Abstract
B-cell activation biomarkers have been associated with increased risk of non-Hodgkin lymphoma (NHL) in HIV-infected populations. However, whether a similar association may exist in general populations has not been established. We conducted a case-control study within the Women’s Health Initiative Observational Study cohort to measure the B-cell activation biomarkers sCD23, sCD27, sCD30, sCD44, and CXCL13 in serum samples collected an average of 6 years before NHL diagnosis, in 491 cases and 491 controls. Using logistic regression to estimate odds ratios, we observed strong associations between NHL and markers, for all B-cell NHL and for major subtypes. Women with marker levels in the highest-versus-lowest quartile categories of CD23, CD27, CD30, or CXCL13 were at 2.8 to 5.5-fold increased risk of B-NHL. Additionally, there were significant trends of risk with increasing levels of these markers present. Associations were strongest for cases with shortest lag times between blood draw and diagnosis (<3 years). However, there were also significant associations for cases with the longest prediagnostic lag (9–13 years). Taken together, our findings indicate a prominent role for B-cell activation among postmenopausal women in the etiology of B-cell NHL and/or in processes reflective of early disease development, as early as 9 years before diagnosis.
Keywords: non-Hodgkin lymphoma, immune activation, cohort, epidemiology
INTRODUCTION
One factor that is a well-known cause of NHL is altered immunity – as evidenced by increased rates of NHL among persons with AIDS, transplant recipients, and autoimmune disease patients (1–3). However, the importance of subclinical immune dysregulation in development of NHL in cases occurring in the general population, who comprise the majority of cases, is unclear.
The risk of developing NHL of the B-cell type is markedly increased in HIV/AIDS, particularly for aggressive subtypes such as diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma. B-cell hyperactivation is commonly seen in persons with HIV, and several studies indicate that those patients with the most marked B-cell activation are at increased risk of developing NHL. For example, increased serum/plasma levels of molecules involved in B-cell activation, including soluble sCD23 (4–7), sCD27 (7,8), sCD30 (9), sCD44 (10), and CXCL13 (11,12), have been associated with subsequent development of AIDS-related NHL. B-cell activation is characterized by lymphocyte proliferation, class switch recombination, and somatic hypermutation, all of which are prone to resultant errors in DNA that may lead to lymphomagenesis.
Recently, reports from two prospective cohort studies of the general population (presumably immunocompetent persons) found that increased levels of sCD30 were associated with increased risk of NHL, when measured in blood samples collected up to 6–10 years before diagnosis (13,14). sCD27 was also associated with increased NHL risk in one of these cohorts (15). Taken together, these studies provide the first evidence that lymphomagenesis in the general population may result from relatively increased levels of B-cell activation in a manner similar to that observed in HIV-positive persons.
We investigated the risk of B-cell NHL associated with biomarkers, measured in prediagnostic serum, that are indicative of an immune stimulatory host environment, including levels of a cytokine-like molecule (sCD23), soluble cytokine receptors (sCD27, sCD30), a molecule involved in lymphocyte activation (sCD44), and a B-cell stimulatory chemokine (CXCL13). We hypothesized that chronic B-cell activation, as evidenced by elevated serum levels of these molecules, increases the risk of B-cell NHL among immunocompetent persons, similar to the risks observed in HIV-positive populations. We further hypothesized that the increased risk would apply across a range of histologic subtypes, including more indolent subtypes less commonly encountered in HIV. We investigated our hypothesis in the Women’s Health Initiative (WHI) Observational Study (OS) cohort using biologic samples collected an average of 6 years before NHL diagnosis.
MATERIAL AND METHODS
The study was designed as a nested case-control study in the WHI OS. All study activities were approved by Human Subjects review by the FHCRC IRB (IR #6897).
Study Population
The design of the WHI OS has been published previously (16). The OS includes 93,676 community-dwelling, postmenopausal women enrolled between 1994 and 1998 at 40 clinical centers distributed widely throughout the U.S., including targeted enrollment of minority women. Participants in the OS were screened for the WHI clinical trials but were determined to be ineligible or were unwilling to participate, or were recruited through a direct invitation for screening into the OS only. All participants of the WHI OS gave written informed consent.
Patient charts of women who reported a cancer diagnosis on an annually mailed questionnaire were reviewed by coders trained in Surveillance, Epidemiology, and End-Results (SEER) program guidelines to classify pathology findings according to the ICD-O-3 coding (17). We included B-cell NHL cases that were diagnosed before April 2009. Of 615 incident cases, 493 met the criteria for our study. Excluded were women with cancer history (except non-melanoma skin cancer) at enrollment (n=88) or incident cancer during follow-up but prior to the B-cell NHL diagnosis (n=34). Two additional cases were eliminated because we did not find a matched control (one DLBCL and one follicular lymphoma, see below); therefore, the final number of cases in our study was 491. Our overall case group included any subtype of B-cell NHL, and subtypes were further grouped based on the World Health Organization and the International Lymphoma Epidemiology Consortium (InterLymph) guidelines (18,19), including 142 chronic lymphocytic leukemia/small lymphocytic lymphoma/prolymphocytic leukemia (CLL/SLL/PLL; 28.8% of cases), 138 DLBCL (28.2%), and 102 follicular lymphoma (20.9%); any case not included in one of these subtypes was classified as ‘other’ B-cell NHL (N=109, 22.2%).
We selected one control for each case from OS cohort members who had never been diagnosed with cancer (except non-melanoma skin cancer) at the time of case diagnosis. Each control was selected using stratified random sampling from the pool of eligible non-cases living at the time of case diagnosis, and was individually matched to its case by birthdate (±1 year), US region (Northeast, South, Midwest, West), and date of enrollment blood draw (±3 months). We successfully matched 491 control participants to the cases (we excluded 2 cases for whom a successful match was not found).
Biomarker Measurement
We measured serum levels of soluble receptor molecules (sCD23, sCD27, sCD30, sCD44) and CXCL13 by enzyme-linked immunosorbent assay (ELISA). Assays were carried out according to the manufacturers’ protocols for sCD23, sCD30, and sCD44 (Bender MedSystems, Burlingame, CA), CXCL13 (Quantikine™, R&D Systems, Minneapolis, MN), and sCD27 using the PeliKine-compact ELISA Kit and Toolset (CLB/Sanquin, RDI/Fitzgerald, Concord, MA). Case and control serum samples were placed in random order, with matched case/control pairs located next to each other. Several precautions were taken to minimize interassay variation (i.e., variation between runs for each analyte). We purchased all of the ELISAs used in the study for a given analyte from a single lot, to eliminate lot-to-lot variation. We included a standard curve for each ELISA plate; therefore, the values of the study specimens were calculated from a standard curve run on the same plate as the specimens. Additionally, replicate laboratory controls were included in every plate, allowing us to monitor interassay coefficients of variation (CVs), which were <5% based on replicates from three plates selected from the first, middle, and last third of assay plates run. The WHI also included 50 blinded duplicate pairs (100 samples), interspersed randomly among the study samples. Overall CVs based on the blind duplicate samples ranged from 7.0% (sCD30) to 11.7% (sCD23).
Statistical Analysis
All analyses were performed using the SAS system, version 9.1 (SAS Institute, Cary, NC). The immune markers were analyzed as continuous variables as well as categorical variables with cutpoints for each assay set at the quartiles of the control distributions. All of the immune marker variables had non-normal distributions with right-skewing. We transformed these variables using the natural log in order to reduce the influence of extreme values in regression analyses.
