Abstract
Tumor-associated myeloid cells are the major type of inflammatory cells involved in the regulation of anti-tumor immune responses. One key characteristic of these cells is the generation of reactive oxygen (ROS) and reactive nitrogen (RNS) species in the tumor microenvironment. Recent studies have demonstrated the important role of ROS and RNS, especially peroxynitrite (PNT), in immune suppression in cancer. ROS and RNS are involved in induction of antigen-specific T-cell tolerance, inhibition of T-cell migration to the tumor site, and tumor cell evasion of recognition by cytotoxic T cells. In pre-clinical settings, a number of potential therapeutic agents demonstrated activity in blocking ROS/RNS in cancer and in improving the efficacy of cancer immune therapy. A better understanding of ROS/RNS-associated pathways in myeloid cells will help to identify more specific and direct targets to facilitate the development of more effective immune therapy of cancer.
Keywords: myeloid-derived suppressor cells, macrophages, neutrophils, reactive oxygen, peroxynitrite, cytotoxic T cells
Background
Paradigms of modern tumor immunology
Several major paradigms currently determine therapeutic efforts in tumor immunology. The major paradigm states that an efficient anti-tumor immunity requires capture and processing of tumor-associated or tumor-specific antigens by professional antigen presenting cells (dendritic cells - DC) with subsequent presentation of these antigens in the lymph nodes to CD8+ cytotoxic T lymphocytes (CTL). Antigen presentation is associated with the activation of CD8+ T cells; among other things, it results in the up-regulation of granzyme B (GrzB) necessary for the cytotoxic activity of CTLs and in the up-regulation of certain chemokine receptors that facilitate T cell migration to the tumor site. Once at the tumor site, T cells are able to recognize tumor cells expressing specific antigens and destroy them. Although this chain of events is observed in some experimental tumors, it is rare in cancer patients. Therefore, therapeutic efforts are focused on increasing the frequency of CTL-mediated tumor distraction by applying cancer vaccines, adoptively transferred antigen-specific T cells, inhibitors of check-point blockade (CTLA4, PD1) or other immune therapeutics (1). However, even in tumor-bearing mice, once tumors are established these interventions have relatively limited efficacy, and the rate of clinical responses in cancer patients, although encouraging, remains relatively low (2, 3). Appreciation of these facts has led to the establishment of another paradigmwhich states that immune responses in cancer are inhibited. Large numbers of different factors have been implicated in this process, including regulatory T cells, myeloid cells, various soluble factors and cytokines, inhibitory molecules expressed by immune and tumor cells, etc. (4–6). This has prompted a concerted effort to target specific molecules, with the goal of improving anti-tumor immune responses. However, the abundance of these inhibitory factors and the fact that most of them do not require antigen specificity to exert their suppressive effects are difficult to reconcile with the fact that in most cases neither tumor-bearing mice nor cancer patients are immune compromised (with the exception of terminal stages of the disease). T cells from the blood of cancer patients and from the spleens or lymph nodes of tumor-bearing mice have relatively strong responses to nonspecific stimuli like lectins, anti-CD3 antibody, etc. However, tumor-specific responses of these T cells are substantially inhibited (7–9). In contrast, T cells isolated from the tumor site in both patients and mice are profoundly suppressed, exhibiting dramatic changes in T-cell receptor (TCR) signaling, proliferation, and effector functions (10–12). This has resulted in the development of the third paradigm in tumor immunology: a compartmentalization of immune suppression in cancer. Immune deficiency at the tumor site is profound and antigen-independent, whereas in the periphery it is much more specialized with respect to tumor-associated antigens. Additional complications come from the fact that T cell infiltration into solid tumors is limited by the effect of tumor stroma and other factors, which will be discussed below.
We recently proposed new paradigm that may have direct implications for cancer immune therapy: tumor cells may resist recognition by potent CTLs in a way that does not affect immune suppression. This process is mediated by reactive oxygen species (ROS) and reactive nitrogen species (RNS) and is associated with tumor infiltration by myeloid cells (13).
