Abstract
STAT1 is a member of the JAK-STAT signaling family and plays a key role in facilitating gene transcription in response to activation of the Type I and Type II interferon (IFN) receptors. TYK2 is essential for type I, but not type II, IFN-induced STAT1 activation. Previous studies show that STAT1-deficient mice are resistant to endotoxin-induced shock. The goal of the present study was to assess the response of STAT1-and TYK2-deficient mice to septic shock caused by cecal ligation and puncture (CLP). Endpoints included survival, core temperature, organ injury, systemic cytokine production and bacterial clearance. Results showed that survival rates were significantly higher in STAT1KO mice compared to wild type controls (80% vs 10%). The improved survival of STAT1KO mice was associated with less hypothermia, metabolic acidosis, hypoglycemia and hepatocellular injury. Plasma IL-6, MIP-2, CXCL10 and IFNα concentrations were significantly lower in STAT1KO mice than in wild type mice. In the absence of antibiotic treatment, blood and lung bacterial counts were significantly lower in STAT1KO mice than in controls. However, treatment with antibiotics ablated that difference. A survival advantage was not observed in TYK2-deficient mice compared to control. However, CLP-induced hypothermia and systemic IL-6 and CXCL10 production were significantly attenuated in TYK2-deficient mice. These results indicate that STAT1 activation is an important factor in the pathogenesis of CLP-induced septic shock and is associated with the development of systemic inflammation and organ injury. TYK2 activation also appears to contribute to CLP-induced inflammation, but to a lesser extent than STAT1.
Keywords: inflammation, bacteremia, organ injury, physiologic dysfunction, interferons, TYK2
Introduction
STAT1 is a transcription factor that is a member of the Janus-signal transducer and activator of transcription (JAK-STAT) signaling pathway and is essential for effective interferon (IFN)-induced intracellular signaling (1). Activation of the type I interferon (IFNα/β) receptor (IFNAR) causes recruitment and formation of activated STAT1/STAT2 heterodimers through trans-phosphorylation of receptor-associated JAK1 and TYK2 moieties (2). Type II IFN (IFNγ) receptor activation induces the phosphorylation of receptor-associated JAK1 and JAK2 proteins, recruitment of STAT1 to the cytoplasmic portion of the activated receptor complex and formation of STAT1 homodimers. The generated STAT1 homo- and STAT1/STAT2 heterodimers translocate from cytoplasm to nucleus and facilitate transcription of IFN-inducible gene products (3). STAT1-mediated gene transcription is crucial for mounting an effective host response to pathogenic viruses and intracellular bacteria such as Mycobacteria and Listeria (4–5). STAT1-deficient mice are vulnerable to a multitude of viral pathogens whereas humans with STAT1 deficiency show increased susceptibility to infection with Mycobacteria and certain pathogenic viruses (2,6).
STAT1-deficient mice are resistant to lipopolysaccharide (LPS)-induced shock. Kamezaki et al (7) showed decreased systemic inflammation and improved survival in STAT1 and TYK2 knockout mice challenged with LPS compared to wild type controls. Karaghiosoff and colleagues (8) also showed that STAT1-, TYK2- and IFNβ-deficient mice are resistant to LPS-induced inflammation, physiologic dysfunction and mortality. In other studies, Shirota and co-workers (9) reported that blockade of STAT1 phosphorylation using synthetic oligonucleotides confers resistance to LPS-induced injury whereas Kim et al (10) demonstrated that LPS-induced production of the pro-inflammatory mediator HMGB1 is attenuated in STAT1-deficient mice. However, the effect of STAT1-deficiency on the host response to more clinically relevant models of sepsis, such as the cecal ligation and puncture (CLP) model, has not been thoroughly investigated. Current studies on the contribution of STAT1 activation to the pathogenesis of CLP-induced sepsis are limited to measurement of systemic cytokine concentrations (11). Therefore, the goal of the present study was to assess the importance of STAT1 activation during the pathogenesis of CLP-induced sepsis with emphasis on evaluation of systemic inflammation, bacterial clearance, physiologic function and survival.
Methods
Mice
Female, 10 to 12 week-old 129S6/SvEvTac wild type and homozygous STAT1 null (129S6/SvEv-STAT1tm1Rds) mice were purchased from Taconic Farms (Hudson, NY). C57BL/10SnJ wild type and B10.D1-H2q/SgJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The B10.D1-H2q/SgJ strain is homozygous for a naturally occurring missense mutation of TYK2 resulting in loss of protein function. All studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and complied with the National Institutes of Health Guide for the Care and Use of Experimental Animals.
Cecal ligation and puncture (CLP)
CLP was performed as previously described (12). Briefly, mice were anesthetized with 2–3% isoflurane in oxygen. After shaving and aseptic preparation, a 1- to 2-cm midline incision was made through the abdominal wall; the cecum was identified and ligated with a 3–0 silk tie at 1 cm from the tip. A double puncture of the cecal wall was performed with a 20-gauge needle. The incision was closed with Autoclips (Becton Dickinson, sparks, MD). Mice were resuscitated with intraperitoneal injection of either 1 ml of lactated Ringer’s (LR) solution alone or LR containing Primaxin (25 mg/kg, Merck & Co, Whitehouse Station, NJ) immediately after CLP. Primaxin is a formulation of imipenem (a potent, broad spectrum thienamycin antibiotic) and cilastatin sodium (the inhibitor of the renal dipeptidase, dehydropeptidase I). Mice received buprenorphene (2.5 ug subcutaneously) 30 minutes before surgery and every 8–12 hours thereafter for analgesia. In survival studies, all mice received treatment with Primaxin.