We generated odds ratios (ORs) and 95% confidence intervals (CIs) as estimates of relative risks of B-cell NHL associated with higher- versus lower levels of each marker, using conditional logistic regression (20). Statistical significance of the odds ratios was evaluated using the Wald χ2–square test. Initially, each specific marker was modeled separately. Relative risks associated with each continuous marker variable were estimated per unit increase in the analyte level based on a log-linear model for the OR, corresponding to a 2.72-fold increase of the untransformed analyte level. Risks associated with marker level categories based on quartiles were estimated using the lowest category as the referent. We conducted tests for trend across quartiles using a continuous variable with assigned values equal to the intra-category median analyte level among controls. We also created a variable reflecting the number of “high” marker levels for each woman, calculated as the number of markers (out of 5 total) for which a subject had levels in the highest quartile category. We analyzed the number of high marker levels as a set of indicator variables for each possibility (1 through 5, using 0 as the referent).
We tested for etiologic heterogeneity by fitting unconditional polytomous logistic regression models for major subtypes of B-cell NHL, including DLBCL (N=138), follicular lymphoma (N=102), CLL/SLL/PLL (N=142), and ‘other’ B-cell NHL (N=109), and testing for heterogeneity of risk estimates between each pair of NHL subtypes.
To describe the patterns of association between immune biomarkers and B-cell NHL according to the number of years between blood sample collection and NHL diagnosis (“prediagnostic lag”), we classified NHL cases according to prediagnostic lag increments of <3, 3–5, 6–8, and 9–13 years, chosen to maximize the range between the shortest and longest lags, while including at least 90 cases in each group. We tested whether there was heterogeneity in the associations between the immune markers and NHL according to the length of the prediagnostic lag by fitting unconditional polytomous logistic regression models with NHL categorized in these lag increments, and computing p-values for heterogeneity of risk estimates between specific lag increments. We also evaluated risk estimates according to the prediagnostic lag for DLBCL, follicular lymphoma, and CLL/SLL/PLL subtypes, separately, for the continuous marker measures and the trend variables only (due to sample size limitations in these subtype-specific analyses).
All estimates were adjusted for the matching factors – age, region, and date of blood draw. We also evaluated a set of potential confounders, selected a priori, including race/ethnicity (coded as indicator variables for Black or African American and Other, with White, not Hispanic as the referent), education (coded as grouped linear with 7 categories, as shown in Table 2), body mass index (BMI, coded as continuous), and smoking status (coded as indicator variables for Former and Current, with Never as the referent). Potential confounding was evaluated by the change in the effect estimate for a specific marker when including the covariate in the model compared to the model without the covariates; changes in risk estimates greater than 10 percent were considered as evidence of possible confounding. Certain medical conditions and medications may influence circulating levels of the immune markers we are studying and may also increase risk of NHL. To further evaluate the magnitude of the main associations of interest among otherwise apparently immunocompetent subjects, we conducted subanalyses of the main effects by excluding subjects who reported at baseline that they had ever been diagnosed with certain conditions or taken medications known to be characterized by/cause altered immune status (such as inflammation). Specifically, we excluded subjects who reported a history of systemic lupus erythematosus, rheumatoid arthritis, glucocorticosteroid medications, disease-modifying antirheumatic drugs, colitis, diverticulitis, pancreatitis, kidney stones, gallbladder disease, stomach ulcer, goiter, and asthma.
Table 2.
Immune marker levels among control subjects (geometric means), by characteristics of the study population
| sCD23 (U/mL) |
sCD27 (U/mL) |
sCD30 (ng/mL) |
sCD44 (ng/mL) |
CXCL13 (pg/ml) |
|
|---|---|---|---|---|---|
| Age (years)a | |||||
| 50–59 | 37.8 | 198.3 | 27.5 | 357.8 | 56.7 |
| 60–69 | 42.5 | 222.5 | 30.2 | 386.3 | 54.2 |
| 70–79 | 42.7 | 246.3 | 35.1 | 413.1 | 64.0 |
| Study regiona | |||||
| Northeast | 40.4 | 224.8 | 31.3 | 385.5 | 56.9 |
| South | 43.7 | 221.2 | 31.3 | 389.2 | 55.0 |
| Midwest | 48.7 | 231.9 | 33.6 | 402.3 | 57.3 |
| West | 36.0 | 221.0 | 28.7 | 379.6 | 60.6 |
| Race | |||||
| White, not Hispanic | 40.9 | 224.3 | 31.6 | 390.9 | 57.2 |
| Black or African American | 43.6 | 215.3 | 25.4 | 352.9 | 59.9 |
| Other | 47.5 | 228.0 | 30.3 | 388.4 | 59.9 |
| Education | |||||
| Less than high school diploma | 45.3 | 231.0 | 30.8 | 399.1 | 60.2 |
| High school diploma or GED | 54.1 | 249.8 | 35.0 | 402.2 | 65.1 |
| Vocational or training school | 45.3 | 249.6 | 33.3 | 413.3 | 57.3 |
| Some college or associate degree | 41.6 | 216.9 | 30.2 | 386.8 | 54.4 |
| Baccalaureate degree | 35.3 | 228.9 | 30.3 | 370.4 | 53.9 |
| Some postgraduate or professional | 36.1 | 201.2 | 28.3 | 395.0 | 59.6 |
| Master’s or doctoral degree | 38.5 | 213.9 | 30.6 | 372.6 | 58.1 |
| Smoking status | |||||
| Never | 47.1 | 231.8 | 32.4 | 391.4 | 56.7 |
| Former | 37.7 | 214.0 | 29.7 | 381.3 | 57.3 |
| Current | 29.0 | 249.9 | 31.2 | 427.5 | 69.5 |
| Body mass index | |||||
| Underweight (<18.5) | 18.3 | 253.8 | 36.8 | 319.9 | 96.7 |
| Normal weight (18.5–24.9) | 36.5 | 219.3 | 30.1 | 380.8 | 55.6 |
| Overweight (25–29.9) | 41.6 | 217.9 | 31.6 | 394.1 | 57.1 |
| Obesity (30–34.9) | 50.6 | 241.1 | 30.7 | 396.9 | 60.5 |
| Extreme obesity (≥35) | 63.0 | 236.9 | 33.6 | 408.3 | 57.4 |
RESULTS
Our study population of postmenopausal women (Table 1) were mostly 60 years and older at the study baseline (>80% of cases and controls), of white, not Hispanic race/ethnicity (91% of cases and 85% of controls), and educated beyond high school (approximately 80% of cases and controls). Approximately half of the women never smoked, and approximately 60% were classified as overweight or obese (BMI ≥25.0 kg/m2).
Table 1.
