Jones, 1995; Shipley et al., 1996) and has been implicated in neural development (Christie et al., 1989) and cognitive performance (Usher et al., 1999). Moreover, it has been reported that LC and PFC present episodes of slow, synchronous oscillatory activity (Lestienne et al., 1997) and that LC-NE neurons modulate neural oscillations in several areas of the brain (Brown et al., 2005; Delagrange et al., 1993; Delagrange et al., 1989; Dzirasa et al., 2010; Kalauzi et al., 2009; Walling et al., 2011). To test whether ATM modulates the interplay between LC and other structures, we analyzed the amount of synchronous activity (coherence) between spike trains and LFPs in LC and between cortical EEG and LC local fields. High levels of coherence within or between brain areas are believed to reflect increased neuronal interaction (‘coupling’) among interconnected networks (Fries, 2005; Womelsdorf et al., 2007).
We found that during the first 30–90 min post-administration, only the high dose of ATM (1 mg/kg) generally increased spike-field coherence across theta, alpha and beta bands. However, during the second time window considered, almost all coherence values returned to pre-drug levels. Coherence between cortical EEG and LFPs recorded in LC was also increased by ATM, but only at the higher doses. 0.3 mg/kg of ATM increased coherence only in the alpha and beta frequencies, whereas 1 mg/kg increased coherence for all frequency bands considered. Both these effects were long lasting and persisted from 30 to 150 min post-administration. The lower dose of ATM (0.1 mg/kg) had the opposite effect, decreasing coherence values across frequencies, except for the beta band. As previously discussed, low doses of NE reuptake inhibitors may decrease NE extracellular content in prefrontal areas (Fernandez-Pastor et al., 2005; Mateo et al., 1998), which would explain the biphasic effect of high and low doses of ATM on EEG-field coherence and sensory-evoked average LFPs. These results are consistent with the finding that hypo-noradrenergic mice show decreased cross-structural coherence across several interconnected brain areas together with behavioural hyperactivity and that these deficits are reversed by treatment with NE precursors (Dzirasa et al., 2011). On the other hand, it has been proposed that the LC-NE system exerts a tonic inhibitory activity on brain areas responsible for specific oscillatory rhythms (Rougeul-Buser and Buser, 1997). This latter mechanism may also contribute to the observed modulatory activity of ATM on coherence levels in the present experiments, especially at high doses that decrease LC spontaneous firing rate. Thus, although the precise mechanisms underlying the modulation of spike-field and EEG-field coherence in the LC after ATM administration are not clear yet, the present results indicate that this drug affects brain areas connected to the LC promoting neural coordination which is known to correlate with cognitive task performance (e.g., Tallon-Baudry et al., 2004; Wu et al., 2008).
In summary, the present study demonstrates for the first time that ATM modulates LC neuronal activity in a way potentially associated with its beneficial effects on cognitive functions as shown by a number of psychopharmacological studies in human (Bidwell et al., 2011; Del Campo et al., 2011; Robbins and Arnsten, 2009) and non-human subjects (Berridge and Devilbiss, 2011; Eagle et al., 2008; Floresco and Jentsch, 2011; Pattij and Vanderschuren, 2008). Increased ‘gain’ or ‘signal-to-noise ratio’ have been observed in several brain areas after NE application or noradrenergic pathway stimulation (Foote et al., 1975; Freedman et al., 1977; Moises et al., 1981; Waterhouse et al., 1981; Waterhouse and Woodward, 1980). The present results indicate that increased P:T ratios in the source of NE (LC neurons) may also contribute to these modulatory effects. Thus, the LC, together with specific regions within the PFC (Bari et al., 2011; Chamberlain et al., 2009; Gamo et al., 2010), is likely to be one of the major substrates for ATM effects on cognition, where it decreases tonic firing rates and enhances the P:T ratio of the sensory-evoked phasic response. The important modulation of sensory-evoked LFPs, spike-field coherence and EEG-field coherence by ATM should encourage further studies at the level of cooperating circuitries in the brain and the way neural synchronization affects performance on cognitive tasks.