ELISA
Heparinized blood was obtained by carotid laceration and plasma was harvested from centrifuged blood (2000 × g for 15 min). Lavage of the peritoneal cavity was performed with 3 ml of sterile phosphate buffered saline. Interleukin-6 (IL-6), MIP-2, IFNα and CXCL10 concentrations in peritoneal lavage fluid and plasma were measured according to the manufacturer’s protocol (eBioscience, San Diego, CA, USA; R&D Systems, Minneapolis, MN, USA). IL-6 and MIP-2 were measured because studies from our laboratory, and others, show that high plasma concentrations of those cytokines correlate with mortality in the CLP model (13). Production of CXCL10 (formerly known as interferon-inducible protein 10 or IP-10) is produced primarily in response to type I and type II interferons and serves as a marker of systemic interferon activity (14). IFNα is an important inducer of STAT1 activation (1). Myeloperoxidase (MPO) concentratons in peritoneal lavage fluid was measured according to manufacturer recommendations (Hycult Biotech, Plymouth Meeting, PA). Cytokine and MPO concentrations were determined by measuring optical density at 450 nm using a microtiter plate reader (Dynatech Laboratories, Chantilly, VA, USA).
Measurement of Temperature and Bacterial Counts
Body temperature was measured by insertion of a rectal temperature probe immediately after induction of anesthesia with 2–3% isoflurane in oxygen. Bacterial counts were performed on blood, peritoneal lavage fluid and lung. Samples of plasma and peritoneal lavage fluid were obtained as described above. Lung tissue was harvested by thoracotomy under aseptic conditions, weighed and homogenized in sterile phosphate buffered saline to achieve a final concentration of 11 mg of tissue per ml of saline. Samples were serially diluted in sterile saline and cultured on tryptic soy agar pour plates. Plates were incubated (37°C) for 24–48 hours and colony counts were performed by direct visualization.
Flow cytometry
Intraperitoneal leukocytes were harvested at 6 hours after intraperitoneal challenge with FITC-labeled Pseudomonas aeruginosa (5 × 107 colony forming units (cfu)) by lavage of the peritoneal cavity with 3 ml of sterile phosphate buffered saline. Cells were counted, placed in polystyrene tubes (1 × 106/tube) and incubated with anti-mouse CD16/32 (eBioscience, San Diego and R&D Systems, Minneapolis) to block nonspecific Fc receptor-mediated antibody binding. After washing, fluorochrome-conjugated labeling antibodies or isotype controls (0.5–1 g /tube) were added, incubated (4°C) for 30 min, and washed with 2 ml of cold PBS. Antibodies used in the analyses included PE-conjugated anti-F4/80 and APC-conjugated anti-Ly6G (eBioScience, San Diego and R&D Systems, Minneapolis). Cells were fixed in 250 μl of 1% paraformaldehyde and analyzed with the EasyCyte Guava HT flow cytometer (Milipore, Billerica, MA). Specific staining was determined by comparison with appropriate antibody isotype controls. Analysis of flow cytometry data was performed using GuavaSoft 2.2.2. (Milipore, Billerica, MA).
Measurement of blood gases and organ injury
After induction of anesthesia with isoflurane in 100% oxygen as described above, arterial blood was obtained by laceration of the carotid artery under direct visualization. Blood was harvested in heparinized syringes and blood gas measurements were performed using CG4+ and Chem8+ iStat cartridges (iStat Corporation, East Windsor, NJ). Remaining blood was centrifuged, plasma harvested and plasma ALT and AST concentrations were measured in the Clinical Pathology Laboratory at the Shriners Hospital for Children.
Statistics
All data were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Data from multiple group experiments were analyzed using one-way ANOVA followed by a post hoc Tukey’s test to compare groups. Paired data were analyzed using a paired t-test. For measurements of bacterial CFU, groups were compared using a nonparametric Kruskal-Wallis test, followed by a post hoc Dunn’s test. Survival data were analyzed using the log rank test. A value of p < 0.05 was considered statistically significant for all experiments. All values are presented as the mean ± SEM, except for bacterial counts, for which median values are designated.
Results
STAT1-deficient mice are resistant to CLP-induced septic shock
Studies were undertaken to assess survival and physiologic function in wild type and STAT1 knockout mice during CLP-induced septic shock. STAT1 knockout mice showed significantly improved survival (80% vs 10%) compared to wild type mice. (Figure 1). Core temperature was also measured because it is a reliable predictor of survival and indicator of physiologic dysfunction during septic shock caused by CLP (12;15). Wild type mice developed significant hypothermia after CLP whereas CLP-induced hypothermia was significantly attenuated in STAT1KO mice (Figure 2). Assessment of acid-base balance at 24 hours after CLP showed that wild type mice developed marked metabolic acidosis as indicated by significantly decreased pH and plasma bicarbonate and a significantly elevated base deficit (Figure 2). Blood pCO2 was significantly lower in STAT1KO mice than in wild type controls (Figure 2). Plasma glucose concentrations were significantly lower in wild type mice after CLP compared to STAT1KO mice and non-septic controls (Figure 2). Wild type mice also showed significant hepatocellular injury as indicated by elevated plasma concentrations of ALT and AST (Figure 2). Plasma AST was significantly increased in STAT1KO mice compared to non-septic controls but was significantly lower than in septic wild type mice. Plasma ALT was not significantly different when comparing non-septic and STAT1KO mice. Plasma creatinine concentrations and PaO2/FiO2 ratios were not significantly different between wild type and STAT1KO mice and were not significantly different from non-septic controls (data not shown).
Figure 1.
