Abstract
Drug addiction continues to be an important public health problem, with an estimated 22.6 million current illicit drug users in the United States alone. For many addictions, including cocaine, methamphetamine, and marijuana addiction, there are no approved pharmacological treatments. Behavioral treatments are effective but effects vary widely across individuals. Treatments that are effective across multiple addictions are greatly needed, and accumulating evidence suggests that one such approach may be pharmacological or behavioral interventions that enhance executive inhibitory control in addicts. Current evidence indicates that most forms of chronic drug use may be associated with significant cognitive impairments, especially in attention, working memory, and response inhibition functions. In some studies, these impairments predict poor treatment retention and outcome. A number of cognitive enhancing agents, including galantamine, modafinil, atomoxetine, methylphenidate, and guanfacine, have shown promising findings in human studies. Specific behavioral interventions, including cognitive remediation, also show promise. However, whether improvement of selective cognitive functions reduces drug use behavior remains to be determined. Cognitive enhancement to improve treatment outcomes is a novel strategy worthy of future research, as are related questions such as whether these approaches may be broadly beneficial to most addicts or best reserved for substance users with specific demonstrated cognitive impairments.
1. Introduction
Drug addiction continues to be an important public health problem, with an estimated 22.6 million current illicit drug users in the United States (SAMHSA, 2011). Effective medications are available for the treatment of nicotine, alcohol, and opioid addictions (Potenza et al., 2011; Sofuoglu and Kosten, 2004). Unfortunately, no medications have been proven to be effective for cocaine addiction despite a large number of medications screened in randomized clinical trials (Sofuoglu and Kosten, 2006). Similarly, no medications have been approved for the treatment of methamphetamine (Hill and Sofuoglu, 2007) or cannabis addiction (Sofuoglu et al., 2010b), although fewer clinical trials have been conducted for those addictions.
A number of effective behavioral treatments have been developed for addictive behaviors (Carroll and Onken, 2005; Dutra et al., 2008; Miller and Wilbourne, 2002). Among those with the strongest level of empirical support from randomized clinical trials are contingency management (CM, where abstinence or other targeted outcomes are reinforced with incentives)(Higgins et al., 1991; Petry, 2006), motivational interviewing (MI, where a specific, nonjudgmental interviewing style is used to enhance motivation and harness the individuals capacity for change)(Hettema et al., 2005; Miller, 1985), and cognitive behavioral therapy (CBT, which teaches specific strategies and skills to reduce substance use) (Carroll et al., 1994; Marlatt and George, 1984). In contrast to the specificity of effects of most medications for drugs of abuse (e.g., methadone or buprenorphine have demonstrated efficacy for opioid dependence with little effect on concomitant cocaine use), empirically validated behavioral therapies tend to be effective across the range of substance use disorders. For example, CBT, CM, and MI have been found to be effective across alcohol, cannabis, and cocaine use disorders (Burke et al., 2003; Dutra et al., 2008; Lussier et al., 2006; Marijuana Treatment Project Research Group, 2004; Miller and Wilbourne, 2002). This effectiveness of behavioral treatments across addictions is also consistent with many common features of addictive disorders, including continued substance use despite consequences, impaired control over behavior, repeated unsuccessful attempts to reduce use, narrowing of activities in favor of drug use, and diminished control over use (Edwards and Gross, 1976). Effect sizes remain modest for most behavioral therapies and outcomes vary widely across individuals (Dutra et al., 2008). Therefore, focusing on individual variables associated with poorer outcomes, including impaired cognition, may be an important strategy to enhance the effectiveness of behavioral treatments.
Disruptions to inhibitory or executive control have been identified as defining features of many theories of addictions, as they address the maintenance of drug use behavior and the difficulty many individuals have in resisting habitual drug use once established (Everitt et al., 2007; Goldstein and Volkow, 2011; Li and Sinha, 2008; Porrino et al., 2007). The inhibitory and executive control functions, concentrated primarily in the prefrontal and parietal cortices, are especially important when the individual needs to override a prepotent response, such as drug-taking behavior in response to drug cues (Sarter et al., 2006). Thus, addressing these critical aspects of cognitive function may be may be a successful strategy for increasing treatment efficacy across addictive disorders (Sofuoglu, 2010).
The goal of this review is to provide an overview of the rationale for targeting cognitive-enhancement strategies for the treatment of drug addiction and to outline some existing pharmacological and behavioral approaches which show promise in achieving cognitive enhancement in drug addicted populations. We first present a summary of studies documenting cognitive impairments associated with addictions and discuss the relevance of these cognitive deficits as predictors of treatment outcome in addiction. We then review potential mechanisms linking cognitive deficits to drug use and conclude with examples of candidate medications and behavioral interventions which show potential as cognitive-enhancing agents and may serve as stand-alone or adjunct treatments for drug dependence. While intended as a broad overview of cognitive enhancement as a treatment strategy across the addictions, it should be noted that most of the empirical work on this topic has focused on cocaine and methamphetamine addictions. This review complements the recent reviews on this topic that focused on individual drugs of abuse (Sofuoglu, 2010; Sofuoglu et al., 2010b) or covered pharmacological treatments (Brady et al., 2011). Cognitive consequences of chronic alcohol use have been reviewed recently and will not be included in this manuscript (Stavro et al., 2012).