Highlights.
Atomoxetine attenuates spontaneous firing rate of locus coeruleus neurons in vivo.
Atomoxetine increases the phasic:tonic ratio of locus coeruleus response to stimuli.
Atomoxetine modulates the average evoked field response in locus coeruleus.
Atomoxetine modulates spike-field and cortical EEG-field coherence in locus coeruleus.
Acknowledgments
The authors would like to thank Dr E. M. Vazey for help with electrophysiology, Dr T. Tompa for advice on LFP analysis and R. Wise for help with histology. AB is recipient of an International Trainee Fellowship Award from the MUSC Neuroscience Institute. This work was supported by PHS grant R01-MH MH092868, R37-DA06214 and the MUSC Neuroscience Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Mescaline and LSD facilitate the activation of locus coeruleus neurons by peripheral stimuli. Brain Res. 1980;186:492–498. doi: 10.1016/0006-8993(80)90997-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Cedarbaum JM, Wang RY. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Res. 1977;136:570–577. doi: 10.1016/0006-8993(77)90083-x. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Roussel B, Lin JS, Chouvet G, Jouvet M. Effect of modafinil and amphetamine on the rat catecholaminergic neuron activity. Neurosci Lett. 1991;123:20–22. doi: 10.1016/0304-3940(91)90148-m. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Modulation of prefrontal cortical-striatal circuits: relevance to therapeutic treatments for Tourette syndrome and attention-deficit hyperactivity disorder. Adv Neurol. 2001;85:333–341. [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Astier B, Ennis M. Inhibition of noradrenergic locus coeruleus neurons by C1 adrenergic cells in the rostral ventral medulla. Neuroscience. 1992;48:371–381. doi: 10.1016/0306-4522(92)90497-p. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005a;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005b;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Bloom FE. Low doses of ethanol disrupt sensory responses of brain noradrenergic neurones. Nature. 1982;296:857–860. doi: 10.1038/296857a0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Segal M, Bloom FE. Brain aminergic axons exhibit marked variability in conduction velocity. Brain Research. 1980;195:215–222. doi: 10.1016/0006-8993(80)90880-x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The rat nervous system. 2. Academic Press; New York: 1995. pp. 183–214. [Google Scholar]
- Baarendse PJ, Vanderschuren LJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D, Andrzejewski M, Muckenhoff K, Scheid P. Rhythms, synchrony and electrical coupling in the Locus coeruleus. Respir Physiol Neurobiol. 2004;143:199–214. doi: 10.1016/j.resp.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM. Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology. 2009a;57:702–707. doi: 10.1016/j.neuropharm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Brown CR, Bruggemann JM, van Rijbroek I. Caffeine effects on resting-state arousal in children. Int J Psychophysiol. 2009b;73:355–361. doi: 10.1016/j.ijpsycho.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e101–111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacol Biochem Behav. 2011;99:262–274. doi: 10.1016/j.pbb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry. 2007;61:1340–1350. doi: 10.1016/j.biopsych.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Brown E, Moehlis J, Holmes P, Clayton E, Rajkowski J, Aston-Jones G. The influence of spike rate and stimulus duration on noradrenergic neurons. J Comput Neurosci. 2004;17:13–29. doi: 10.1023/B:JCNS.0000023867.25863.a4. [DOI] [PubMed] [Google Scholar]