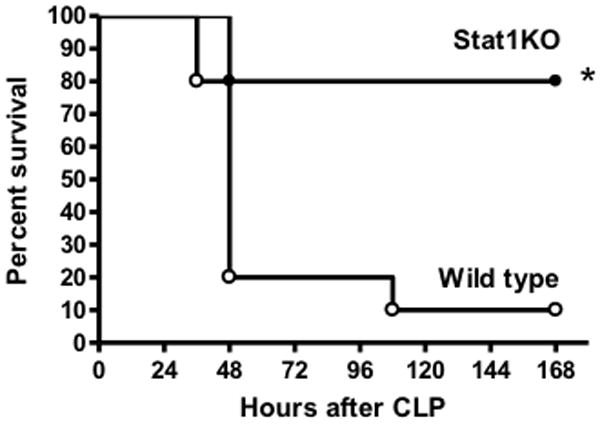
Survival of wild type and STAT1 knockout (STAT1KO) mice after CLP. Mice were resuscitated with lactated Ringer’s plus Primaxin (25 mg/kg) immediately after CLP. *p<0.05 compared to wild type, n = 15 mice per group.
Figure 2.
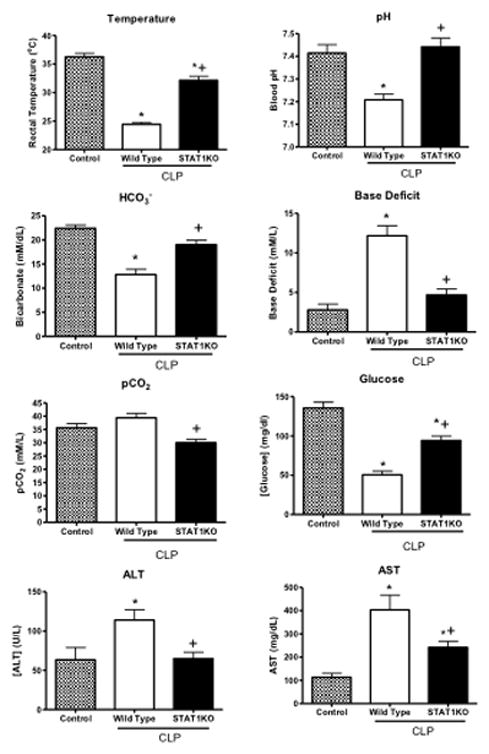
Physiologic function and organ injury in wild type and STAT1 knockout (STAT1KO) mice at 24 hours after CLP. Mice were resuscitated with lactated Ringer’s plus Primaxin (25 mg/kg) immediately after CLP. *p<0.05 compared to control (no CLP), +p<0.05 compared to wild type mice, n = 10 mice per group, values are expressed as mean ± SEM.
Systemic cytokine production and bacterial clearance in STAT1KO mice during CLP-induced sepsis
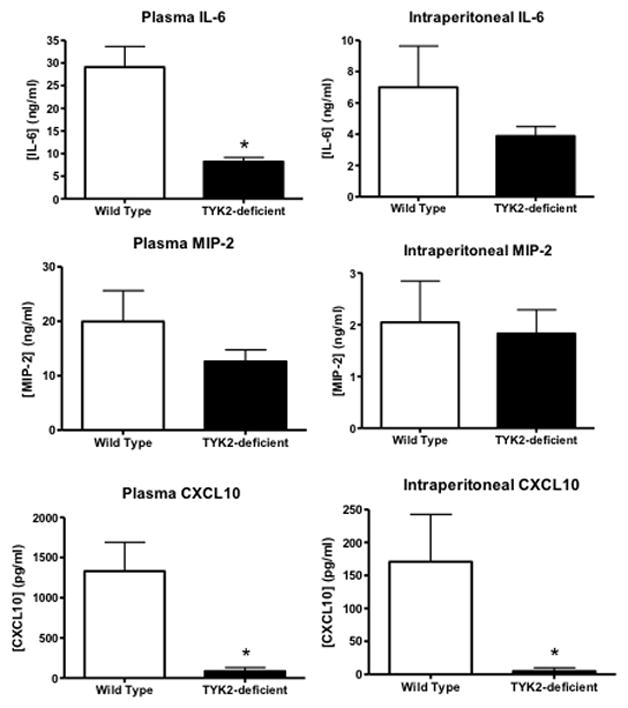
Plasma cytokine concentrations and systemic bacterial counts were measured in wild type and STAT1KO mice after CLP. Plasma concentrations of IL-6, MIP-2, CXCL10 and IFNα were significantly lower in STAT1KO mice than in wild type mice, regardless of whether Primaxin was given (Figure 3). Intraperitoneal concentrations of IL-6 were significantly lower in STAT1KO mice than in wild type controls in mice receiving LR alone or LR plus Primaxin (Figure 3). However, intraperitoneal MIP-2, CXCL10 and IFNα concentrations were not significantly different between wild type and STAT1KO mice after CLP with the exception of CXCL10 in mice that were treated with Primaxin, in which intraperitoneal CXCL10 concentrations were lower in STAT1KO mice than in wild type controls (Figure 3).
Figure 3.