2. Cognitive deficits in addicted individuals
Multiple studies have reported that chronic drug use, especially cocaine, methamphetamine, cannabis use, and cigarette smoking are associated with deficits in cognitive functioning, including in decision-making, response inhibition, planning, working memory, and attention (Durazzo et al., 2010; Fernandez-Serrano et al., 2012; Jovanovski et al., 2005; Nordahl et al., 2003; Price et al., 2011; Simon et al., 2002; Stavro et al., 2012). While many studies report the results of statistical significance testing, effect size analyses better describe the magnitude of differences between drug users and controls (Zakzanis, 2001). A meta-analysis by Jovanoski et al. (2005) comparing cocaine users (n=481) with healthy controls (n=586) reported large effect sizes for attentional function (Cohen’s d≥0.8), moderate effect sizes for visual and working memory (0.8>d≥0.5) and small effect sizes for language and sensory-perceptual functions (0.5>d≥0.2) (Cohen 1988). A separate meta-analysis comparing methamphetamine users (N=487) with healthy controls (N=464) observed moderate effect sizes for learning, executive function, memory, and speed of information processing domains and small effect sizes for motor skills, attention, working memory, visuo-construction, and language domains (Scott et al., 2007). In a recent study, cigarette smokers performed worse than non-smokers on several domains of cognitive function with large effect sizes for performance on auditory–verbal and visuospatial learning, visuospatial memory, cognitive efficiency, executive skills, general intelligence, and processing speed (Durazzo et al., 2012). These findings are consistent with several previous studies with cigarette smokers and matched controls (Nooyens et al., 2008; Paul et al., 2006; Sabia et al., 2008). However, comparing drug users and healthy controls on cognitive function requires careful consideration of many potential confounds. As discussed in a recent review by Hart et al. (2012), studies examining the neurocognitive effects of chronic methamphetamine use often do not control for differences between drug users and controls in education, IQ, and other psychiatric comorbidities or length of abstinence within substance users; may employ suboptimal cognitive assessment tools; and are often limited by small sample sizes. Thus, findings from these studies should be interpreted with such possible limitations in mind (Hart et al., 2012).
Studies on the influence of chronic cannabis use on cognitive function have found mixed results. Some studies reported that chronic heavy marijuana use is associated with impairments in verbal learning and memory, sustained attention, and executive functioning (Bolla et al., 2002; Pope et al., 1995; Pope and Yurgelun-Todd, 1996; Solowij, 1995; Solowij et al., 1995; Solowij et al., 2002). In contrast, other studies reported minimal (Grant et al., 2003) or no lasting effects of chronic cannabis use on overall IQ, attention, working memory, and abstract reasoning (Fried et al., 2005; Jager et al., 2006). Cannabis-induced cognitive impairments may depend on age of onset; with those beginning cannabis use before age 17 demonstrating greater impairment (Kempel et al., 2003; Pope et al., 2003). Thus, age of onset and other baseline variables, particularly IQ (Bolla et al., 2002), may explain the conflicting findings regarding long-term cannabis use on cognitive function.
Functional neuroimaging studies have examined the neural substrates of these deficits. A resting state positron emission tomography (PET) study demonstrated low glucose metabolism in the anterior cingulate cortex (ACC) and high glucose metabolism in the lateral orbitofrontal area, middle and posterior cingulate, amygdala, ventral striatum, and cerebellum of recently abstinent methamphetamine abusers (London et al., 2004). Methamphetamine (Nestor et al., 2011) and chronic cocaine (Bolla et al., 2004) users demonstrate prefrontal cortical (PFC) hypoactivation during Stroop task performance, a measure of cognitive control and response inhibition. Similarly, long-term cannabis users show hypoactivity in the ACC and the left lateral PFC during the Stroop task (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005). These and many other studies provide evidence for PFC dysfunction in chronic drug users.
Despite evidence of a strong association of cognitive deficits in substance dependent populations, particularly in their most severe form, the clinical implications of these findings has received limited attention, perhaps due to the subtle nature of many of these deficits, variability across individuals, and observations that that at least some of these deficits may be reversible following cessation of drug use. However, several studies suggest that these cognitive deficits are not reversible after short-term abstinence. For example, methamphetamine dependent individuals failed to demonstrate significant improvement in cognitive performance following one month of abstinence (Simon et al., 2010). Similarly, in a PET study, abstinent individuals who were previously methamphetamine-dependent, displayed persistent neurocognitive deficits despite nearly full recovery of dopamine transporter (DAT) deficiency (Volkow et al., 2001). Furthermore, some cognitive impairments associated with cannabis use do not appear reversible with short-term abstinence, further emphasizing that some impairments are not entirely accounted for by the acute effects of the drugs (Medina et al., 2007).
Another consideration is the extent to which cognitive impairments are caused by chronic drug use. While chronic drug use may cause cognitive impairment, individuals with cognitive deficits may also be more vulnerable to initiating drug use and/or becoming drug-dependent (Wagner et al., 2012). Furthermore, drug use is often associated with psychiatric co-morbidity (e.g., depression, attention deficit hyperactivity disorder) and cognitive impairments may be primarily accounted for by these co-morbid disorders. Some evidence suggests that especially smokers may have preexisting mild cognitive impairments (Wagner et al., 2012; Yakir et al., 2007). A recent study reported that smokers (n=1002) were more likely than non-smokers (n=1161) to have impairments in visual attention and impulsivity (Wagner et al., 2012). However these impairments were also present in smokers with low levels of cigarette consumption and lifetime cigarette consumption was not correlated with cognitive function. As these findings suggest, cognitive impairments may predate the initiation of drug use, rendering individuals more vulnerable for drug addiction (Wagner et al., 2012). Carefully controlled longitudinal studies are needed to disentangle the associations between cognitive impairments and drug use. If cognition influences substance use outcomes and general functioning, cognitive enhancement will serve as an important treatment target, regardless of whether cognitive impairments in addicted populations reflect persistent brain dysfunction arising secondary to chronic substance use; acute drug effects; short-term withdrawal effects; pre-existing vulnerability factors for addiction; or, perhaps most plausibly, a combination of several of such factors.