Myeloid cells in cancer and production of reactive oxygen species
It is now clear that inflammation plays a major role in tumor progression (14). Myeloid cells are a major component of inflammatory reactions, and in recent years ample evidence has placed them at a prominent position in the regulation of tumor-associated immune suppression, tumor growth, and metastasis. Myeloid cells in cancer are represented by populations of mature cells (macrophages, granulocytes, and DC), as well by as pathologically-activated immature myeloid cells (myeloid-derived suppressor cells - MDSC). Some populations of mature myeloid cells in cancer may be different from their control counterparts by acquiring immune suppressive activity (15–19). MDSC is the group of cells that is not present under normal conditions. They comprise a heterogeneous population of immature myeloid cells and myeloid progenitor cells. In healthy hosts, cells with the same phenotype are not immune suppressive and rapidly differentiate into mature myeloid cells. In cancer, these cells are expanded and activated, which results in the acquisition of potent immune suppressive activity and the altered ability to differentiate. Based on the morphology and phenotype, in mice and cancer patients MDSC can be subdivided into two major groups: polymorphonuclear MDSC (PMN-MDSC) and monocytic MDSCs (M-MDSC) (20–23). M-MDSC represent a population of pathologically-activated monocytes with high levels of arginase I and iNOS (Nos2). In the tumor microenvironment, these cells differentiate into immune suppressive tumor-associated macrophages (TAM) (24). PMN-MDSC represent a population of pathologically-activated immature neutrophils and in culture rapidly differentiate into neutrophils (PMN). PMN-MDSC have substantially higher levels of ROS and myeloperoxidase and reduced phagocytosis compared to PMN. PMN-MDSC also contain more lysosomal enzymes than PMN and exhibit increased chemotaxis toward supernatants from human carcinomas (25, 26).
One of the predominant functions shared by all myeloid cells is the generation of ROS and RNS. In tumor-bearing hosts, ROS and RNS are the major mediators of myeloid cell-induced immune suppression (18, 27–30). In myeloid cells, NADPH oxidase (Nox1) is the primary producer of ROS. Nox1 reduces oxygen to superoxide anion (O2.−). Superoxide anion then readily reacts with nitric oxide (NO) primarily produced by Nos2, and this results in the generation of peroxynitrite (ONOO−). Peroxynitrite (PNT) is one of the most potent oxidants in the body. If not efficiently controlled by an antioxidant system, PNT can cause severe damage by oxidizing biological molecules such as membrane phospholipids, nucleic acids and proteins. In particular, PNT induces the nitration of four aminoacids: tyrosine, cysteine, methionine and tryptophan (31). Nitrotyrosine in tissues has been used as a marker of PNT activity (32).
The metabolism of L-arginine in myeloid cells is also associated with the production of ROS/RNS. L-arginine acts as a substrate for two enzymes, arginase 1 and Nos2. Arginase 1 converts L-arginine to urea and L-ornithine, and directs the production of superoxide anion, while Nos2 causes the generation of high levels of NO. The cooperative effect of arginase 1 and Nos2 drives the production of PNT (33). PNT has been detected in many cancers, and high PNT levels in tumor tissues are associated with poor prognosis (34–38).