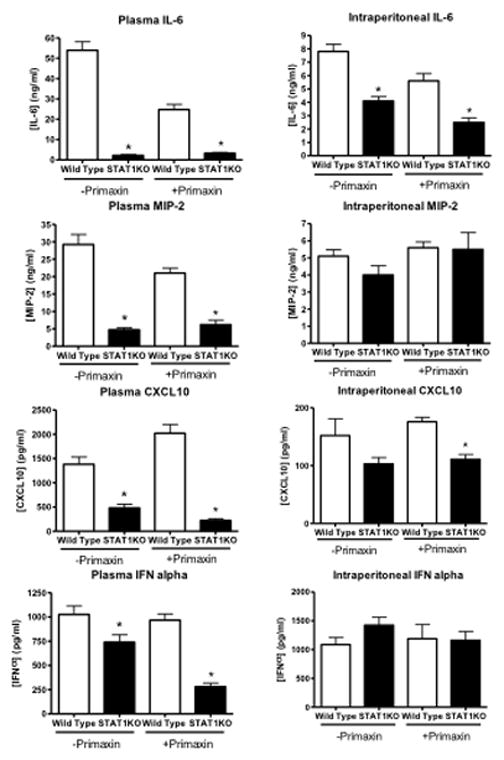
Plasma and peritoneal lavage IL-6, MIP-2, CXCL10 and IFNα concentrations in wild type and STAT1 knockout (STAT1KO) mice at 18 hours after CLP. Mice were resuscitated with lactated Ringer’s solution alone (-Primaxin) or with lactated Ringer’s solution plus Primaxin (25 mg/kg, +Primaxin). Cytokine concentrations were measured by ELISA. *p<0.05 compared to wild type, n = 10 mice per group, values are expressed as mean ± SEM.
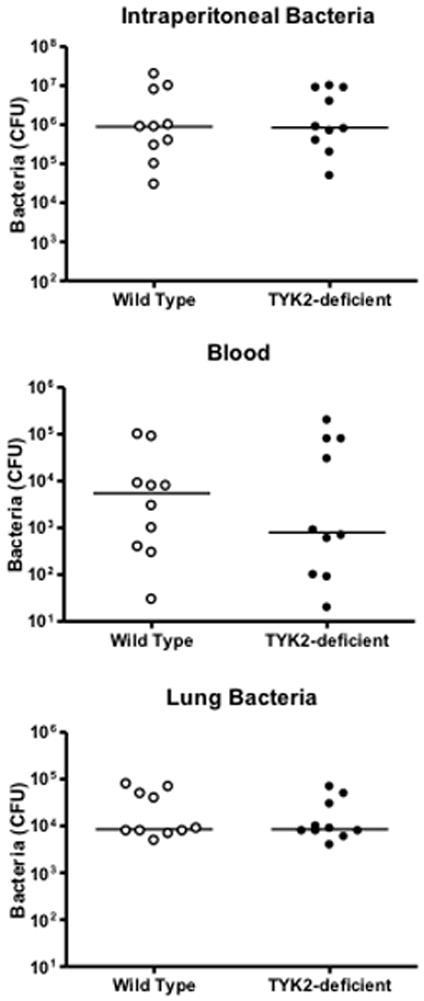
Assessment of bacterial counts showed no differences in intraperitoneal colony forming units (CFU) between wild type and STAT1KO mice, regardless of whether Primaxin was given (Figure 4). However, bacteria CFU in blood and lungs were signficantly lower in STAT1KO mice than in wild type mice when mice did not receive Primaxin treatment (Figure 4). Treatment of mice with Primaxin significantly decreased intraperitoneal, blood and lung CFU compared to mice that did not receive Primaxin treatment and no significant difference in bacterial counts were observed in peritoneal lavage fluid, blood and lungs when comparing STAT1KO and wild type mice that were treated with Primaxin (Figure 4).
Figure 4.
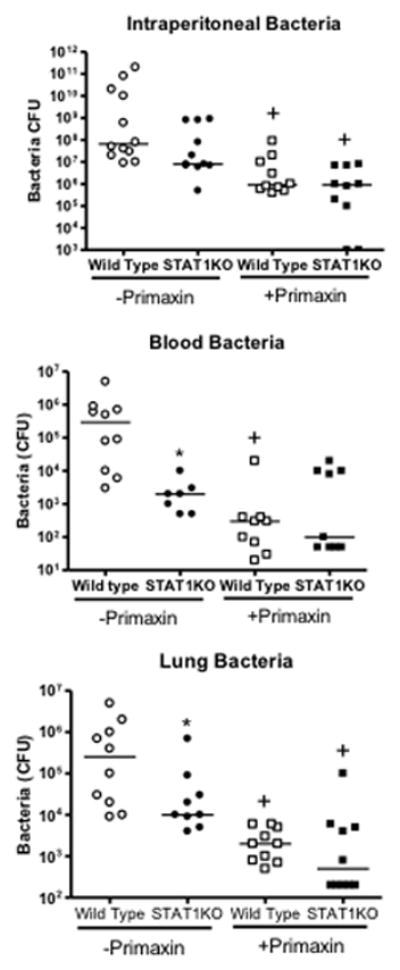
Bacterial colony forming units (CFU) in blood, peritoneal lavage fluid and lung at 18 hours after CLP in wild type and STAT1 knockout (STAT1KO) mice. Mice were resuscitated with lactated Ringer’s solution alone (-Primaxin) or with lactated Ringer’s solution plus Primaxin (25 mg/kg, +Primaxin). n = 8–10 mice per group, the median value is designated. *p<0.05 compared to wild type, +p<0.05 compared to mice of the same genotype that did not receive Primaxin,
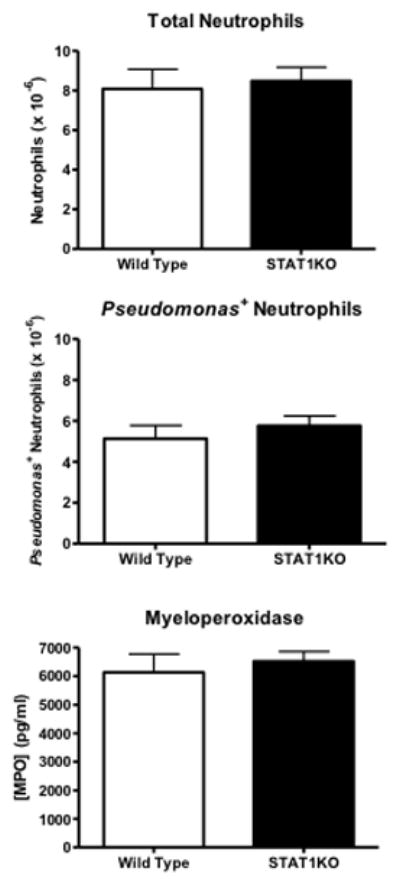
Studies were undertaken to assess neutrophil recruitment and functional activity. Mice received intraperitoneal challenge with FITC-labeled Pseudomonas aeruginosa. Total neutrophil numbers, the numbers of Pseudomonas+ neutrophils and intraperitoneal myeloperoxidase production were measured (Figure 5). Analysis of results showed no differences between STAT1KO and wild type mice in regard to total neutrophil recruitment, Pseudomonas+ neutrophils and intraperitoneal myeloperoxidase concentration (Figure 5).