3. Cognitive Impairments as Predictors of Relapse and Treatment Outcomes
While the clinical literature linking cognitive functioning to treatment outcome has shown mixed results, cognitive impairments are generally associated with poorer treatment retention in most substance-using samples. For example, Aharonovich and colleagues reported that cognitive impairments in cocaine or cannabis users are associated with poorer response to behavioral treatment (Aharonovich et al., 2008; Aharonovich et al., 2006; Aharonovich et al., 2003). Among cocaine users offered CBT (n=56), treatment non-completers performed significantly worse on laboratory measures of attention, memory, spatial ability, speed, accuracy, global functioning, and cognitive proficiency compared with treatment completers (Aharonovich et al., 2006). Similarly, cannabis dependent treatment non-completers performed significantly worse than treatment completers on measures of abstract reasoning and processing accuracy (Aharonovich et al., 2008). However, these measures of cognitive function were not related to rates of abstinence during the treatment trial in these cocaine and cannabis using samples (Aharonovich et al., 2008; Aharonovich et al., 2006; Aharonovich et al., 2003). While self-reported baseline drug use did not differ between treatment completers and non-completers, these studies did not directly investigate whether cognitive function is a predictor of treatment outcomes independently of drug use severity. A more recent study by Carroll et al extended the Aharonovich study by including both treatment as usual (TAU) and computerized CBT treatment for cocaine addiction (n=77) (Carroll et al., 2011). Baseline performance on a subset of the cognitive measures assessed (i.e. BART, a task of risk-taking; CPT, a sustained attention task) differentially predicted retention (e.g. days in treatment), treatment engagement (e.g. CBT modules and homework completed) and treatment outcomes (e.g. drug-positive urines) in the CBT arm but were less predictive of outcome in the TAU arm. However, composite score of overall cognitive performance at baseline was not significantly predictive of treatment response in either treatment condition (Carroll et al., 2011). Furthermore, an association between IQ at pre-treatment and drug-positive urines during post-treatment follow-up was mediated by the quality of coping skills demonstrated at the end of treatment in the CBT arm (after controlling for baseline coping skills), but this relationship did not hold within the TAU arm (Kiluk et al., 2011) These findings highlight the importance of carefully assessing differential relationships between cognitive domains and aspects of the clinical course (e.g. vulnerability to initiate use or transition to dependence; ability to understand or engage in certain treatments; drug use outcomes). In methamphetamine dependent individuals (N=60), measures of cognitive function predicted treatment outcome and study retention less robustly than an indicator of baseline methamphetamine use (urine drug screening) (Dean et al., 2009). This study raised questions about the independent contribution of cognitive function as a treatment outcome predictor. More recently, in 131 cocaine-dependent individuals, baseline executive function predicted treatment retention (Verdejo-Garcia et al., 2012). Of note, baseline demographics and cognitive function explained 14 % of the variation in treatment outcome. These findings are consistent with other studies suggesting that impairments in cognitive functioning and inhibitory control tend to be associated with higher drop-out rates (Brewer et al., 2008; Streeter et al., 2008; Turner et al., 2009). However, the effect sizes in these studies seem to be modest and questions remain for the independent contribution of cognitive function as a predictor of treatment outcome.
4. Mechanisms Linking Cognitive Impairments to Drug Use
Both executive and implicit/automatic cognitive processes play a role in controlling drug use. We review these processes as they relate to addiction (for a broader review, see (Field and Cox, 2008; Miller and Cohen, 2001; Wiers and Stacy, 2006).
4.1 Executive Control
Many contemporary theories of addiction emphasize a disrupted inhibitory or executive control in compulsive drug use (Everitt et al., 2007; Goldstein and Volkow, 2011; Li and Sinha, 2008; Porrino et al., 2007). The executive control is coordinated by two parallel networks of the PFC: the dorsolateral “executive” and the orbitofrontal “limbic” network primarily contained in the orbito frontal cortex (OFC) (Abernathy et al., 2010). While the dorsolateral PFC is the primary regulator of goal-directed behavior, the ACC is critical in conflict resolution. The “limbic” OFC is connected with many sensory cortical areas and limbic regions including hippocampus and determines the salience of information about environmental contingencies. PFC control over executive function is a result of the coordination between the “executive” and “limbic” networks as well as the top-down control over the ascending neuromodulators which include monoamines (dopamine, serotonin, norepinephrine), orexin, and acetylcholine (Robbins and Arnsten, 2009). These neuromodulators have powerful influences over PFC functions (Robbins and Arnsten, 2009).
Executive control, rather than being a unitary function, can best be characterized as a collection of related but separable functions (Friedman et al., 2008) including response inhibition, working memory, attention, problem solving, decision making, set shifting, and other functions. Among executive functions, response inhibition, working memory, and sustained attention are particularly relevant for addictive disorders (de Wit, 2009; Eagle et al., 2008; Gregoire et al., 2012). These functions have been operationalized by separate tasks and may serve as potential treatment targets for cognitive-enhancement approaches.
4.1.1 Response Inhibition
Response inhibition refers to the ability to voluntarily inhibit a dominant, automatic, or pre-potent response (Friedman et al., 2008) and is often assessed via tasks such as the Stop-Signal Task (SST) or Go/No-Go (Eagle et al., 2008). The SST is a speeded choice response task (e.g. press right button as quickly as possible for a right-pointing arrow and left button for a left-pointing arrow). Similarly, the Go/No-Go task presents a majority of ‘go’ trials, but intermixes a small proportion of ‘stop’ trials. In both tasks the motor response is correct in the majority of trials, therefore it becomes pre-potent as the task is learned and this response must be actively inhibited in the minority of ‘stop’ or ‘no-go’ trials.