- Brown RA, Walling SG, Milway JS, Harley CW. Locus ceruleus activation suppresses feedforward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci. 2005;25:1985–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Holdnack J, Saylor K, Adler L, Spencer T, Williams DW, Padival AK, Schuh K, Trzepacz PT, Kelsey D. Effect of atomoxetine on executive function impairments in adults with ADHD. J Atten Disord. 2011;15:130–138. doi: 10.1177/1087054709356165. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Noradrenergic neurons of the locus coeruleus: inhibition by epinephrine and activation by the alpha-antagonist piperoxane. Brain Res. 1976;112:413–419. doi: 10.1016/0006-8993(76)90297-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J Neurosci. 1989;9:3584–3589. doi: 10.1523/JNEUROSCI.09-10-03584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Clarke DC, Croft RJ. Effects of stimulant medications on children with attention-deficit/hyperactivity disorder and excessive beta activity in their EEG. Clin Neurophysiol. 2003;114:1729–1737. doi: 10.1016/s1388-2457(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt OD, Watanabe S, Lux HD. Relations between EEG phenomena and potentials of single cortical cells. I. Evoked responses after thalamic and erpicortical stimulation. Electroencephalogr Clin Neurophysiol. 1966;20:1–18. doi: 10.1016/0013-4694(66)90136-2. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Conti E, Valentino RJ. Cocaine effects on brain noradrenergic neurons of anesthetized and unanesthetized rats. Neuropharmacology. 1993;32:419–428. doi: 10.1016/0028-3908(93)90165-y. [DOI] [PubMed] [Google Scholar]
- Dafny N. Catecholamine-modulated field potentials in the hypothalamus. Neuroendocrinology. 1975;18:42–54. doi: 10.1159/000122382. [DOI] [PubMed] [Google Scholar]
- Dafny N, Burks TF. Neurophysiological changes in caudate nucleus and substantia nigra following morphine treatment. Neuropharmacology. 1976;15:547–554. doi: 10.1016/0028-3908(76)90106-4. [DOI] [PubMed] [Google Scholar]
- De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Delagrange P, Canu MH, Rougeul A, Buser P, Bouyer JJ. Effects of locus coeruleus lesions on vigilance and attentive behaviour in cat. Behav Brain Res. 1993;53:155–165. doi: 10.1016/s0166-4328(05)80275-x. [DOI] [PubMed] [Google Scholar]
- Delagrange P, Tadjer D, Bouyer JJ, Rougeul A, Conrath M. Effect of DSP4, a neurotoxic agent, on attentive behaviour and related electrocortical activity in cat. Behav Brain Res. 1989;33:33–43. doi: 10.1016/s0166-4328(89)80016-6. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J Pharmacol Exp Ther. 2006;319:1327–1335. doi: 10.1124/jpet.106.110015. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Fuentes R, Kumar S, Potes JM, Nicolelis MA. Chronic in vivo multi-circuit neurophysiological recordings in mice. J Neurosci Methods. 2011;195:36–46. doi: 10.1016/j.jneumeth.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Phillips HW, Sotnikova TD, Salahpour A, Kumar S, Gainetdinov RR, Caron MG, Nicolelis MA. Noradrenergic control of cortico-striato-thalamic and mesolimbic cross-structural synchrony. J Neurosci. 2010;30:6387–6397. doi: 10.1523/JNEUROSCI.0764-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Res. 1986;374:299–305. doi: 10.1016/0006-8993(86)90424-5. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Michelson D, Adler L, Reimherr F, Seidman L. Atomoxetine and stroop task performance in adult attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15:664–670. doi: 10.1089/cap.2005.15.664. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pastor B, Mateo Y, Gomez-Urquijo S, Javier Meana J. Characterization of noradrenaline release in the locus coeruleus of freely moving awake rats by in vivo microdialysis. Psychopharmacology (Berl) 2005;180:570–579. doi: 10.1007/s00213-005-2181-y. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipolito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl) 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975;86:229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hoffer BJ, Woodward DJ, Puro D. Interaction of norepinephrine with cerebellar activity evoked by mossy and climbing fibers. Exp Neurol. 1977;55:269–288. doi: 10.1016/0014-4886(77)90175-3. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Shang CY. Improvement of executive functions in boys with attention deficit hyperactivity disorder: an open-label follow-up study with once-daily atomoxetine. Int J Neuropsychopharmacol. 2010;13:243–256. doi: 10.1017/S1461145709990836. [DOI] [PubMed] [Google Scholar]
- Graham AW, Aghajanian GK. Effects of amphetamine on single cell activity in a catecholamine nucleus, the locus coeruleus. Nature. 1971;234:100–102. doi: 10.1038/234100b0. [DOI] [PubMed] [Google Scholar]