Figure 5.
Neutrophil recruitment, phagocytic activity and myeloperoxidase production in wild type and STAT1KO mice after intraperitoneal challenge. Mice received intraperitoneal challenge with 5 × 107 FITC-labeled Pseudomonas aeruginosa. At 6 hours after bacterial challenge, intraperitoneal leukocytes were harvested and evaluated using flow cytometry. Intraperitonal myeloperoxidase activity was measured using ELISA. n = 5 mice per group.
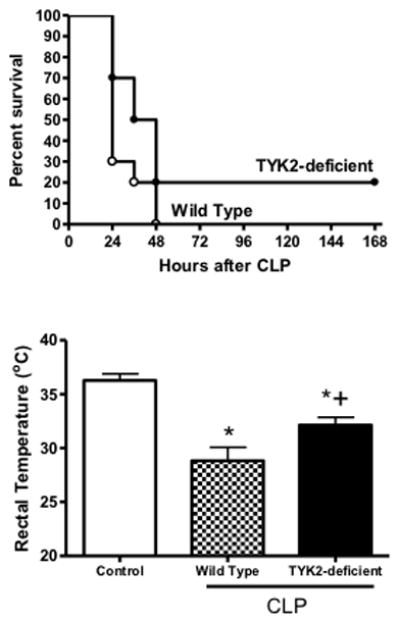
Response of TYK2-deficient mice to CLP-induced septic shock
Survival was not significantly different between TYK2-deficient and wild type mice after CLP (Figure 6). Both strains showed a significant decline in core body temperature compared to non-septic control mice at 18 hours after CLP and core temperature was significantly higher in TYK2-deficient mice than in wild type controls (Figure 6). Examination of bacterial burden showed that colony forming units in the peritoneal cavity, blood and lungs were not significantly different between TYK2-deficient mice and controls (Figure 7). Plasma IL-6 and CXCL10 concentrations were significantly lower in TYK2-deficient mice than in wild type mice but intraperitoneal IL-6 as well as plasma and intraperitoneal MIP-2 concentrations were not significantly different between groups (Figure 8). Intraperitoneal CXCL10 concnetrations were significantly lower in TYK2-deficient mice than in wild type mice (Figure 8).
Figure 6.
Survival and rectal temperature in control and TYK2-defcient mice. C57BL/10SnJ (control) and B10.D1-H2q/SgJ (TYK2-defiicent) mice were resuscitated with lactated Ringer’s plus Primaxin (25 mg/kg) immediately after CLP. Rectal temperature was measured at 18 hours after CLP. n = 10 mice per group. *p<0.05 compared to control (no CLP), +p<0.05 compared to wild type mice, n = 10 mice per group, values are expressed as mean ± SEM for rectal temperature measurements.
Figure 7.
Plasma and peritoneal lavage IL-6 and MIP-2 concentrations in control and TYK2-deficient mice. C57BL/10SnJ (control) and B10.D1-H2q/SgJ (TYK2-defiicent) mice were resuscitated with lactated Ringer’s plus Primaxin (25 mg/kg) immediately after CLP. Cytokine concentrations were measured by ELISA. *p<0.05 compared to C57BL/10SnJ, n = 10 mice per group, values are expressed as mean ± SEM.
Figure 8.
Bacterial colony forming units (CFU) in blood, peritoneal lavage fluid and lung at 18 hours after CLP in control and TYK2-deficient mice. C57BL/10SnJ (control) and B10.D1-H2q/SgJ (TYK2-defiicent) mice were resuscitated with lactated Ringer’s plus Primaxin (25 mg/kg) immediately after CLP. n = 10 mice per group, the median value is designated.
Discussion
Results of this study show that STAT1-deficient mice are resistant to septic shock caused by CLP as indicated by significant improvements in survival and physiologic function compared with wild type mice. The improved response observed in STAT1-deficient mice was associated with markedly attenuated systemic production of pro-inflammatory cytokines and chemokines as well as decreased organ injury. Bacterial burden was also decreased in STAT1-deficient mice that did not receive antibiotic treatment but not in mice that were treated with Primaxin. In contrast, TYK2-deficiency did not confer a survival benefit in this model. Temperature and systemic cytokine production were marginally improved in TYK2-deficient mice compared to controls and the magnitude of improvement was much less than that observed in STAT1-deficient mice.