Reduced response inhibition function, as determined by the SST or Go-No/Go task, have been repeatedly demonstrated in cocaine (e.g. Fernandez-Serrano et al., 2012; Li et al., 2006; Fillmore et al 2007) and methamphetamine dependent individuals (Monterosso et al., 2005), compared to non-addicted controls.
The norepinephrine (NE) system is thought to play a key role for response inhibition function (Aston-Jones et al., 2009). For example, norepinephrine transporter (NET) inhibitor atomoxetine improves response inhibition function in healthy controls as well as in patients with ADHD (Chamberlain et al., 2006). Atomoxetine increases dopamine (DA) and NE levels in the PFC; however, increases in NE likely mediate the improvement in response inhibition function (Bari et al., 2011).
4.1.2 Working Memory
Working memory refers to the ability to keep in mind an event that had just been experienced or retrieve information from long-term memory storage and use this information to regulate behavior (Arnsten, 2011). Classic measures of working memory include auditory or visuo-spatial span tasks, which require information to be held ‘on line’ while it is actively updated or manipulated.
As mentioned above, meta-analyses found impaired working memory function in cocaine (Jovanovski et al., 2005) and methamphetamine users, with medium to small effect sizes (Scott et al., 2007). Many studies have suggested that working memory function is linked to inhibitory control in that high working memory demand or reduced working memory function may facilitate drug craving or relapse (Chambers et al., 2009). For example, under high working demand, cocaine users have reduced response inhibition, measured by a Go-No/Go task, compared to healthy controls (Hester and Garavan, 2004). In abstinent smokers, poorer working memory function is predictive of relapse (Patterson et al., 2010).
The ascending DA and NE pathways are the main neuromodulators for working memory function. Monoamine transporter inhibitors atomoxetine, modafinil, and methyphenidate, as well as the alpha2-adrenergic agonist, guanfacine, may improve working memory function (Marquand et al., 2011; Minzenberg and Carter, 2008; Swartz et al., 2008b).
4.1.3 Sustained Attention
Sustained attention is controlled by both bottom-up and top-down processes (Posner and Rothbart, 1998). Bottom-up processing, also known as exogenous or stimulus-driven attention, refers to an automatic process driven by external stimuli (e.g. visual drug cues). Top-down processing, also known as the endogenous or executive attention, is controlled via engagement of PFC and basal ganglia neural circuitry and is closely linked to working memory and response inhibition functions (Rueda et al., 2005). Sustained attention is often measured by continuous performance tasks, such as Rapid Visual Information Processing task (RVIP;(Turner et al., 2005)) where subjects are asked to attend to rapidly presented visual stimuli and respond to specific stimuli which are presented infrequently.
Individuals addicted to methamphetamine, cocaine, cannabis, or nicotine have shown impairments in sustained attention function (Bolla et al., 2002; Durazzo et al., 2012; Jovanovski et al., 2005; Simon et al., 2010; Scott et al., 2007), with effect sizes ranging from large in cocaine users (Jovanovski et al., 2005) to small in methamphetamine users (Scott et al., 2007). Sustained attention has a bidirectional interaction with drug craving. For example, craving for drugs demands attentional resources and takes attention away from non-drug stimuli resulting in impaired performance in sustained attention tasks (Sayette et al., 2010). Lapses in attention during early abstinence have been linked to relapse, possibly by reducing behavioral inhibition (de Wit, 2009), leading to apparent ‘hijacking’ of the brain by drug cues.
Sustained attention function is modulated by acetylcholine (ACh), NE, DA, glutamate, and gamma-aminobutyric acid (GABA) (Levin et al., 2011). Mounting evidence supports the role of Ach release in the PFC is an essential step in mediating attentional processes (Kozak et al. 2006; Sarter et al. 2006; Sarter et al. 2009). Medications enhancing DA, NE and ACh transmission have been shown to improve sustained attention (Levin et al., 2011).
4.2 Automatic/Implicit Cognitive Processes
Recently, addiction researchers have highlighted the role of automatic/implicit cognitive processes in drug addiction (Wiers and Stacy, 2006). Automatic/implicit cognitive processes are fast, parallel, effortless, and may not engage conscious awareness. These processes are often measured with modified Stroop tasks, where subjects are shown words written in colored ink and are asked to identify the ink color (Wiers and Stacy, 2006). Attentional bias is indicated by the degree to which performance slows or diminishes in accuracy for drug-related words relative to neutral words. Other common tasks of attentional bias (e.g. dot probe task) measure the relative speed at which subjects visually attend to a neutral stimulus (e.g. dot) when it is presented in the same location as a drug-related visual cue as compared to when it is presented in the neutral-cue location.
Meta-analyses have confirmed that measures of automatic/implicit cognition are associated with craving (small-to-medium effect size of r=.19 from 68 datasets; (Field et al., 2009)) and substance use (medium effect size of r=.31 from 89 datasets; (Rooke et al., 2008)). Measures of automatic/implicit cognition are also associated with relapse (Carpenter et al., 2006; Cox et al., 2002; Cox et al., 2007; Janes et al., 2010; Powell et al., 2010; Waters et al., 2003). One widely studied automatic process is “attentional bias”. In addiction, attentional bias refers to exaggerated attention to drug cues, an important cognitive mechanism in drug addiction (Field and Cox, 2008; Franken, 2003; Ryan, 2002). An individual with high attentional bias for a specific drug will be more likely than an individual with low attentional bias to attend to drug cues, which in turn may provoke drug craving. Another important automatic/implicit process is approach bias (Wiers et al., 2010). An individual with a large approach bias to drug cues will be more likely than an individual with a small approach bias to automatically approach drug cues when exposed to those cues. Therefore, attentional bias increases exposure to drug cues, and approach bias increases approach behavior to those cues. The neuropharmacology of automatic/implicit processes has not been well characterized. However, one study reported that attentional bias was attenuated by a DA antagonist (Franken et al., 2004)
5. Cognitive-Enhancement Treatments
5.1 Pharmacological Treatments
Table 1 summaries some of the promising cognitive enhancing pharmacotherapies for addictions.