- Grandoso L, Pineda J, Ugedo L. Comparative study of the effects of desipramine and reboxetine on locus coeruleus neurons in rat brain slices. Neuropharmacology. 2004;46:815–823. doi: 10.1016/j.neuropharm.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH, Malison RT, Carson RE, Ding YS. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68:854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC, editor. Statistical Methods for Psychology. Duxbury Press; Belmont, CA: 1997. [Google Scholar]
- Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J Neurosci. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Extranuclear dendrites of locus coeruleus neurons: activation by glutamate and modulation of activity by alpha adrenoceptors. J Neurophysiol. 1995;74:2427–2436. doi: 10.1152/jn.1995.74.6.2427. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Aarde SM, Seu E. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 2009;202:497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- Kalauzi A, Kesic S, Saponjic J. Cortico-pontine theta synchronization phase shift following monoaminergic lesion in rat. J Physiol Pharmacol. 2009;60:79–84. [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja FA, Tsui JM, Pack CC. Pattern motion selectivity of spiking outputs and local field potentials in macaque visual cortex. J Neurosci. 2009;29:13702–13709. doi: 10.1523/JNEUROSCI.2844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee MR, Offenloch K, Tigges J. Cross-Correlation Analysis of Electroencephalographic Potentials and Slow Membrane Transients. Science. 1965;147:519–521. doi: 10.1126/science.147.3657.519. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Katz JL. Response requirement and increases in accuracy produced by stimulant drugs in a 5-choice serial reaction-time task in rats. Psychopharmacology (Berl) 2011;213:723–733. doi: 10.1007/s00213-010-2027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf J, Bunney BS, Aghajanian GK. Noradrenergic neurons: morphine inhibition of spontaneous activity. Eur J Pharmacol. 1974;25:165–169. doi: 10.1016/0014-2999(74)90045-4. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix D, Ferron A. Electrophysiological effects of methylphenidate on the coeruleo-cortical noradrenergic system in the rat. Eur J Pharmacol. 1988;149:277–285. doi: 10.1016/0014-2999(88)90658-9. [DOI] [PubMed] [Google Scholar]
- Lestienne R, Herve-Minvielle A, Robinson D, Briois L, Sara SJ. Slow oscillations as a probe of the dynamics of the locus coeruleus-frontal cortex interaction in anesthetized rats. J Physiol Paris. 1997;91:273–284. doi: 10.1016/s0928-4257(97)82407-2. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Pineda J, Meana JJ. Somatodendritic alpha2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J Neurochem. 1998;71:790–798. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Miguelez C, Fernandez-Aedo I, Torrecilla M, Grandoso L, Ugedo L. alpha(2)-Adrenoceptors mediate the acute inhibitory effect of fluoxetine on locus coeruleus noradrenergic neurons. Neuropharmacology. 2009;56:1068–1073. doi: 10.1016/j.neuropharm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Mohamed AD, Sahakian BJ. The ethics of elective psychopharmacology. Int J Neuropsychopharmacol. 2011:1–13. doi: 10.1017/S146114571100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moises HC, Waterhouse BD, Woodward DJ. Locus coeruleus stimulation potentiates Purkinje cell responses to afferent input: the climbing fiber system. Brain Res. 1981;222:43–64. doi: 10.1016/0006-8993(81)90939-2. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Csicsvari J, Penttonen M, Hetke J, Wise K, Buzsaki G. Extracellular recording and analysis of neuronal activity: From single cells to ensembles. In: Eichenbaum HB, Davis JL, editors. Neuronal ensembles: Strategies for recording and decoding. Wiley-Liss; New York, NY: 1998. [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl) 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005a;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Gilzenrat MS, Holmes BD, Cohen JD. The role of the locus coeruleus in mediating the attentional blink: a neurocomputational theory. J Exp Psychol Gen. 2005b;134:291–307. doi: 10.1037/0096-3445.134.3.291. [DOI] [PubMed] [Google Scholar]