Characteristics of study population (N [%])
| Cases (N=491) | Controls (N=491) | |
|---|---|---|
| Age (years)a | ||
| 50–59 | 95 (19.4) | 95 (19.4) |
| 60–69 | 247 (50.3) | 244 (49.7) |
| 70–79 | 149 (30.4) | 152 (31.0) |
| Study regiona | ||
| Northeast | 124 (25.3) | 124 (25.3) |
| South | 109 (22.2) | 109 (22.2) |
| Midwest | 119 (24.2) | 119 (24.2) |
| West | 139 (28.3) | 139 (28.3) |
| Race | ||
| White, not Hispanic | 449 (91.4) | 416 (84.7) |
| Black or African American | 19 (3.9) | 32 (6.5) |
| Other | 22 (4.5) | 38 (7.8) |
| Missing | 1 (0.2) | 5 (1.0) |
| Education | ||
| Less than high school diploma | 16 (3.3) | 29 (5.9) |
| High school diploma or GED | 87 (17.7) | 66 (13.5) |
| Vocational or training school | 50 (10.2) | 51 (10.4) |
| Some college or associate degree | 141 (28.7) | 142 (28.9) |
| Baccalaureate degree | 58 (11.8) | 64 (13.0) |
| Some postgraduate or professional | 59 (12.0) | 50 (10.2) |
| Master’s or doctoral degree | 78 (15.9) | 86 (17.5) |
| Missing | 2 (0.4) | 3 (0.6) |
| Smoking status | ||
| Never | 255 (51.9) | 246 (50.1) |
| Former | 206 (42.0) | 215 (43.8) |
| Current | 26 (5.3) | 24 (4.9) |
| Missing | 4 (0.8) | 6 (1.2) |
| Body mass index | ||
| Underweight (<18.5) | 8 (1.6) | 10 (2.0) |
| Normal weight (18.5–24.9) | 184 (37.5) | 182 (37.1) |
| Overweight (25–29.9) | 187 (38.1) | 170 (34.6) |
| Obesity (30–34.9) | 71 (14.5) | 79 (16.1) |
| Extreme obesity (≥35) | 32 (6.5) | 44 (9.0) |
| Missing | 9 (1.8) | 6 (1.2) |
| Histologic subtype | ||
| CLL/SLL/PLL | 142 (28.8) | |
| Diffuse large B-cell (DLBCL) | 138 (28.2) | |
| Follicular lymphoma (FL) | 102 (20.9) | |
| Marginal zone lymphoma (MZL) | 45 (9.1) | |
| B-NHL, not otherwise specified (NOS) | 30 (6.1) | |
| Lymphoplasmacytic lymphoma (LPL)/Waldenstrom macroglobulinemia (WM) | 16 (3.3) | |
| Mantle cell lymphoma (MCL) | 9 (1.8) | |
| Burkitt lymphoma (BL) | 5 (1.0) | |
| Hairy cell leukemia (HCL) | 4 (0.8) |
Controls were matched to cases on birth year, blood draw date, and study region
The levels of the immune markers we studied (Table 2) differed significantly by age, in that older women had higher levels (sCD27, sCD30, sCD44, CXCL13). Black or African American subjects had lower levels of sCD30 than white or other race/ethnicity groups. Smoking status was a significant predictor of sCD23 and sCD27 levels, with opposite patterns (current smokers had the lowest levels of sCD23 and the highest levels of sCD27). There were some differences of immune marker levels by education, but with no clear pattern. Body mass index was associated with sCD23 (p=0.0002) and sCD44 (p=0.07), with marker levels increasing by BMI category and notably low levels for the small number of underweight controls (N=10). CXCL13 levels were also significantly associated with BMI; however, the only apparent variability in the marker was that underweight women had higher levels than the rest of the BMI categories. The five markers were all significantly correlated with eachother (not shown in tables), with Spearman rank correlation coefficients ranging from r=0.26 (sCD23 with CXCL13) to r=0.59 (sCD27 with sCD30).
Each of the immune markers was significantly associated with increased risk of B-cell NHL, all types combined (Table 3). There was no evidence of confounding by the a priori covariates (race/ethnicity, education, BMI, and smoking status) in any of our analyses of all B-NHL, NHL subtypes, or NHL cases grouped by years since blood draw; therefore, we present risk estimates adjusted for the matching factors only (age, region, blood draw date). The continuous marker variables were all associated with NHL risk, with 2.07 to 5.94-fold increases in risk for each unit increase on the natural log scale (Table 3). When all five markers were modeled together in continuous form (not shown in tables), the risk estimates for B-cell NHL were reduced somewhat but remained increased, with the exception of sCD44 for which the association was reversed (sCD23, OR=1.71 [95% CI: 1.44–2.04]; sCD27, OR=2.64 [(95% CI: 1.43–4.87]; sCD30, OR=1.45 [95% CI: 0.95–2.21]; sCD44, OR=0.42 [95% CI: 0.2–0.92]; CXCL13, OR=1.51 [95% CI: 1.09–2.08]). Women with marker levels in the highest-versus-lowest quartile categories of CD23, CD27, CD30, or CXCL13 were at 2.8 to 5.5-fold increased risk of NHL, and there were significant trends of risk by increasing categories of these markers. CD44 was significantly associated with NHL in each quartile category; however, the risk did not increase across categories (p-trend=0.39). Women with a high level (highest quartile) for any one of the 5 markers had 1.9-fold increased risk of B-cell NHL (95% CI: 1.3–2.8) compared to women without high levels for any of the markers, and the relative risk increased with each additional number of high marker levels (2 high, OR=2.1 [95% CI: 1.4–3.2]; 3 high, OR=2.5 [95% CI: 1.5–4.2]; 4 high, OR=5.5 [95% CI: 3.1–9.7]; 5 high, OR=10.0 [95% CI: 5.0–20.2]).
Table 3.