Table 1.
Medications that may be used as cognitive enhancers in addicted individuals
| Target | Medication | Dose/Design | Improved Cognitive Domain |
Cognitive Measure (E.S.) |
Participants in relevant analyses |
Caveats/Limitations |
|---|---|---|---|---|---|---|
| Acetylcholine | Galantamine (Sofuoglu et al., 2011) |
8 mg/day for 10 days. Between- subject. |
Sustained attention |
RVIP A’ (d=0.91) | 26 abstinent CD (13 placebo, 13 galantamine) |
Small sample, no substance use outcomes were included. |
| Varenicline (Patterson et al., 2009) |
1 mg/twice daily for 21 days. Between- subject |
Sustained attention Working memory |
CPT # True Positives (d=0.5) N-Back Correct RT (d=0.41) |
67 treatment-seeking abstinent cigarette smokers |
Seven days of smoking abstinence as the main outcome. No long-term smoking cessation outcomes. |
|
| Monoamine transporters |
Modafinil (Dean et al., 2011) | 200 mg, single dose. Within-subject |
Sustained attention |
CPT reaction time variability (MA d= 0.59; HC d= 0.82) |
24 short-term abstinent MA; 17 HC |
Small sample. MA and HC groups not matched for cigarette smoking or IQ. Heavier MA use was associated with greater cognitive improvement on modafinil. |
| Modafinil (Hester et al., 2010) |
200 mg/day for 6 days. Between- subject. |
Immediate verbal memory |
RAVLT Recall B (MA d=1.2) |
14 one week abstinent MA (residential withdrawal unit) |
Small sample | |
| Methylphenidate (Li et al., 2010) |
0.5 mg/kg intravenous. Within-subject. |
Response inhibition |
Stop Signal Reaction Time (d=1.49) |
10 non-treatment seeking, ≥5 days abstinent CD |
Small sample. IV administration may limit generalizibility. |
|
| Atomoxetine Chamberlain et al., 2007) |
60 mg, single dose. Within- subject |
Response inhibition |
Stop Signal Reaction Time (d=0.8) |
20 adult ADHD | Non- addicted sample. Mixed medication status at study entry. |
|
| Adrenergic receptors |
Guanfacine Swartz et al., 2008b |
2–3 mg, single dose. Within- subject |
Visual memory | Delayed match to sample accuracy (HC d= 0.74) |
10 HC, 14 frontal lobe epilepsy (FLE), 13 temporal lobe epilepsy (TLE) |
Non-addicted sample. Tested before and after guanfacine without placebo-control |
| Guanfacine (Scahill et al., 2001) |
1 mg 3 times/day for 8 weeks. Between subject. |
Motor impulsivity Sustained attention |
CPT commission errors (d=0.9) CPT omission errors (d=0.8) |
34 children with ADHD combined type and tic disorder (17 guanfacine; 17 placebo) |
Non-addicted sample of children (mean age 10.4). |
|
| Glutamate and others |
D-cycloserine (Onur et al., 2010) |
250 mg single dose. Between- subject. |
Declarative memory |
Item category association task response accuracy (d=0.89) |
40 HC | Non-addicted sample. |
| Minocycline (Sofuoglu et al., 2010a) |
200 mg/day for 5 days. Within- subject |
Attention/ psychomotor speed |
“Go” reaction time on Go/No-Go task (d=1.0) |
10 HC | Small sample size. Non-addicted sample. |
Participant Groups (MA: methamphetamine dependent; CD: cocaine dependent; HC: healthy control; ADHD: attention-deficit hyperactivity)
Cognitive Measures (RAVLT: Rey Auditory Verbal Learning test; RVIP A': Rapid Visual Information Processing, signal detection measure; CPT: continuous performance task)
E.S.=Effect size.
5.1.1 Cholinergic Medications
5.1.1.1 Galantamine
Galantamine, in addition to its acetylcholinesterase inhibitor effects, is also an allosteric potentiator of the nicotinic acetylcholine receptor (nAChR), especially α7 and α4β2 subtypes (Schilstrom et al., 2007). In a series of studies, we examined the potential use of galantamine as a cognitive-enhancing treatment for drug addiction. In a recent double-blind, placebo-controlled study, ten days of galantamine treatment (8 mg/day) improved sustained attention and working memory functions in abstinent cocaine users (N=28), as assessed by the Rapid Visual Information Processing (RVIP) test (Sofuoglu et al., 2011). In a separate 8-week, double-blind study in opioid and cocaine dependent individuals (N=14), those receiving galantamine (16 mg/day) submitted fewer cocaine-positive urine specimens and reported less cocaine use than those assigned to placebo, and the medication was well-tolerated (Sofuoglu and Carroll, 2011). Together, these results indicate the feasibility, safety, and promise of galantamine as a potential cognitive enhancer for the treatment of cocaine addiction. Randomized clinical trials are underway to test the efficacy of galantamine for the treatment of cocaine addiction.
Furthermore, in a placebo-controlled study in abstinent cigarette smokers, galantamine (8 mg/day) improved sustained attention and response inhibition as assessed by Go/No-Go (Sofuoglu et al., 2012). Galantamine also attenuated the subjective effects of nicotine administered intravenously, consistent with galantamine’s enhancement of cholinergic transmission. These findings support the potential utility of galantamine for the treatment of nicotine addiction.