This report extends current knowledge by characterizing the effect of STAT1 deficiency in a clinically relevant experimental model of septic shock. Previous studies have shown that STAT1-deficient mice are resistant to LPS-induced shock (7,8). Systemic cytokine production, physiologic dysfunction and mortality were attenuated in STAT1-deficient mice receiving challenge with normally lethal doses of LPS (8). However, LPS-induced sepsis does not fully mimic clinical sepsis because live infection is not present. The present study shows that STAT1 deficiency improves outcome in a model of sepsis characterized by high local and systemic bacterial counts, systemic inflammation and organ injury. Peck-Palmer and colleagues (11) previously reported that systemic cytokine production is attenuated during CLP-induced sepsis in STAT1-deficient mice. However, the functional significance of that observation was not determined in their study. In agreement with our results, they showed that STAT1-deficient mice had profound suppression of plasma IL-6 concentrations after CLP that was rivaled only by deficiency of the toll-like receptor (TLR) coupling protein MyD88 among the inflammatory signaling proteins studied (11). The present study extends their findings by showing that systemic inflammation, physiologic dysfunction and organ injury are attenuated in STAT1-deficient mice despite the presence of high systemic bacterial counts.
STAT1-deficient mice were resistant to septic shock regardless of whether antibiotics were administered. Interestingly, bacterial burden in blood and lungs was lower in STAT1-deficient mice compared to wild type mice when Primaxin was not given. That observation implies that STAT1 deficiency augments bacterial clearance during CLP-induced septic shock through mechanisms that are currently undefined but appear to be independent of neutrophil recruitment and function. Despite decreased bacterial burden in blood and lungs, intraperitoneal neutrophil counts, myeloperoxidase activity and phagocytosis were not different when comparing STAT1KO and wild type mice. It is well known that STAT1-deficient mice and humans with dysfunctional STAT1 mutations are susceptible to infection with pathogenic viruses and intracellular bacteria such as Salmonella and Mycobacteria (16–17). However, the importance of STAT1 for host resistance to the common extracellular pathogens that lead to most cases of sepsis in the clinical setting has not been investigated thoroughly (18). Treatment of STAT1-deficient mice with Primaxin markedly decreased bacterial burden but did not alter systemic cytokine production during CLP-induced sepsis. The numbers of bacteria in the peritoneal cavity, blood and lungs were not significantly different between wild type and STAT1-deficient mice when Primaxin was given but pro-inflammatory cytokine production was markedly decreased in STAT1-deficient mice under those conditions. That finding implies that the protective effect of STAT1 deficiency is due to attenuated systemic inflammation. However, further studies are needed to fully define the mechanisms by which STAT1 deficiency confers resistance to severe sepsis.
STAT1 plays a crucial and unique role in intracellular signaling after activation of Type I and Type II IFN receptors. Interferons are known to be important mediators of the host response to viral and bacterial infections. However, the role that interferons play in the pathogenesis of severe sepsis is controversial. Numerous previous investigators have reported that IFNγ (type II IFN) plays a key role in shock caused by systemic administration of high doses of LPS (19–21). LPS challenge induces very high plasma levels of IFNγ and neutralization or ablation of IFNγ provides protection from LPS-induced shock (22). Our research group previously showed that IFNγ also contributes to systemic inflammation, physiologic dysfunction and organ injury during CLP-induced septic shock (23). IFNγ was shown to be an important factor for inducing systemic inflammation through activation of myeloid cells at the site of infection. Blockade or ablation of IFNγ improved survival during CLP-induced septic shock. Marquez-Velasco et al (24) have also shown that late treatment of mice with anti-IFNγ F(ab)2 provides protection during CLP-induced sepsis. Likewise, Yin and colleagues (25) reported that IFNγ neutralization confers protection from CLP-induced septic shock, possibly by inhibiting the production of HMGB1. In all of the cited studies, IFNγ neutralization was associated with attenuated systemic inflammation. However, the overall benefit associated with IFNγ deficiency or neutralization in most prior studies was not at the level observed in STAT1-deficient mice in the present study, which implies that both type I and II IFNs act together to promote systemic inflammation during severe sepsis.
The role of type I IFNs (IFNα/β) in the pathogenesis of severe sepsis is equally controversial. As is true for IFNγ, investigators have reported that type I IFN signaling is associated with hyperinflammation and injury during LPS- or TNFα-induced shock. Karaghiosoff et al (8) have shown that type I IFN signaling plays a central role in the pathogenesis of LPS-induced shock. Kim et al (10) reported that LPS or E. coli-induced HMGB-1 production and systemic inflammation is dependent on the type I IFN signaling pathway. However, other investigators have reported that type I IFN receptor activation can be beneficial in less severe models of sepsis characterized by slowly progressive bacteremia. Kelly-Scumpia and colleagues (26) reported that type I IFN deficiency improves survival during LPS-induced shock but is detrimental in a sublethal model of CLP-induced sepsis. In the latter model, lack of type I IFN signaling resulted in impaired myeloid cell recruitment and impaired bacterial clearance. Likewise, Mancuso et al (27) reported that type I IFN signaling is crucial for resistance against different species of pathogenic bacteria Group B Streptococci and Pneumococcus. In the present study, a model of CLP-induced septic shock was used. The results indicate that STAT1 deficiency results in decreased systemic cytokine production and attenuated organ injury and physiologic dysfunction. The effect of STAT1 deficiency on neutrophil recruitment and the host response to a sublethal model of CLP-induced peritonitis remains to be determined.