Association between immune marker levels at study baseline and NHL incidence, for all B-cell NHL and NHL subtypesa
| Controls | All B-cell NHL | CLL/SLL/PLL | DLBCL | Follicular lymphoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | N | OR (95% CI) | p trend | N | OR (95% CI) | p trend | N | OR (95% CI) | p trend | N | OR (95% CI) | p trend |
| sCD23 (U/ml) | 490 | 486 | 2.07 (1.75–2.45)b | 140 | 4.99 (3.84–6.47)b | 137 | 1.30 (1.06–1.58)b | 102 | 1.97 (1.53–2.53)b | ||||
| <29.2 | 122 | 57 | Referent | 7 | Referent | 22 | Referent | 10 | Referent | ||||
| 29.2 to <51.8 | 123 | 93 | 1.7 (1.1–2.7) | 11 | 1.6 (0.6–4.2) | 31 | 1.4 (0.8–2.5) | 27 | 2.7 (1.3–5.9) | ||||
| 51.8 to <75.0 | 122 | 82 | 1.6 (1.0–2.5) | 13 | 1.9 (0.7–4.9) | 39 | 1.7 (1.0–3.1) | 15 | 1.5 (0.7–3.5) | ||||
| ≥75.0 | 123 | 254 | 5.4 (3.5–8.4) | <0.0001 | 109 | 16.1 (7.2–36.2) | <0.0001 | 45 | 2.0 (1.1–3.6) | 0.08 | 50 | 5.1 (2.5–10.6) | <0.0001 |
| sCD27 (U/ml) | 490 | 487 | 5.94 (3.79–9.31)b | 140 | 6.04 (3.68–9.92)b | 138 | 3.64 (2.19–6.06)b | 102 | 4.13 (2.36–7.22)b | ||||
| <177 | 122 | 50 | Referent | 9 | Referent | 12 | Referent | 17 | Referent | ||||
| 177 to <224 | 123 | 103 | 2.1 (1.4–3.4) | 36 | 4.1 (1.9–9.0) | 31 | 2.6 (1.3–5.3) | 19 | 1.2 (0.6–2.3) | ||||
| 224 to <275 | 122 | 110 | 2.8 (1.7–4.4) | 29 | 3.5 (1.6–7.9) | 33 | 2.8 (1.4–5.7) | 21 | 1.3 (0.7–2.7) | ||||
| ≥275 | 123 | 224 | 5.5 (3.5–8.7) | <0.0001 | 66 | 8.7 (4.1–18.5) | <0.0001 | 62 | 5.3 (2.7–10.4) | 0.001 | 45 | 3.0 (1.6–5.6) | 0.0004 |
| sCD30 (ng/ml) | 490 | 487 | 2.74 (2.04–3.68)b | 140 | 1.99 (1.35–3.93)b | 138 | 2.78 (1.91–4.06)b | 102 | 4.23 (2.84–6.30)b | ||||
| <24.1 | 122 | 72 | Referent | 21 | Referent | 22 | Referent | 11 | Referent | ||||
| 24.1 to <30.2 | 123 | 101 | 1.4 (0.9–2.0) | 38 | 1.8 (1.0–3.4) | 28 | 1.2 (0.7–2.3) | 12 | 1.1 (0.5–2.7) | ||||
| 30.2 to <40.2 | 122 | 114 | 1.6 (1.0–2.3) | 36 | 1.8 (1.0–3.3) | 27 | 1.2 (0.6–2.2) | 28 | 2.8 (1.3–5.9) | ||||
| ≥40.2 | 123 | 200 | 2.8 (1.9–4.2) | <0.0001 | 45 | 2.3 (1.3–4.1) | 0.15 | 61 | 2.7 (1.5–4.8) | <0.0001 | 51 | 5.1 (2.5–10.5) | <0.0001 |
| sCD44 (ng/ml) | 490 | 487 | 2.64 (1.53–4.56)b | 140 | 1.47 (0.73–2.96)b | 138 | 3.07 (1.55–6.07)b | 102 | 2.46 (1.14–5.34)b | ||||
| <326 | 122 | 84 | Referent | 32 | Referent | 17 | Referent | 17 | Referent | ||||
| 326 to <391 | 123 | 139 | 1.7 (1.2–2.5) | 40 | 1.2 (0.7–2.1) | 34 | 2.0 (1.1–3.8) | 34 | 2.0 (1.1–3.8) | ||||
| 391 to <469 | 122 | 131 | 1.6 (1.1–2.4) | 34 | 1.1 (0.6–1.9) | 40 | 2.3 (1.2–4.4) | 26 | 1.6 (0.8–3.0) | ||||
| ≥469 | 123 | 133 | 1.7 (1.1–2.6) | 0.39 | 34 | 1.1 (0.6–1.9) | 0.65 | 47 | 2.7 (1.5–5.0) | 0.75 | 25 | 1.5 (0.8–3.0) | 0.28 |
| CXCL13 (pg/ml) | 490 | 487 | 2.40 (1.85–3.12)b | 140 | 1.54 (1.09–2.16)b | 138 | 2.40 (1.77–3.26)b | 102 | 2.88 (2.09–3.96)b | ||||
| <41.8 | 122 | 67 | Referent | 25 | Referent | 18 | Referent | 10 | Referent | ||||
| 41.8 to <55.8 | 123 | 95 | 1.6 (1.0–2.4) | 34 | 1.3 (0.7–2.3) | 21 | 1.1 (0.6–2.3) | 18 | 1.8 (0.8–4.1) | ||||
| 55.8 to <75.4 | 122 | 122 | 2.1 (1.3–3.2) | 39 | 1.6 (0.9–2.8) | 29 | 1.6 (0.8–3.1) | 31 | 3.2 (1.5–6.9) | ||||
| ≥75.4 | 123 | 203 | 3.4 (2.2–5.2) | <0.0001 | 42 | 1.7 (1.0–3.0) | 0.22 | 70 | 3.9 (2.2–6.9) | <0.0001 | 43 | 4.4 (2.1–9.2) | 0.0009 |
Adjusted for the matching factors (birth year, blood draw date, and study region).
OR for association with 1-unit in the immune marker on the natural log scale (i.e., with 2.72-fold increase on the untransformed scale).
We saw increased risks associated with CD23, CD27, CD30, and CXCL13 for all the NHL subtypes examined (Table 3); however, there were some significant differences between subtypes in the magnitude of risk estimates (p<0.05 for tests of heterogeneity between subtypes in polytomous regression analyses). sCD23 was more strongly associated with CLL/SLL/PLL than with DLBCL or follicular lymphoma for the continuous measure, the highest-versus-lowest quartile, and the trend variable, and in turn, sCD23 was more strongly associated with follicular lymphoma than DLBCL. For example, each per-unit increase in the continuous sCD23 measure (on the natural log scale) was associated with 4.99-fold increased risk of CLL/SLL/PLL, which was significantly greater than the 1.97-fold increased risk estimated for follicular lymphoma (p-heterogeneity < 0.0001), and this risk estimate for follicular lymphoma was significantly greater than the 1.30-fold risk associated with DLBCL (p-heterogeneity < 0.005). sCD27 associations were stronger for CLL/SLL/PLL than follicular lymphoma for the quartile variables. The relative risk estimate associated with the sCD30 continuous measure was significantly higher for follicular lymphoma than CLL/SLL/PLL, and the trend across quartiles was stronger for follicular lymphoma or DLBCL than for CLL/SLL/PLL. sCD44 results were stronger for the highest-versus-lowest quartile for DLBCL than CLL; however, there was no clear risk trend across quartiles for DLBCL. CXCL13 associations were stronger for DLBCL and follicular lymphoma than for CLL/SLL/PLL for the continuous measure and the highest-versus-lowest quartile, and the trend across quartiles was stronger for DLBCL than for CLL/SLL/PLL. Similar to observed associations for the three subtypes examined, the risk of ‘other’ B-cell NHL was significantly elevated by continuous marker levels of sCD23 (OR=1.50, 95% CI: 1.20–1.89), sCD27 (OR=5.23, 95% CI: 3.04–9.01), sCD30 (OR=2.02, 95% CI: 1.32–3.10), and CXCL13 (OR=2.66, 95% CI: 1.93–3.68), but not sCD44 (OR=1.94, 95% CI: 0.89–4.25).
In polytomous regression analyses with B-NHL cases grouped by the lag time between (prediagnostic) blood draw and diagnosis (Table 4), CD23, CD27, CD30, and CXCL13 were all significantly associated with increased risk of NHL when measured in samples collected as early as 9–13 years before diagnosis, whereas CD44 was associated with NHL risk (albeit inconsistently) only when measured in samples collected within 8 years of the diagnosis. Nevertheless, there was significant heterogeneity in the results by the length of the prediagnostic lag (based on p<0.05 for tests of heterogeneity between cases grouped by lag time in polytomous regression analyses) that indicated generally decreasing strength of associations with increasing time between blood draw and NHL diagnosis. The continuous measure for each marker was more strongly associated with NHL cases with <3 years prediagnostic lag than for cases with 9–13 years lag; for example, sCD30 was associated with 4.08-fold increased risk of NHL for cases with <3 years prediagnostic lag, versus 2.03-fold increased risk for cases with 9–13 years lag (p-heterogeneity = 0.009). The most consistent pattern of risk decreases by increasing lag time were observed for sCD23 and sCD27, for which the continuous marker measure was more strongly associated with NHL cases whose blood samples were collected in the <3 years before diagnosis than for any longer lag, and the trend across quartiles was less strong for cases with 9–13 years lag than for cases with any shorter lag. Similar results were seen for sCD30, CD44, and CXCL13 with statistical significance for some, but not all pairwise lag increment comparisons.