5.1.1.2 Varenicline
Varenicline, a partial agonist of α4β2 and full agonist for the α7 nAChR subtypes, is used for smoking cessation. In a recent study of cigarette smokers, 10 days of varenicline treatment improved working memory and attention impairments induced by nicotine withdrawal (Patterson et al., 2009). In a functional MRI study using a visual working memory task, abstinent smokers receiving varenicline treatment, compared to placebo, showed greater activation of many PFC regions which was associated with improved task performance in highly dependent smokers (Loughead et al., 2010). Medications targeting α4β2 and α7 nAChR are under investigation as cognitive-enhancing treatments (Dunbar et al., 2007; Wallace and Porter, 2011).
5.1.2 Monoamine Transporter Inhibitors
5.1.2.1 Modafinil
Modafinil is a wakefulness-promoting agent, approved for the treatment of narcolepsy, sleep apnea, and shift work-induced sleep disorder. Modafinil, is a weak inhibitor of DAT and NET and has additional effects on the brain GABA, glutamate, and orexin (Minzenberg and Carter, 2008). Modafinil’s cognitive enhancing effects have been well-recognized in individuals with neuropsychiatric disorders, including those with addictions (Minzenberg and Carter, 2008). In a series of studies, the cognitive-enhancing effects of modafinil were examined in methamphetamine dependent individuals. In a small inpatient study, 7 days of modafinil treatment improved immediate verbal memory function (Hester et al., 2010). In another study a single 200 mg oral dose of modafinil improved sustained attention (Dean et al., 2011). Furthermore, treatment with 400 mg/day of modafinil for 3 days improved working memory function in methamphetamine dependent individuals who had impaired working memory function at baseline (Kalechstein et al., 2010). Consistent with these findings, a single 200 mg dose of modafinil enhanced activation in the ventrolateral PFC and ACC (Ghahremani et al., 2011), brain regions associated with executive functions. Modafinil has also shown promise in randomized clinical trials for the treatment of methamphetamine addiction (Heinzerling et al., 2010; Shearer et al., 2009).
5.1.2.2 Methylphenidate
Methylphenidate is a stimulant drug similar to amphetamines. It is marketed for the treatment of attention-deficit hyperactivity disorder (ADHD) and has been repeatedly shown to improve response inhibition in individuals with ADHD (e.g. (DeVito et al., 2009)). Methylphenidate, similar to cocaine, inhibits DAT and NET (Challman and Lipsky, 2000). In a functional MRI study with cocaine users, a single 20 mg oral methylphenidate treatment ameliorated ACC hypoactivation and improved behavioral measures of response inhibition (Goldstein et al., 2010). In another study with cocaine dependent individuals, intravenous methylphenidate, compared to placebo, improved response inhibition, as assessed with the SST (Li et al., 2010). Methylphenidate also showed promise in reducing cocaine use in individuals with co-morbid ADHD and cocaine addiction (Levin et al., 2007).
5.1.2.3 Atomoxetine
Atomoxetine is used for the treatment of ADHD. It is a selective NET inhibitor that increases synaptic NE levels in the PFC (Bymaster et al., 2002; Swanson et al., 2006). Atomoxetine also increases dopamine levels in the PFC, but not in the nucleus accumbens or other striatal regions (Bymaster et al., 2002; Swanson et al., 2006). This discrepancy has been attributed to sparse distribution of dopamine transporters in the PFC, indicating that NET significantly contributes to clearance of extracellular dopamine in the PFC (Carboni et al., 1990). In contrast, amphetamines increase both DA and NE levels in the nucleus accumbens and in the PFC (Kuczenski and Segal, 1997), which may contribute to the differences between the pharmacological effects of atomoxetine and amphetamines, including the lower abuse liability of atomoxetine (Heil et al., 2002; Wee and Woolverton, 2004).
In healthy controls and patients with ADHD, atomoxetine improved response inhibition, measured with the SST (Chamberlain et al., 2007) or Stroop (Faraone et al., 2005), (see also (Nandam et al., 2011). As both methamphetamine and cocaine users have been reported to have poorer response inhibition than healthy controls, as indicated by slower SST reaction times (Li et al., 2006; Monterosso et al., 2005), it would be of interest to examine atomoxetine’s ability to improve performance on this task in stimulant users. Atomoxetine remains to be evaluated in clinical trials for stimulant addiction.
5.1.3 Alpha2-adrenergic Agonist
5.1.3.1 Guanfacine
Guanfacine, an alpha2-adrenergic agonist, is used for the treatment of hypertension and ADHD. Guanfacine reduces NE activity by stimulating presynaptic alpha2-adrenergic receptors. Stimulation of post-synaptic alpha2 adrenergic receptors may mediate the beneficial effects of guanfacine on working memory and attention (Ramos and Arnsten, 2007). Guanfacine has been demonstrated to improve working memory performance in healthy volunteers (Jakala et al., 1999; Swartz et al., 2008a) and sustained attention in individuals with schizophrenia (Friedman et al., 2001) or ADHD (Scahill et al., 2001). Guanfacine’s effects on cognitive function in addictive individuals remain to be determined.
5.1.4 Glutamatergic Medications
Several medications targeting the glutamate system are also under investigation as cognitive-enhancing treatments.
5.1.4.1 Memantine
Memantine, a non-competitive NMDA antagonist is marketed as a cognitive enhancer for Alzheimer’s disease. Memantine may also have antidepressant properties (Hashimoto, 2009). Memantine’s efficacy as a cognitive enhancer has not been examined in addicted individuals. However, clinical trials with memantine have been negative for alcohol (Evans et al., 2007) or cocaine dependence (Bisaga et al., 2010).
5.1.4.2 D-cycloserine
D-cycloserine (DCS) is a partial agonist at the glycine site of the NMDA-type glutamate receptors. DCS enhances the effectiveness of behavioral treatments for phobias and other anxiety disorders (McNally, 2007; Ressler et al., 2004; Wilhelm et al., 2008), without demonstrated therapeutic effects as a monotherapy for these disorders. In a pilot study, DCS attenuated smoking urges and physiological reactivity to smoking cues in cigarette smokers (Santa Ana et al., 2009). In another study, DSC improved declarative memory function in healthy controls (Onur et al., 2010), although DSC's cognitive-enhancing effects in addicted individuals remain to be examined.