Possible role of PNT in cancer
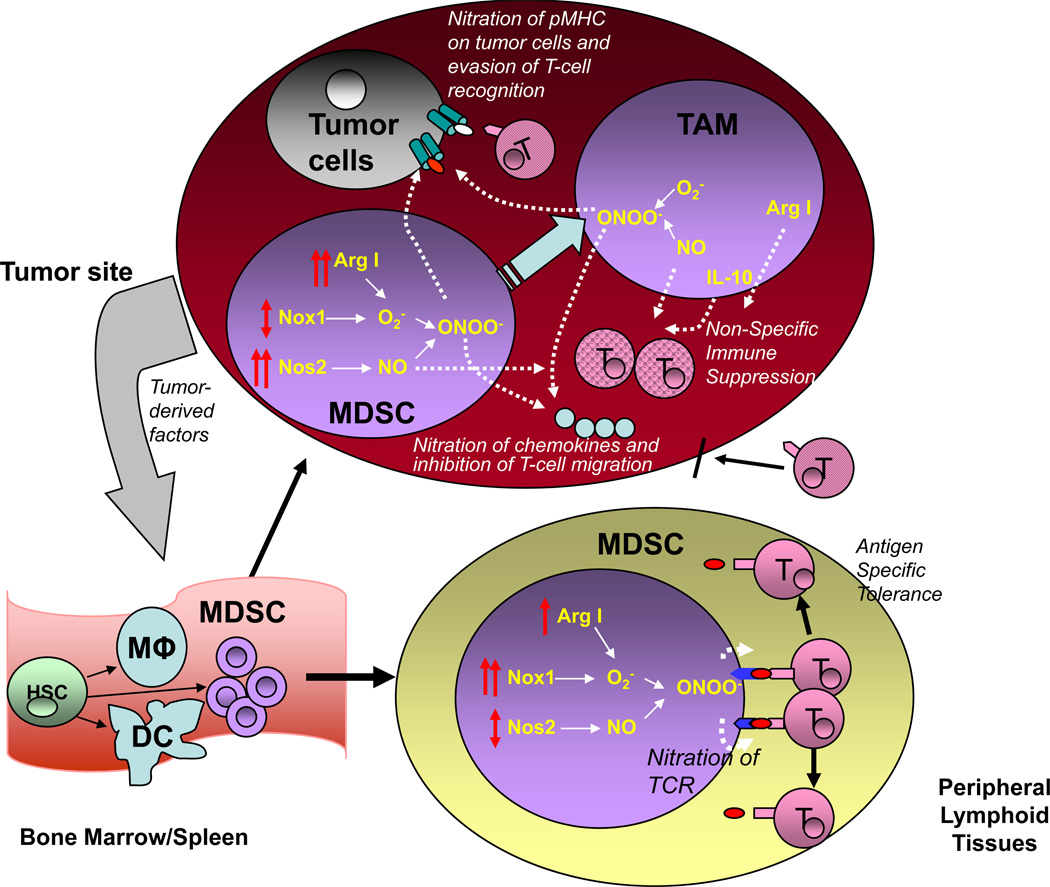
At least three negative effects of myeloid cell-produced PNT have been demonstrated in the tumor microenvironment (Fig. 1).
Figure. Effects of myeloid-cell derived PNT in immune responses in cancer.
Tumor-derived factors affect myelopoiesis by promoting accumulation of MDSC. These cells migrate to either peripheral lymphoid organs (lymph nodes) or tumor site. In lymphoid organs MDSC express high level of Nox1 and produce large amount of ROS. Nos1 expression and NO level remain relatively unchanged. However, increased superoxide production leads to generation of increased amount of PNT that induce nitration of TCR/CD8 on the surface of T cells after their close contact (antigen-specific) with MDSC. T cells with nitrated TCR/CD8 are not able to bind specific peptide that causes antigen-specific tolerance. In tumor site MDSC have lower levels of Nox1 and ROS than in periphery, however, it is compensated by dramatically increased expression of Nos1 and NO and thus results in high production of PNT. In tumor site MDSC rapidly differentiate to tumor associated macrophages that produce large array of different immune suppressive cytokines (for example IL-10), NO, and arginase I that contribute to immune suppression. NO and arginase I cause T-cell suppression in tumor site regardless of antigen-specificity. PNT produced by MDSC and macrophage causes nitration of MHC I on tumor cells that allow tumor cells to escape recognition by tumor-infiltrating T cells. PNT also causes nitration of chemokines that prevent migration of T cells to the tumor site.