The results of this study imply that STAT1 inhibitors could be attractive for treatment of inflammatory syndromes such as sepsis. However, selective and non-toxic STAT1 inhibitors are currenty not available for in vivo administration. Protein inhibitor of activated STAT1 (PIAS1) is as an endogenous inhibitor of STAT1 that blocks binding of activated STAT1 to promoter regions of target genes (28–29). Upregulation of PIAS1 using adenovirus overexpression strategies has been shown to decrease pancreatitis-induced acute lung injury (29). However, PIAS1 is not selective for STAT1 and also interacts with the nuclear proteins small ubiquitin modifier 1, Sp3, UBE2, Nf-κB and p53 (30–33). Compounds such as epigallocatechin-3-gallate (EGCG) from green tea leaves and hyperforin from St. John’s Wort also possess STAT1 blocking activity and possess anti-inflammatory properties. However, neither compound selectively blocks STAT1 activity (34–35). The chemotherapeutic purine analog fludarabine possesses STAT1 blocking properties and is anti-inflammatory (36–37). However, fludarabine also inhibits DNA polymerase activity and has been used primarily in ex vivo studies due to its toxicity in vivo. Therefore, there are significant limitations associated with currently available STAT1 inhibitors. Nevertheless, further research aimed at developing selective and non-toxic STAT1 inhibitors could reveal effective new agents for treatment of severe sepsis.
Results of the present study show that TYK2 deficiency does not confer survival benefit during CLP-induced septic shock but TYK2 knockout mice showed significant attenuation of hypothermia and systemic cytokine production. Those findings indicate that TYK2 activation contributes, in part, to the induction of the pro-inflammatory response during severe sepsis. However, the attenuation of CLP-induced inflammation was more profound in STAT1-deficient mice than in mice with non-functional TYK2. TYK2 is crucial for effective STAT1 activation in response to Type I interferons (IFN α/β), but not IFNγ (1;38) Therefore, our findings indicate that Type I IFNs do not act independently to activate STAT1 during CLP-induced sepsis. That contention is supported by our previous report that showed IFNγ contributes to systemic inflammation, physiologic dysfunction and organ injury during CLP-induced septic shock (21). Taken together, these results imply that activation of STAT1 by both Type I and Type II IFNs contributes to the pathogenesis of CLP-induced shock.
The response of TYK2-deficient mice to CLP-induced sepsis has not been previously reported. As noted above, resistance to CLP-induced sepsis was only marginally improved in TYK2-deficient mice in our study. Other investigators have shown that TYK2-deficient mice are resistant to LPS-induced shock. Kamezaki et al (7) reported that TYK2-deficent mice are more resistant to LPS-induced shock than STAT1-deficient mice. The resistance was associated with decreased production of pro-inflammatory mediators such as cytokines and nitric oxide. Thus, previous investigators have postulated that the resistance of TYK2-deficient mice to LPS-induced shock is caused by an attenuated systemic inflammatory response. The response of TYK2-deficient mice to live bacterial infections has received limited attention. Nakamura and colleagues (39) reported that TYK2-deficient mice have an impaired ability to clear E. coli after intraperitoneal challenge. The altered bacterial clearance was associated with impaired IL-17-induced neutrophil recruitment. In the present study, TYK2-deficient mice did not show significant alterations in bacterial burden compared to controls.
In conclusion, this study shows that STAT1-deficient mice are resistant to CLP-induced septic shock as indicated by improved survival. The resistance is characterized by attenuated systemic cytokine production, physiologic dysfunction and organ injury. TYK2-deficient mice showed only marginal resistance to CLP-induced septic shock but showed attenuation of systemic inflammation at a lesser degree than observed in STAT1KO mice. In combination with our previous report that IFNγ deficiency confers partial resistance to CLP-induced shock, results of the present study raise the likelihood that both type I and type II IFNs induce STAT1 activation in response to CLP.
Acknowledgments
Supported by NIH Grant R01 GM66885 and Grant 8780 from the Shriners of North America
Footnotes
There is no conflict of interest
References
- 1.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 2.Najjar I, Fagard R. STAT1 and pathogens, not a friendly relationship. Biochimie. 2010;92:425–44. doi: 10.1016/j.biochi.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghislain J, Sussman G, Goelz S, Ling LE, Fish EN. Configuration of the interferon-alpha/beta receptor complex determines the context of the biological response. J Biol Chem. 1995;270:21785–92. doi: 10.1074/jbc.270.37.21785. [DOI] [PubMed] [Google Scholar]
- 4.Bradfute SB, Stuthman KS, Shurtleff AC, Bavari S. A STAT-1 knockout mouse model for Machupo virus pathogenesis. Virol J. 2011;8:300. doi: 10.1186/1743-422X-8-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker T, Stockinger S, Karaghiosoff M, Muller M, Kovarik P. IFNs and STATs in innate immunity to microorganisms. J Clin Invest. 2002;109:1271–7. doi: 10.1172/JCI15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vairo D, Tassone L, Tabellini G, et al. Severe impairment of IFN-gamma and IFN-alpha responses in cells of a patient with a novel STAT1 splicing mutation. Blood. 2001;118(7):1806–17. doi: 10.1182/blood-2011-01-330571. [DOI] [PubMed] [Google Scholar]
- 7.Kamezaki K, Shimoda K, Numata A, Matsuda T, Nakayama K, Harada M. The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. Int Immunol. 2004;16:1173–9. doi: 10.1093/intimm/dxh118. [DOI] [PubMed] [Google Scholar]