5.1.4.3 Minocycline
Minocycline, an antibiotic which is used to treat acne, is also under investigation for the treatment of neurodegenerative and neuropsychiatric disorders. Minocycline has significant inhibitory effects on dopamine and glutamate transmission, although the exact mechanism of action is unknown. Minocycline improved methamphetamine-induced recognition memory impairments (Mizoguchi et al., 2008) and neurotoxicity in mice (Zhang et al., 2006). In healthy controls, 4 days of minocycline (200mg/day) improved response inhibition function as measured by Go/No-Go (Sofuoglu et al., 2010a). The effects of minocycline in addicted individuals remain to be determined.
To summarize, although several cognitive-enhancing medications are available for clinical use, it remains to be determined whether these medications can reduce drug use through improvement of selective cognitive functions. Many of these cognitive-enhancing medications may have mood-elevating and antidepressant effects as well (e.g., atomoxetine, memantine, or modafinil) (Dell'Osso et al., 2011; Hashimoto, 2009). Given the close association between depression and drug use (Davis et al., 2008), mood elevation may potentially contribute to the proposed efficacy of cognitive-enhancers for drug addiction (Mitchell and Phillips, 2007). To probe these possibilities, future research should address both whether cognitive-enhancing medications improve drug use outcomes and whether these effects are mediated by improvement in selective cognitive functions or mood states.
5.2 Behavioral Treatments
The accumulating data on cognitive deficits among substance users has also led to articulation of how behavioral approaches might be developed or modified to address these issues in clinical samples.
5.2.1 Cognitive Behavioral Therapy
While Cognitive Behavioral Therapy (CBT) for drug abuse is often thought of as teaching specific coping strategies, many of the putative ‘active ingredients’ of CBT may exert their effects via strengthening aspects of executive control over behavior, as there is some evidence that acquisition of these types of skills in CBT mediates long term-outcomes (Kiluk et al., 2010).
For example, a promising explanation for CBT’s durability is its focus on conveying generalizable strategies to (1) exert cognitive control over over-learned patterns of substance use via functional analysis of behavior (e.g., understanding episodes of substance use in terms of antecedents and consequences), (2) reduce impulsive responding in response to drug cues via implementing strategies to control craving (regulation of craving and negative affect), (3) improve general decision-making and problem-solving skills, (4) and recognize, challenge, and exert control over cognitions associated with drug use.
CBT requires a comparatively high cognitive workload. The learning, practicing, and implementation of new cognitive skills is complex and requires that patients be able to attend to and understand the therapist’s instructions, then remember and execute these new skills in difficult situations. As such, several investigators have hypothesized that CBT would be a sub-optimal treatment for patients with greater cognitive impairment (Kadden et al., 2001). The Aharonovich studies which linking poorer CBT retention to higher levels of cognitive impairment may be consistent with these expectations (Aharonovich et al., 2008; Aharonovich et al., 2006; Aharonovich et al., 2003). Rather than avoid using CBT with cognitively impaired drug users who might most benefit from strengthening of executive control, our group has sought to develop modified versions of CBT that are suitable for use by individuals with a broad range of cognitive abilities. For example, we have delivered a computerized version of CBT that can be tailored for individuals with mild cognitive impairments. This modification of CBT capitalizes on the multimedia capabilities of computer-based learning to present information in formats compatible with a range of learning styles. Computer-based CBT also allows users to pace the presentation of information, repeat material as often as required, be presented with multiple examples of effective skill implementation through videotaped demonstrations, and thus have multiple opportunities for clarification, evaluation and consolidation of learning (Carroll et al., 2008). A recent study showing little evidence of relationships between level of cognitive function and treatment retention or outcome suggests that this computer-based CBT may ‘level the playing field’ amongst cognitively impaired patients (Carroll et al., 2010). Nevertheless, there was some evidence that cognitive capacity was negatively associated with acquisition of CBT skills, highlighting the challenges of learning cognitively complex skills amongst many drug using individuals (Kiluk et al., 2011).
5.2.2. General Cognitive Training
Another behavioral strategy that may prove useful as an adjunct to, or preparation for, addiction treatment is cognitive rehabilitation training. Cognitive rehabilitation typically involves repeated practice of cognitive tasks involving memory, problem-solving, response inhibition, visuo-construction and perception, visual tracking, and discrimination skills for several hours per week over the course of several months. In general, these approaches have been associated with improvements among individuals with traumatic brain injury, and more recently, schizophrenia (Bell et al., 2005; Lynch, 2002). Neuroimaging data from these studies suggests that cognitive rehabilitation may normalize regional brain activation in the PFC (Wexler et al., 2000). Recent technological advances in computer-delivered cognitive rehabilitation allow for a high level of individualization of the program to account for patients patterns of cognitive strengths and deficits. In a recent trial, Bickel demonstrated that focused training on computerized memory tasks resulted in significant reductions in an aspect of impulsivity, delay discounting (i.e. preference for immediate versus delayed rewards), among stimulant users (Bickel et al., 2011). Adherence to the computerized memory training was facilitated by integrating the computerized cognitive rehabilitation with contingency management.