1. PNT can cause T cell suppression and tolerance. Inside the tumor site, high levels of PNT induce the nitration of tumor-infiltrating lymphocytes and render these cells non-responsive to various nonspecific stimuli (39). In the peripheral lymphoid organs, the level of PNT is much lower. It is produced by myeloid cells, primarily MDSC, and it requires close cell-cell contact between MDSC and T cells to affect T cell function. Macrophages and neutrophils can also produce PNT, albeit at substantially lower levels than MDSC, and potentially can contribute to this effect. PNT can induce T cell tolerance via nitration of the TCRs and CD8 on the surface of T cells, rendering CD8+ T cells unable to bind to peptide-MHC complexes (40). It is known that a single nitrated tyrosine can alter the recognition of MHC class I- and MHC class II-restricted peptides by CD8+ and CD4+ T cells, respectively (41, 42). CTLs with nitrated surface molecules demonstrated no responses to the specific peptides, while non-nitrated CTLs responded normally to the peptide stimulation (9). However, after interaction with MDSC, T cells retain their ability to respond to nonspecific stimuli.
2. PNT can affect tumor cells directly. We recently demonstrated that PNT-treated tumor cells were resistant to lysis by tumor-specific CTLs. PNT treatment did not affect the expression of MHC class I on tumor cells, but it did dramatically reduce the binding efficiency of peptides to MHC class I molecules (13). This effect was primarily mediated by tumor-infiltrating MDSC and to lesser extent by macrophages. In several patient tumor types, including lung, breast, and pancreatic carcinomas, myeloid cells but not tumor cells were the main source of PNT (13). Similar results were observed in mouse tumor tissues. The inability of CTLs to kill tumor cells was not caused by inhibition of their functional activity, and was observed even in experimental conditions where mice were treated with total body irradiation and adoptive T cell transfer. Pharmacological or genetic inhibition of ROS and PNT production in myeloid cells substantially reduced tumor cell resistance to CTLs. Moreover, inhibition of ROS and PNT production significantly enhanced the effect of cancer immune therapy (13). These data suggested that even if potent CTLs were generated by cancer vaccines or adoptive T-cell therapy, PNT-induced post-translational modifications to peptide-MHC I complexes on tumor cells would render CTLs unable to recognize tumor-specific antigens and eliminate tumors.
3. PNT can affect T cell migration to the tumor site. Molon et al. have demonstrated that intra-tumoral production of PNT induced CCL2 chemokine nitration and inactivation, which blocked T cell infiltration of the tumors (43). CCL2 is a chemoattractant for myeloid cells, activated T cells, and NK cells (44). Both human and mouse T cells were unable to migrate to the nitrated-CCL2. However, the migration of myeloid cells was not affected, which may explain why myeloid cells but not T cells are easily infiltrate tumors (43).
Clinical-Translational Advances
Because ROS and RNS play a major role in tumor-associated immune suppression and tumor progression, they have become an attractive target for therapeutic intervention. Several agents have demonstrated therapeutic potential in pre-clinical studies, and some of them are currently being tested in clinical trials.
One group of agents is represented by synthetic triterpenoids. These compounds work through activation of the transcription factor Nrf2 (nuclear factor (erythroid derived)-like 2), which induces the up-regulation of an array of antioxidant molecules, such as glutathione, thioredoxin, catalase, superoxide dismutase, etc. (45). The most potent synthetic triterpenoid, CDDO-Me (C-28 methyl ester of 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid), showed a strong protective effect from inflammation-induced ROS in human neutrophils (46). Our group demonstrated that in several tumor models CDDO-Me abrogated myeloid cell-induced immunosuppression. Similar effect was observed in some patients with pancreatic adenocarcinoma treated with CDDO-Me (47). That clinical trial, however, was not specifically designed to assess function of these cells. CDDO-Me treatment also dramatically enhanced the therapeutic effect of total body irradiation and T cell transfer in mice with established tumors. This effect was associated with a reduction of ROS and PNT levels at the tumor site. This prevented myeloid cell-induced nitration of MHC class I molecules on tumor cells, enabling tumor cells to present tumor-specific antigens and thereby increasing their susceptibility to killing by CTLs (13).