Table 4.
Association between immune marker levels at study baseline and B-NHL incidence, with cases classified according to lag time between blood draw and diagnosisa
| Controls | Cases, 0–2 years lag | Cases, 3–5 years lag | Cases, 6–8 years lag | Cases, 9–13 years lag | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | N | OR (95% CI) | p trend | N | OR (95% CI) | p trend | N | OR (95% CI) | p trend | N | OR (95% CI) | P trend |
| sCD23 (U/ml) | 490 | 91 | 2.69 (2.12–3.40)b | 158 | 1.99 (1.64–2.42)b | 142 | 1.78 (1.46–2.18)b | 95 | 1.58 (1.26–1.98)b | ||||
| <29.2 | 122 | 11 | Referent | 20 | Referent | 11 | Referent | 15 | Referent | ||||
| 29.2 to <51.8 | 123 | 15 | 1.3 (0.6–3.0) | 30 | 1.5 (0.8–2.8) | 29 | 2.6 (1.2–5.5) | 19 | 1.3 (0.6–2.7) | ||||
| 51.8 to <75.0 | 122 | 13 | 1.2 (0.5–2.7) | 18 | 0.9 (0.5–1.8) | 27 | 2.5 (1.2–5.2) | 24 | 1.6 (0.8–3.2) | ||||
| ≥75.0 | 123 | 52 | 4.6 (2.3–9.3) | <0.0001 | 90 | 4.6 (2.6–7.9) | <0.0001 | 75 | 6.8 (3.4–13.5) | <0.0001 | 37 | 2.5 (1.3–4.9) | 0.005 |
| sCD27 (U/ml) | 490 | 91 | 10.3 (5.88–18.2)b | 159 | 5.06 (3.09–8.26)b | 142 | 3.83 (2.29–6.41)b | 95 | 2.60 (1.40–4.83)b | ||||
| <177 | 122 | 7 | Referent | 17 | Referent | 14 | Referent | 12 | Referent | ||||
| 177 to <224 | 123 | 13 | 1.8 (0.7–4.8) | 31 | 1.9 (1.0–3.6) | 34 | 2.5 (1.3–4.8) | 24 | 2.1 (1.0–4.4) | ||||
| 224 to <275 | 122 | 22 | 3.1 (1.3–7.7) | 35 | 2.2 (1.2–4.2) | 25 | 1.9 (0.9–3.8) | 28 | 2.4 (1.1–5.0) | ||||
| ≥275 | 123 | 49 | 7.0 (3.0–16.3) | <0.0001 | 76 | 4.9 (2.7–9.0) | <0.0001 | 69 | 5.3 (2.8–10.1) | <0.0001 | 30 | 2.7 (1.3–5.6) | 0.56 |
| sCD30 (ng/ml) | 490 | 91 | 4.08 (2.70–6.16)b | 159 | 2.72 (1.90–3.91)b | 142 | 2.08 (1.42–3.06)b | 95 | 2.03 (1.28–3.21)b | ||||
| <24.1 | 122 | 13 | Referent | 19 | Referent | 25 | Referent | 15 | Referent | ||||
| 24.1 to <30.2 | 123 | 14 | 1.1 (0.5–2.4) | 29 | 1.7 (0.9–3.1) | 27 | 1.1 (0.6–2.0) | 31 | 1.9 (1.0–3.7) | ||||
| 30.2 to <40.2 | 122 | 17 | 1.3 (0.6–2.9) | 49 | 2.9 (1.6–5.3) | 29 | 1.2 (0.7–2.2) | 19 | 1.1 (0.5–2.3) | ||||
| ≥40.2 | 123 | 47 | 3.6 (1.8–7.2) | <0.0001 | 62 | 3.7 (2.1–6.7) | 0.002 | 61 | 2.5 (1.5–4.4) | <0.0001 | 30 | 1.8 (0.9–3.5) | 0.40 |
| sCD44 (ng/ml) | 490 | 91 | 3.84 (1.74–8.46)b | 159 | 2.45 (1.26–4.76)b | 142 | 2.16 (1.08–4.29)b | 95 | 1.00 (0.43–2.30)b | ||||
| <326 | 122 | 13 | Referent | 23 | Referent | 25 | Referent | 23 | Referent | ||||
| 326 to <391 | 123 | 29 | 2.2 (1.1–4.4) | 42 | 1.8 (1.0–3.2) | 31 | 1.2 (0.7–2.2) | 37 | 1.6 (0.9–2.9) | ||||
| 391 to <469 | 122 | 21 | 1.6 (0.7–3.3) | 51 | 2.3 (1.3–4.0) | 43 | 1.7 (1.0–3.0) | 16 | 0.7 (0.3–1.4) | ||||
| ≥469 | 123 | 28 | 2.0 (1.0–4.2) | 0.85 | 43 | 2.0 (1.1–3.5) | 0.40 | 43 | 1.7 (1.0–4.4) | 0.77 | 19 | 0.8 (0.4–1.5) | 0.17 |
| CXCL13 (pg/ml) | 490 | 91 | 3.20 (2.32–4.41)b | 159 | 2.38 (1.76–3.20)b | 142 | 1.83 (1.32–2.53)b | 95 | 1.98 (1.38–2.85)b | ||||
| <41.8 | 122 | 6 | Referent | 21 | Referent | 24 | Referent | 16 | Referent | ||||
| 41.8 to <55.8 | 123 | 11 | 1.8 (0.6–5.0) | 37 | 1.7 (1.0–3.2) | 27 | 1.1 (0.6–2.0) | 20 | 1.2 (0.6–2.5) | ||||
| 55.8 to <75.4 | 122 | 27 | 4.5 (1.8–11.3) | 32 | 1.5 (0.8–2.8) | 37 | 1.6 (0.9–2.8) | 26 | 1.7 (0.8–3.3) | ||||
| ≥75.4 | 123 | 47 | 7.9 (3.3–19.3) | <0.0001 | 69 | 3.2 (1.8–5.6) | <0.0001 | 54 | 2.3 (1.3–3.9) | 0.003 | 33 | 2.0 (1.1–3.9) | 0.07 |
Adjusted for the matching factors (birth year, blood draw date, and study region).
OR for association with 1-unit in the immune marker on the natural log scale (i.e., with 2.72-fold increase on the untransformed scale).