- 8.Karaghiosoff M, Steinborn R, Kovarik P, et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003;4:471–7. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 9.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–83. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Kim SJ, Lee IS, et al. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182:2458–66. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 11.Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J Leukoc Biol. 2008;83:1009–18. doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- 12.Herzig DS, Driver BR, Fang G, Toliver-Kinsky TE, Shute EN, Sherwood ER. Regulation of l lymphocyte trafficking by CXC chemokine receptor 3 during septic shock. Am J Respir Crit Care Med. 2012;185:291–300. doi: 10.1164/rccm.201108-1560OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etogo AO, Nunez J, Lin CY, Toliver-Kinsky TE, Sherwood ER. NK but not CD1-restricted NKT cells facilitate systemic inflammation during polymicrobial intra-abdominal sepsis. J Immunol. 2008;180:6334–45. doi: 10.4049/jimmunol.180.9.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhwald M, Aabye MG, Ravn P. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev Mol Diagn. 2012;12:175–87. doi: 10.1586/erm.11.97. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira-Pelegrin GR, Ravanelli MI, Branco LG, Rocha MJ. Thermoregulation and vasopressin secretion during polymicrobial sepsis. Neuroimmunomodulation. 2009;16:45–53. doi: 10.1159/000179666. [DOI] [PubMed] [Google Scholar]
- 16.Averbuch D, Chapgier A, Boisson-Dupuis S, Casanova JL, Engelhard D. The clinical spectrum of patients with deficiency of Signal Transducer and Activator of Transcription-1. Pediatr Infect Dis J. 2011;30:352–5. doi: 10.1097/INF.0b013e3181fdff4a. [DOI] [PubMed] [Google Scholar]
- 17.Chapgier A, Kong XF, Boisson-Dupuis S, et al. A partial form of recessive STAT1 deficiency in humans. J Clin Invest. 2009;119:1502–14. doi: 10.1172/JCI37083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsukawa A. STAT proteins in innate immunity during sepsis: lessons from gene knockout mice. Acta Med Okayama. 2007;61(5):239–45. doi: 10.18926/AMO/32897. [DOI] [PubMed] [Google Scholar]
- 19.Bucklin SE, Russell SW, Morrison DC. Participation of IFN-gamma in the pathogenesis of LPS lethality. Prog Clin Biol Res. 1994;388:399–406. [PubMed] [Google Scholar]
- 20.Car BD, Eng VM, Schnyder B, et al. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–44. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–4. [PubMed] [Google Scholar]
- 22.Kawa K, Tsutsui H, Uchiyama R, et al. IFN-gamma is a master regulator of endotoxin shock syndrome in mice primed with heat-killed Propionibacterium acnes. Int Immunol. 2010;22:157–66. doi: 10.1093/intimm/dxp122. [DOI] [PubMed] [Google Scholar]
- 23.Romero CR, Herzig DS, Etogo A, et al. The role of interferon-gamma in the pathogenesis of acute intra-abdominal sepsis. J Leukoc Biol. 2010;88:725–35. doi: 10.1189/jlb.0509307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquez-Velasco R, Martinez-Velazquez AX, mezcua-Guerra LM, et al. Enhanced survival from CLP-induced sepsis following late administration of low doses of anti-IFNgamma F(ab’)2 antibody fragments. Inflamm Res. 2011;60:947–53. doi: 10.1007/s00011-011-0355-0. [DOI] [PubMed] [Google Scholar]
- 25.Yin K, Gribbin E, Wang H. Interferon-gamma inhibition attenuates lethality after cecal ligation and puncture in rats: implication of high mobility group box-1. Shock. 2005;24:396–401. doi: 10.1097/01.shk.0000175556.03300.c6. [DOI] [PubMed] [Google Scholar]
- 26.Kelly-Scumpia KM, Scumpia PO, Delano MJ, et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–26. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancuso G, Midiri A, Biondo C, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol. 2007;178:3126–33. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 28.Hoefer J, Schafer G, Klocker H, et al. PIAS1 Is Increased in Human Prostate Cancer and Enhances Proliferation through Inhibition of p21. Am J Pathol. 2012;180:2097–107. doi: 10.1016/j.ajpath.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Chen P, Huang L, Zhang Y, Qiao M, Yuan Y. SiRNA-mediated PIAS1 silencing promotes inflammatory response and leads to injury of cerulein-stimulated pancreatic acinar cells via regulation of the P38MAPK signaling pathway. Int J Mol Med. 2010;26:619–26. doi: 10.3892/ijmm_00000507. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Shuai K. Targeting the PIAS1 SUMO ligase pathway to control inflammation. Trends Pharmacol Sci. 2008;29:505–9. doi: 10.1016/j.tips.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapetschnig A, Rischitor G, Braun H, et al. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 2002;21:5206–15. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Shuai K. Targeting the PIAS1 SUMO ligase pathway to control inflammation. Trends Pharmacol Sci. 2008;29:505–9. doi: 10.1016/j.tips.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Yang Y, Chernishof V, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–14. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 34.Wang JH, Cheng J, Li CR, Ye M, Ma Z, Cai F. Modulation of Ca Signals by Epigallocatechin-3-gallate(EGCG) in Cultured Rat Hippocampal Neurons. Int J Mol Sci. 2011;12:742–54. doi: 10.3390/ijms12010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dell’Aica I, Niero R, Piazza F, et al. Hyperforin blocks neutrophil activation of matrix metalloproteinase-9, motility and recruitment, and restrains inflammation-triggered angiogenesis and lung fibrosis. J Pharmacol Exp Ther. 2007;321:492–500. doi: 10.1124/jpet.106.116459. [DOI] [PubMed] [Google Scholar]
- 36.Frank DA, Mahajan S, Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med. 1999;5:444–7. doi: 10.1038/7445. [DOI] [PubMed] [Google Scholar]
- 37.Torella D, Curcio A, Gasparri C, et al. Fludarabine prevents smooth muscle proliferation in vitro and neointimal hyperplasia in vivo through specific inhibition of STAT-1 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2935–H2943. doi: 10.1152/ajpheart.00887.2006. [DOI] [PubMed] [Google Scholar]
- 38.Mori Y, Hirose K, Suzuki K, et al. Tyk2 is essential for IFN-alpha-induced gene expression in mast cells. Int Arch Allergy Immunol. 2004;134(Suppl 1):25–9. doi: 10.1159/000077789. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J Immunol. 2008;181:2071–5. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]