Nitroaspirins are a group of non-steroid anti-inflammatory drugs coupled with an NO-releasing moiety. It has been demonstrated that nitroaspirins are not only able to inhibit the production of inflammatory cytokines by mononuclear cells, but they are also effective at inhibiting ROS and RNS (48). They blocked inflammation-induced superoxide and Nox1 expression in smooth muscle cells and endothelial cells (49). Also, nitroaspirins suppress arginase 1 and Nos2 activity in myeloid cells. Their strong anti-ROS/RNS effect neutralized myeloid cell-induced T cell dysfunction in mice with colon carcinoma, and enhanced the therapeutic effect of anti-cancer immune vaccines (48).
[3-(aminocarbonyl)furoxan-4-yl]methyl salicylate, a member of a recently described new class of NO-donor aspirin, containing the furoxan system, demonstrated a strong ability to block the production of PNT in vivo through the inhibition of arginase I and Nos2. It was found to be an effective adjuvant in adoptive cell therapy (43). The administration of this compound in tumor-bearing mice significantly increased tumor infiltration by CTLs. This effect was mediated by the reduced presence of nitrated-CCL2 in tumors (43).
Phosphodiesterase-5 (PED5) inhibitors such as sildenafil and tadalafil, which are used in the clinic for non-cancer-related conditions, were found to down-regulate the expression of arginase 1 and Nos2 in myeloid cells. They were able to enhance the anti-tumor effect of CTLs in tumor-bearing mice, increase the infiltration and activation of CTLs inside the tumors, and improve the therapeutic effect of adoptive T-cell therapy (50). In limited settings of phase I clinical trial of patients with multiple myeloma and head and neck cancers, PED5 inhibitors restored lymphocyte proliferation, likely by blocking the suppressive effect of CD14+ myeloid cells (50).
Cyclooxygenase 2 (COX-2) has been shown to induce the production of arginase I and ROS in tumor-infiltrating myeloid cells. COX-2 inhibitors such as SC58236, SC58125 and celecoxib were able to down-regulate the expression of arginase I, ROS, and PGE2, resulting in the improvement of anti-tumor T cell effects and the efficacy of immunotherapy (51–53).
Signal transducer and activator of transcription 3 (STAT3) plays an important role in cancer inflammation and in the suppression of anti-tumor immune responses. STAT3 is involved in the generation of ROS in myeloid cells by increasing Nox1 activity, either directly through up-regulation of the expression of Nox1 subunits, or indirectly through up-regulation of the expression of the calcium-binding pro-inflammatory proteins S100A9 and S100A8 (54, 55). Sunitinib, a tyrsosine kinase inhibitor, demonstrated substantial reversal of MDSC-mediated immune suppression (56). This effect could be mediated by blockade of STAT3 signaling activity (57).
In summary, ROS/RNS-induced modifications of proteins play a major role in the inability of the immune system to recognize and eliminate tumor cells, and most likely act as an important factor limiting the effect of immune therapeutics. Targeting ROS/RNS may substantially improve the effect of cancer immune therapy. Several compounds that do so have demonstrated potent activity in vitro and in vivo in mouse tumor models, but their potential effects in cancer patients need to be determined. In many types of cancer, myeloid cells act as the major source of ROS and RNS in tumor tissues. Therefore, elimination of tumor-infiltrating myeloid cells could also contribute to a reduction in ROS/RNS. Although several mechanisms of ROS/RNS-mediated effects on the immune system in cancer have been suggested, the precise role of these mechanisms needs further elucidation.