- Nyback HV, Walters JR, Aghajanian GK, Roth RH. Tricyclic antidepressants: effects on the firing rate of brain noradrenergic neurons. Eur J Pharmacol. 1975;32:302–312. doi: 10.1016/0014-2999(75)90297-6. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Jones RS, Steinmann MW. The locus coeruleus: actions of psychoactive drugs. Experientia. 1983;39:242–249. doi: 10.1007/BF01955287. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Steinmann MW, Jones RS. Locus coeruleus as a target for psychogeriatric agents. Ann N Y Acad Sci. 1985;444:394–405. doi: 10.1111/j.1749-6632.1985.tb37603.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Wetzler C, Hackett A, Hanania T. Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. Int J Neuropsychopharmacol. 2011:1–15. doi: 10.1017/S1461145711001635. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Schoffelmeer AN, van Gaalen MM. On the improvement of inhibitory response control and visuospatial attention by indirect and direct adrenoceptor agonists. Psychopharmacology (Berl) 2012;219:327–340. doi: 10.1007/s00213-011-2405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Petsche H, Pockberger H, Rappelsberger P. On the search for the sources of the electroencephalogram. Neuroscience. 1984;11:1–27. doi: 10.1016/0306-4522(84)90212-4. [DOI] [PubMed] [Google Scholar]
- Pitts DK, Marwah J. Electrophysiological actions of cocaine on noradrenergic neurons in rat locus ceruleus. J Pharmacol Exp Ther. 1987;240:345–351. [PubMed] [Google Scholar]
- Pliszka SR. Non-stimulant treatment of attention-deficit/hyperactivity disorder. CNS Spectr. 2003;8:253–258. doi: 10.1017/s1092852900018460. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, McCracken JT, Maas JW. Catecholamines in attention-deficit hyperactivity disorder: current perspectives. J Am Acad Child Adolesc Psychiatry. 1996;35:264–272. doi: 10.1097/00004583-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res Bull. 1994;35:607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–371. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hsiao SS, Crone NE, Franaszczuk PJ, Niebur E. Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J Neurosci. 2008;28:7334–7343. doi: 10.1523/JNEUROSCI.1588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Central norepinephrine neurons and behavior. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. [Google Scholar]
- Robinson ES. Blockade of noradrenaline re-uptake sites improves accuracy and impulse control in rats performing a five-choice serial reaction time tasks. Psychopharmacology (Berl) 2012;219:303–312. doi: 10.1007/s00213-011-2420-3. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rougeul-Buser A, Buser P. Rhythms in the alpha band in cats and their behavioural correlates. Int J Psychophysiol. 1997;26:191–203. doi: 10.1016/s0167-8760(97)00764-2. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Tepper JM, Young SJ, Groves PM. Amphetamine’s effects on terminal excitability of noradrenergic locus coeruleus neurons are impulse-dependent at low but not high doses. Brain Res. 1985;341:155–163. doi: 10.1016/0006-8993(85)91483-0. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morein-Zamir S. Neuroethical issues in cognitive enhancement. J Psychopharmacol. 2011;25:197–204. doi: 10.1177/0269881109106926. [DOI] [PubMed] [Google Scholar]
- Salek RL, Claussen CM, Perez A, Dafny N. Acute and chronic methylphenidate alters prefrontal cortex neuronal activity recorded from freely behaving rats. Eur J Pharmacol. 2012;679:60–67. doi: 10.1016/j.ejphar.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuvee-Moreau JJ, Dresse AE. Effect of various antidepressant drugs on the spontaneous firing rate of locus coeruleus and dorsal raphe neurons of the rat. Eur J Pharmacol. 1979;57:219–225. doi: 10.1016/0014-2999(79)90368-6. [DOI] [PubMed] [Google Scholar]