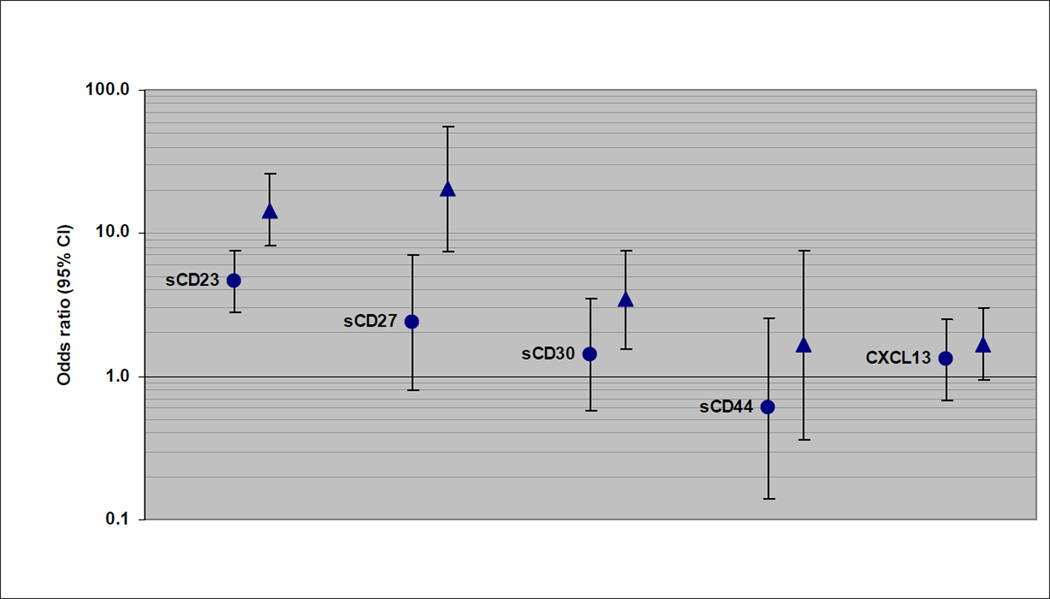
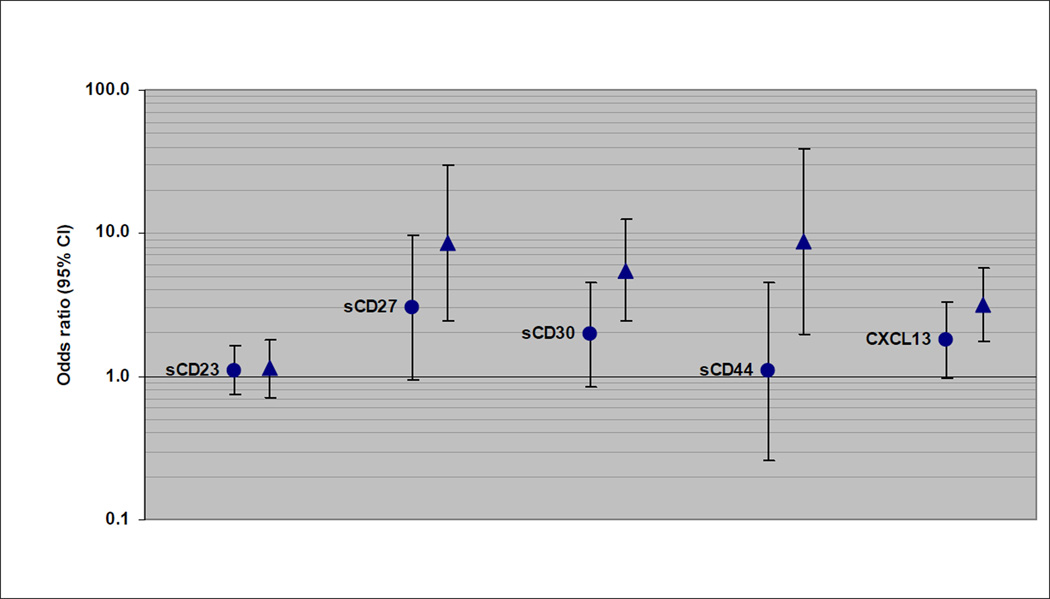
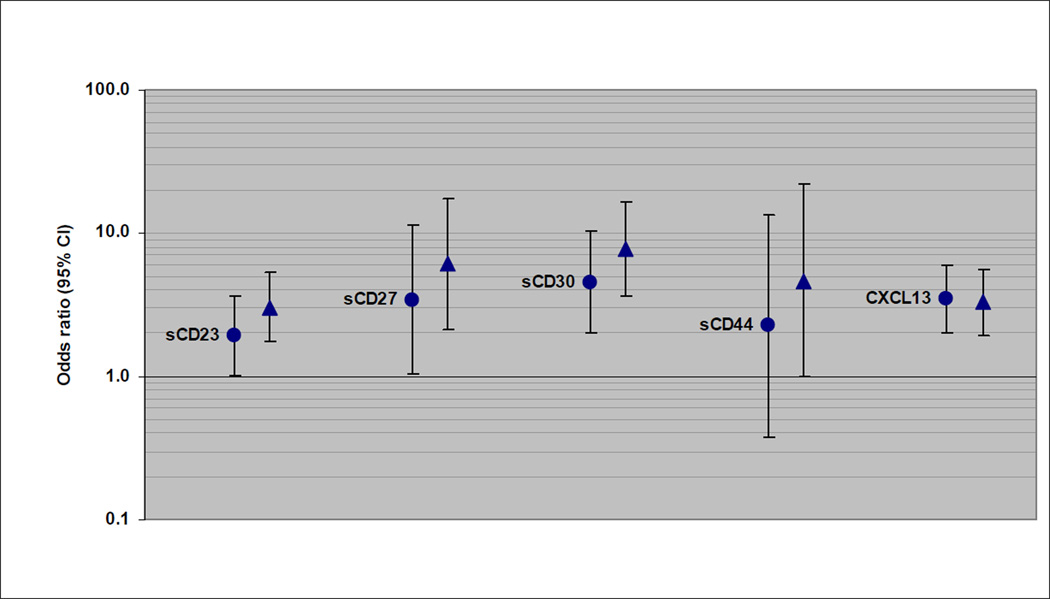
Of the subtypes examined, CLL/SLL/PLL was most strongly associated with sCD23. The continuous sCD23 measure was significantly associated with CLL/SLL/PLL within each prediagnostic lag category (lags of <3 years and 9–13 years are shown in Figure 1); however, the association was significantly stronger for CLL/SLL/PLL cases with blood draw <3 years before diagnosis (OR=14.5, 95% CI: 8.10–25.8) than for longer lags (for example, for cases with 9–13 years lag, OR=4.62, 95% CI: 2.81–7.62; p-heterogeneity = 0.0003). The trend of increasing risk across quartiles of sCD23 was significant for CLL/SLL/PLL cases within each prediagnostic lag increment, although the trend was significantly stronger for cases with <3 years lag compared to those with 9–13 years lag (p-heterogeneity = 0.04). Similar heterogeneity by prediagnostic lag was seen for sCD27 and sCD30 associations with CLL/SLL/PLL (Figure 1). DLBCL associations with each immune marker tended to drop off with a longer lag between blood draw and diagnosis (Figure 2). There were no statistically significant associations with the continuous marker measures for DLBCL cases with 9–13 years lag since blood draw, and no indication of a trend across quartiles for the 9–13 years lag, except for CXCL13 (p-trend = 0.07). For example, the association with sCD44 was significantly stronger for DLBCL cases with blood draw <3 years before diagnosis (OR=8.74, 95% CI: 1.95–39.2) than for 9–13 years lag (OR=1.08, 95% CI: 0.26–4.51; p-heterogeneity = 0.04). DLBCL associations with CXCL13 were not significantly different between lag increments. There was no significant heterogeneity by length of the prediagnostic lag for any of the immune marker associations with follicular lymphoma (Figure 3). Nevertheless, associations did decrease slightly with longer lags for sCD23, sCD27, and sCD30. For example, there was 7.71-fold increased risk associated with the continuous sCD30 measure for follicular lymphoma cases with <3 years prediagnostic lag (95% CI: 3.61–16.5) and 4.53-fold increased risk observed for cases with 9–13 years lag (95% CI: 2.01–10.2), and the trend of increasing risk across quartile categories was significant for all lag increments except 9–13 years.