Acknowledgments
Supported by NCI grant CA CA084488
Footnotes
Authors declare no conflicts of interest
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinowich H, Banks M, Reichert TE, Logan TF, Kirkwood JM, Whiteside TL. Expression and activity of signaling molecules in T lymphocytes obtained from patients with metastatic melanoma before and after interleukin 2 therapy. Clin Cancer Res. 1996;2:1263–1274. [PubMed] [Google Scholar]
- 11.Lopez CB, Rao TD, Feiner H, Shapiro R, Marks JR, Frey AB. Repression of interleukin-2 mRNA translation in primary human breast carcinoma tumor-infiltrating lymphocytes. Cell Immunol. 1998;190:141–155. doi: 10.1006/cimm.1998.1390. [DOI] [PubMed] [Google Scholar]
- 12.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–3145. [PubMed] [Google Scholar]
- 13.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 17.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immunol. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- 23.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011 doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 27.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995;181:1881–1886. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci U S A. 1996;93:13119–13124. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 30.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, et al. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6:4768–4775. [PubMed] [Google Scholar]
- 32.Haqqani AS, Kelly JF, Birnboim HC. Selective nitration of histone tyrosine residues in vivo in mutatect tumors. J Biol Chem. 2002;277:3614–3621. doi: 10.1074/jbc.M105730200. [DOI] [PubMed] [Google Scholar]
- 33.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 34.Vickers SM, MacMillan-Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg. 1999;134:245–251. doi: 10.1001/archsurg.134.3.245. [DOI] [PubMed] [Google Scholar]
- 35.Cobbs CS, Whisenhunt TR, Wesemann DR, Harkins LE, Van Meir EG, Samanta M. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 2003;63:8670–8673. [PubMed] [Google Scholar]
- 36.Kinnula VL, Torkkeli T, Kristo P, Sormunen R, Soini Y, Paakko P, et al. Ultrastructural and chromosomal studies on manganese superoxide dismutase in malignant mesothelioma. Am J Respir Cell Mol Biol. 2004;31:147–153. doi: 10.1165/rcmb.2003-0409OC. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Nakao K, et al. Nitric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin Cancer Res. 2006;12:1201–1207. doi: 10.1158/1078-0432.CCR-05-1269. [DOI] [PubMed] [Google Scholar]
- 38.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119:861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 39.Bronte V, Casic T, Gri G, Gallana K, Borsellino G, Marrigo I, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birnboim HC, Lemay AM, Lam DK, Goldstein R, Webb JR. Cutting edge: MHC class II-restricted peptides containing the inflammation-associated marker 3-nitrotyrosine evade central tolerance and elicit a robust cell-mediated immune response. J Immunol. 2003;171:528–532. doi: 10.4049/jimmunol.171.2.528. [DOI] [PubMed] [Google Scholar]
- 42.Hardy LL, Wick DA, Webb JR. Conversion of tyrosine to the inflammation-associated analog 3'-nitrotyrosine at either TCR- or MHC-contact positions can profoundly affect recognition of the MHC class I-restricted epitope of lymphocytic choriomeningitis virus glycoprotein 33 by CD8 T cells. J Immunol. 2008;180:5956–5962. doi: 10.4049/jimmunol.180.9.5956. [DOI] [PubMed] [Google Scholar]
- 43.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, et al. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 45.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 46.Thimmulappa RK, Fuchs RJ, Malhotra D, Scollick C, Traore K, Bream JH, et al. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid Redox Signal. 2007;9:1963–1970. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muzaffar S, Shukla N, Angelini G, Jeremy JY. Nitroaspirins and morpholinosydnonimine but not aspirin inhibit the formation of superoxide and the expression of gp91phox induced by endotoxin and cytokines in pig pulmonary artery vascular smooth muscle cells and endothelial cells. Circulation. 2004;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- 50.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 52.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 57.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]