- Segal M, Bloom FE. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res. 1974;72:79–97. doi: 10.1016/0006-8993(74)90652-0. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects gain signal to noise ratio and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Fu L, Ennis M, Liu WL, Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J Comp Neurol. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Simpson D, Perry CM. Atomoxetine. Paediatr Drugs. 2003;5:407–415. doi: 10.2165/00128072-200305060-00005. discussion 416–407. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy JP, Mrini A, Lafaille F, Doucet G, Descarries L. Comparative evaluation of [3H]WIN 35428 and [3H]GBR 12935 as markers of dopamine innervation density in brain. Synapse. 1997;25:163–175. doi: 10.1002/(SICI)1098-2396(199702)25:2<163::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Harding M, Faraone SV, Seidman L. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155:693–695. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- Starke K, Montel H. Involvement of alpha-receptors in clonidine-induced inhibition of transmitter release from central monoamine neurones. Neuropharmacology. 1973;12:1073–1080. doi: 10.1016/0028-3908(73)90051-8. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Bunney BS, Aghajanian GK. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Res. 1975;92:291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Usdin T. Feedback inhibition of brain noradrenaline neurons by tricyclic antidepressants: alpha-receptor mediation. Science. 1978;202:1089–1091. doi: 10.1126/science.213833. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology. 2006;50:755–760. doi: 10.1016/j.neuropharm.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Overshiner CD, Davis RJ, Perry KW, Wolff M, McKinzie DL, Witkin JM, Nomikos GG. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol Psychiatry. 2006;11:187–195. doi: 10.1038/sj.mp.4001763. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL. Pharmacology of locus coeruleus spontaneous and sensory-evoked activity. Prog Brain Res. 1991;88:249–256. doi: 10.1016/s0079-6123(08)63814-5. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Parris DG, Wehby RG. Antidepressant actions on brain noradrenergic neurons. J Pharmacol Exp Ther. 1990;253:833–840. [PubMed] [Google Scholar]
- Walling SG, Brown RA, Milway JS, Earle AG, Harley CW. Selective tuning of hippocampal oscillations by phasic locus coeruleus activation in awake male rats. Hippocampus. 2011;21:1250–1262. doi: 10.1002/hipo.20816. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative neurotransmitters. Exp Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20:907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res. 1998;790:33–44. doi: 10.1016/s0006-8993(98)00117-6. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull. 1988;21:425–432. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Woodward DJ. Interaction of norepinephrine with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol. 1980;67:11–34. doi: 10.1016/0014-4886(80)90159-4. [DOI] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J Neurosci. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP, Hyslop DK, Franklin S, Porsolt RD, Bonsignori A, Carfagna N, McArthur RA. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Moises HC, Waterhouse BD, Yeh HH, Cheun JE. Modulatory actions of norepinephrine on neural circuits. Adv Exp Med Biol. 1991;287:193–208. doi: 10.1007/978-1-4684-5907-4_16. [DOI] [PubMed] [Google Scholar]
- Wu W, Wheeler DW, Staedtler ES, Munk MH, Pipa G. Behavioral performance modulates spike field coherence in monkey prefrontal cortex. Neuroreport. 2008;19:235–238. doi: 10.1097/WNR.0b013e3282f49b29. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic methylphenidate modulates locomotor activity and sensory evoked responses in the VTA and NAc of freely behaving rats. Neuropharmacology. 2006a;51:546–556. doi: 10.1016/j.neuropharm.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Sensory-evoked potentials recordings from the ventral tegmental area, nucleus accumbens, prefrontal cortex, and caudate nucleus and locomotor activity are modulated in dose-response characteristics by methylphenidate. Brain Res. 2006b;1073–1074:164–174. doi: 10.1016/j.brainres.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Rapoport JL. Neurobiology of attention deficit disorder with hyperactivity: where have we come in 50 years? J Am Acad Child Adolesc Psychiatry. 1987;26:676–686. doi: 10.1097/00004583-198709000-00011. [DOI] [PubMed] [Google Scholar]