Figure 1.
CLL/SLL/PLL risk associated with 1-unit (natural log scale) increase in immune marker level (OR and 95% CI), with cases categorized by decreasing lag time between blood draw and diagnosis (circle: 9–13 years; triangle: <3 years).
Figure 2.
DLBCL risk associated with 1-unit (natural log scale) increase in immune marker level (OR and 95% CI), with cases categorized by decreasing lag time between blood draw and diagnosis (circle: 9–13 years; triangle: <3 years).
Figure 3.
Follicular lymphoma risk associated with 1-unit (natural log scale) increase in immune marker level (OR and 95% CI), with cases categorized by decreasing lag time between blood draw and diagnosis (circle: 9–13 years; triangle: <3 years).
The associations we observed were robust to exclusion of study subjects reporting immune-related medical conditions or medications at baseline (not shown in tables). For example, in these analyses (n=346 cases, 349 controls), risk of B-cell NHL was significantly elevated for the continuous measure, the highest-versus-lowest quartile, and the trend variable for sCD23, sCD27, sCD30, and CXCL13, with risk estimates for the highest-versus-lowest quartiles ranging from 2.5 for sCD30 (95% CI: 1.5–4.2) to 6.3 for sCD23 (95% CI: 3.4–11.8).
DISCUSSION
Our research was inspired by previous prospective studies that have found these and other immune biomarkers of B-cell activation to be associated with risk of NHL among HIV patients (5–7,9–11,21). Based on the findings of the studies among immune compromised patients, we hypothesized that a B-cell stimulatory host environment increases the risk of B-cell NHL among immunocompetent persons. The prospective design of the WHI OS and the banked samples offered a valuable resource to identify markers of subclinical B-cell activation associated with NHL incidence in a sizable study. We found strong associations between B-cell NHL and sCD23, sCD27, sCD30, and CXCL13, with risks that increased significantly by increasing levels of the markers.
The markers we studied are all indicative of, or involved in B-cell activation. CD23 is an Fc receptor for IgE that is upregulated on activated B cells. Its soluble form, sCD23, is released from activated B cells and can itself induce further B-cell stimulation, including enhancement of Ig class switch recombination (22). CD27 and CD30 are both members of the TNF-receptor superfamily (23,24). CD27 interacts with other molecules to provide co-stimulatory signaling in the activation of both T- and B cells to proliferate and produce cytokines or immunoglobulins.(8) CD30 is preferentially expressed by activated type-2 T cells which produce cytokines that support or enhance B-cell activation and/or differentiation (25). Higher levels of both sCD27 and sCD30 have been found in persons with immune system activation, such as those with autoimmune disorders, hepatitis B and C viruses, and HIV (26,27). CXCL13 is a chemokine that plays a central role in the homeostatic trafficking of B cells, through interactions with its only known receptor, CXCR5, which is commonly expressed on resting B cells (28). The markers we studied are correlated, which was not unexpected, given their related roles in immune activation. Regardless of these correlations and the possibility of one marker serving as a surrogate for a true association with a related molecule, an association with any of the markers we studied would suggest a role of B-cell activation in development of B-cell NHL. Furthermore, even surrogate markers may be useful in development of screening tests for NHL. There remains the possibility that these markers may be predictive of other types of cancer (or other diseases) in addition to NHL, and the specificity of the associations to NHL would need to be examined before the utility of the markers for screening could be determined.
An important question in studies such as ours is whether the markers (or B-cell activation, in general) are etiologically associated with the disease in question (i.e., causal) or whether they are simply elevated in response to as-yet undiagnosed disease (i.e., reverse causation). While this is impossible to determine in an observational study, we assumed that if risk estimates were stronger for cases with a short lag between blood draw and diagnosis than for those with longer lags, this was likely indicative of elevations in the marker due to undiagnosed disease. Some of these molecules, such as sCD27, have been previously associated with lymphoma tumor load (29), providing a basis for speculation that associations we observed may reflect undiagnosed B-NHL or a precursor lesion. We did observe the strongest associations for cases with the shortest prediagnosis lag (<3 years). However, there were also relatively strong, significant associations for cases with the longest lag (9–13 years), suggesting either an etiologic role of B-cell activation, correlation with another etiologic process (i.e. association due to confounding), or a prolonged occult phase of B-NHL. Theoretically, the same molecule could play a causal role in NHL development, as well as be subsequently upregulated after tumor formation, and our results may reflect both scenarios.
The presence of a consistent association of these markers with B-cell NHL across histologic subtypes and over a number of years argues that these associations cannot be attributed to undiagnosed disease in all instances. There is currently no evidence that most lymphoma subtypes, including DLBCL or follicular lymphoma, are preceded by significant circulating subclinical clonal lymphocyte expansions. However, specific associations that we note with a short time lag may reflect undiagnosed disease, particularly for CLL/SLL. It is now well established that CLL/SLL is preceded in virtually all instances by small, preclinical clonal lymphocyte expansions termed “monoclonal B-cell lymphocytosis” (MBL) (30,31). CD23 is known to be expressed by the neoplastic lymphocytes in CLL/SLL, and increased levels of sCD23 have been associated with CLL/SLL diagnosis and may be of prognostic significance (32,33). It is therefore likely that the marked elevation in risk of CLL/SLL associated with increased sCD23 close to the time of diagnosis reflects the presence of preclinical disease in these patients. The same may also apply to the associations with sCD27, as CD27 has also been shown to be expressed on clonal lymphocytes in both MBL and CLL/SLL (33). However, even if this is the case it does not necessarily imply that weaker associations observed with a longer time lag reflect undiagnosed disease, even for CLL/SLL. At this time it is not well-documented whether MBL populations may precede a clinical diagnosis of CLL/SLL by as much as 9 years, and it is also unclear whether very small MBL populations would contribute significantly to circulating levels of these markers.
Our study is the largest to date of the biomarkers we studied in relation to risk of NHL and was well-powered, as evidenced by narrow confidence intervals for our main analyses (confidence interval widths of <3). Nevertheless, B-cell NHL is quite heterogeneous, and we had adequate power for separate analyses of only the more common NHL subtypes. Our results provide evidence that the association with B-cell activation is not limited to the aggressive forms of NHL that predominate in HIV, based on similar associations we observed with indolent lymphomas such as follicular lymphoma and CLL/SLL. In our study, follicular lymphoma was the subtype with the most consistent associations regardless of the lag time between blood draw and diagnosis. We also observed the strongest associations for sCD30 with follicular lymphoma, as did Purdue et al (13). However, we had only 15 follicular lymphoma cases with 9–13 years between blood draw and NHL diagnosis, limiting our power to detect differences by lag time. Much larger samples will be needed to further characterize the heterogeneity by both NHL subtype and the lag between blood draw and diagnosis.
Our findings indicate a prominent role for B-cell activation in the etiology of B-cell NHL and/or in processes reflective of early disease. Our study population consisted of post-menopausal women who were primarily of white race and well-educated, and the generalizability of our findings to the general population is unknown. Further investigation of this biologic pathway in prospective studies is warranted, both for increasing understanding of NHL etiology and well as for development of diagnostic/predictive markers.
ACKNOWLEDGEMENTS
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf.
GRANT SUPPORT
This WHI ancillary study was funded through a contract with the National Heart, Lung, and Blood Institute under the Broad Agency Announcement mechanism (“Markers of B-Cell Stimulation as Potential Predictors of Non-Hodgkin Lymphoma”, BA13). The work was carried out, in part, in the facilities of the UCLA AIDS Institute, which were supported, in part, by funds from the James B. Pendleton Charitable Trust and the McCarthy Family Foundation. The WHI program is supported by contracts from the National Heart, Lung and Blood Institute, NIH.
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Schwartz RS. Immunodeficiency, immunosuppression, and susceptibility to neoplasms. J Natl Cancer Inst Monogr. 2001:5–9. doi: 10.1093/oxfordjournals.jncimonographs.a024257. [DOI] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 3.Koshiol J, Lam TK, Gridley G, Check D, Brown LM, Landgren O. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin's lymphoma in veterans from the United States. J Clin Oncol. 2011;29:378–385. doi: 10.1200/JCO.2010.30.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yawetz S, Cumberland WG, van der MM, Martinez-Maza O. Elevated serum levels of soluble CD23 (sCD23) precede the appearance ofacquired immunodeficiency syndrome--associated non-Hodgkin's lymphoma. Blood. 1995;85:1843–1849. [PubMed] [Google Scholar]
- 5.Schroeder JR, Saah AJ, Hoover DR, Margolick JB, Ambinder RF, Martinez-Maza O, et al. Serum soluble CD23 level correlates with subsequent development of AIDS-related non-Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev. 1999;8:979–984. [PubMed] [Google Scholar]
- 6.Breen EC, van der MM, Cumberland W, Kishimoto T, Detels R, Martinez-Maza O. The development of AIDS-associated Burkitt's/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6. Clin Immunol. 1999;92:293–299. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 7.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widney D, Gundapp G, Said JW, van der MM, Bonavida B, Demidem A, et al. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol. 1999;93:114–123. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 9.Breen EC, Fatahi S, Epeldegui M, Boscardin WJ, Detels R, Martinez-Maza O. Elevated serum soluble CD30 precedes the development of AIDS-associated non-Hodgkin's B cell lymphoma. Tumour Biol. 2006;27:187–194. doi: 10.1159/000093022. [DOI] [PubMed] [Google Scholar]
- 10.Breen EC, Epeldegui M, Boscardin WJ, Widney DP, Detels R, Martinez-Maza O. Elevated levels of soluble CD44 precede the development of AIDS-associated non-Hodgkin's B-cell lymphoma. AIDS. 2005;19:1711–1712. doi: 10.1097/01.aids.0000184924.04983.7c. [DOI] [PubMed] [Google Scholar]
- 11.Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB, et al. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res. 2005;25:702–706. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 12.Widney DP, Gui D, Popoviciu LM, Said JW, Breen EC, Huang X, et al. Expression and Function of the Chemokine, CXCL13, and Its Receptor, CXCR5, in Aids-Associated Non-Hodgkin's Lymphoma. AIDS Res Treat. 2010;2010 doi: 10.1155/2010/164586. 164586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdue MP, Lan Q, Martinez-Maza O, Oken MM, Hocking W, Huang WY, et al. A prospective study of serum soluble CD30 concentration and risk of non-Hodgkin lymphoma. Blood. 2009;114:2730–2732. doi: 10.1182/blood-2009-04-217521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeulen R, Hosnijeh FS, Portengen L, Krogh V, Palli D, Panico S, et al. Circulating soluble CD30 and future risk of lymphoma; evidence from two prospective studies in the general population. Cancer Epidemiol Biomarkers Prev. 2011;20:1925–1927. doi: 10.1158/1055-9965.EPI-11-0396. [DOI] [PubMed] [Google Scholar]
- 15.Purdue MP, Lan Q, Bagni R, Hocking WG, Baris D, Reding DJ, et al. Prediagnostic serum levels of cytokines and other immune markers and risk of non-hodgkin lymphoma. Cancer Res. 2011;71:4898–4907. doi: 10.1158/0008-5472.CAN-11-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.International Classification of Diseases for Oncology, 3rd Edition. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol 1. The Analysis of Case-Control Studies. Lyon, France: 1980. [PubMed] [Google Scholar]
- 21.Breen EC, Magpantay L, Hu Z, Jacobson L, Detels R, Rabkin C, Kaslow R, Ambinder R, Jacobson L. Serum levels of molecules that are markers of B cell activation are elevated prior to the development of acquired immunodeficiency syndrome-associated non-Hodgkin's B cell lymphoma; American Association of Cancer Research, Annual Meeting 2006 #165; 2006. [Google Scholar]
- 22.Gordon J. CD23 and B cell activation. Clin Exp Allergy. 1992;22:199–204. doi: 10.1111/j.1365-2222.1992.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 23.Lens SM, Drillenburg P, den Drijver BF, van Schijndel G, Pals ST, van Lier RA, et al. Aberrant expression and reverse signalling of CD70 on malignant B cells. Br J Haematol. 1999;106:491–503. doi: 10.1046/j.1365-2141.1999.01573.x. [DOI] [PubMed] [Google Scholar]
- 24.Nawrocki JF, Kirsten ES, Fisher RI. Biochemical and structural properties of a Hodgkin's disease-related membrane protein. J Immunol. 1988;141:672–680. [PubMed] [Google Scholar]
- 25.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 26.Younes A, Aggarwall BB. Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer. 2003;98:458–467. doi: 10.1002/cncr.11524. [DOI] [PubMed] [Google Scholar]
- 27.Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27–CD70 interactions. Semin Immunol. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 28.Muller G, Hopken UE, Stein H, Lipp M. Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors. J Leukoc Biol. 2002;72:1–8. [PubMed] [Google Scholar]
- 29.van Oers MH, Pals ST, Evers LM, van der Schoot CE, Koopman G, Bonfrer JM, et al. Expression and release of CD27 in human B-cell malignancies. Blood. 1993;82:3430–3436. [PubMed] [Google Scholar]
- 30.Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Ghia P, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360:659–667. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawstron AC, Bennett FL, O'Connor SJ, Kwok M, Fenton JA, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 32.Meuleman N, Stamatopoulos B, Dejeneffe M, El HH, Lagneaux L, Bron D. Doubling time of soluble CD23: a powerful prognostic factor for newly diagnosed and untreated stage A chronic lymphocytic leukemia patients. Leukemia. 2008;22:1882–1890. doi: 10.1038/leu.2008.190. [DOI] [PubMed] [Google Scholar]
- 33.Rawstron AC, Shingles J, de TR, Bennett F, Jack AS, Hillmen P. Chronic lymphocytic leukaemia (CLL) and CLL-type monoclonal B-cell lymphocytosis (MBL) show differential expression of molecules involved in lymphoid tissue homing. Cytometry B Clin Cytom. 2010;78(Suppl 1):S42–S46. doi: 10.1002/cyto.b.20534. [DOI] [PubMed] [Google Scholar]