5.2.3 Attentional Retraining and Cognitive Bias Modification
Recently, novel behavioral Interventions have been developed to specifically target automatic/implicit processes as described earlier, such as attentional bias (Schoenmakers et al., 2010) and approach bias (Wiers et al., 2010). This Cognitive Bias Modification (CBM) strategy has shown promise in the treatment of addiction (Wiers et al., 2011) and other psychopathologies (Hakamata et al., 2010). For example, in a meta-analysis of 12 attentional retraining studies in individuals with anxiety disorders, attentional retraining yielded significantly greater reductions in anxiety than control training, with a medium-to-large effect size (d =.61). In a randomized trial involving 43 alcoholic patients, 5 sessions of attentional retraining (delivered over 21 days) reduced attentional bias and reduced the time to discharge from treatment (Schoenmakers et al., 2010). In a randomized trial with alcohol-dependent individuals (N=214) (Wiers et al., 2011), 4 brief sessions of training to automatically avoid alcohol cues, improved treatment outcomes at a 1-year follow-up (OR = 2.14) (Wiers et al., 2011). Thus, CBM may useful as an adjunctive treatment for addiction.
5.2.4 Combined Approaches
The bulk of the literature on treatments for addictive disorders suggests that combinations of behavioral therapies and pharmacotherapies may be more effective than single approaches (Carroll, 2001). For example, combining ‘bottom up’ anti-craving medications with ‘top down’ approaches like contingency management or CBT may improve outcome. For instance, reduction of the drive to use drugs via medication may make it easier for patients to deploy cognitive control in the early phases of treatment while these skills are still developing. Combining existing pharmacologic and behavioral addiction treatments with cognitive enhancement treatments could similarly improve treatment outcomes. For example, we are currently conducting a randomized placebo-controlled trial of galantamine with and without computerized CBT, hypothesizing that improved capacity for memory and attention via galantamine may facilitate learning of cognitive skills and strategies via the computerized CBT program. Similarly, we would also predict that combining CBM with cognitive enhancers would be beneficial, because CBM targets automatic/implicit processes and cognitive enhancers target executive control.
6. Conclusions
Long-term drug use is associated with a wide-range of cognitive impairments. These cognitive impairments seem to be predictive of poorer treatment retention and outcome. Among the many executive-control functions, response inhibition, working memory, and sustained attention are potential targets for the treatment of addictive disorder. Medications, (e.g., cholinesterase inhibitors, nicotinic agonists and monoamine transporter inhibitors (modafinil, atomoxetine, methylphenidate)) and behavioral approaches (e.g., CBT and CBM) which target these cognitive functions may have utility for the treatment of addictions. However, there is a dearth of research directly assessing the capacity for cognitive enhancing treatments to improve substance use outcomes via their modulation of cognition and this this gap in our knowledge presents a clinically important topic for future research. If the efficacy of these approaches bears out in clinical trials, future research could further refine this area by addressing issues of specific subgroups of substance users who might show particular benefit from these approaches (e.g., mild versus moderate cognitive impairment) or when in the course of treatment cognitive enhancers should be implemented (e.g., as preparation for treatment or after an initial period of abstinence has been established).
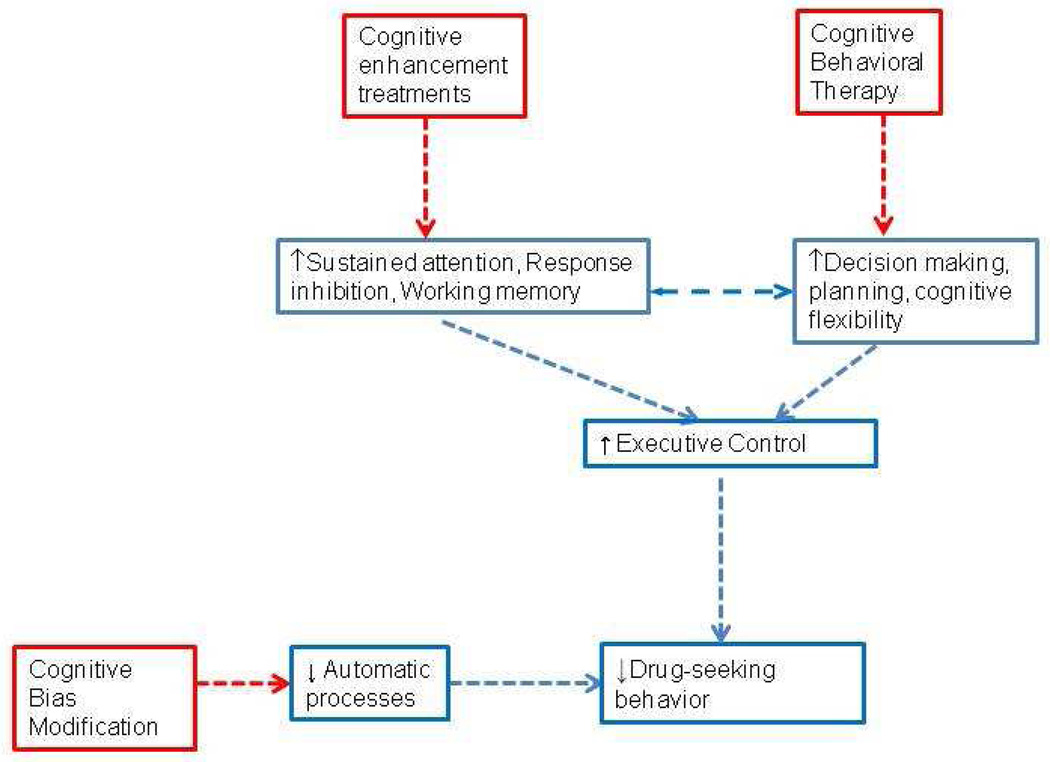
Figure 1.
This figure illustrates the potential therapeutic mechanisms for behavioral and pharmacological cognitive-enhancing treatments for addictions. See text for details.
Long-term drug use is associated with a wide-range of cognitive impairments.
Cognitive impairments are potential targets for the treatment of addictive disorders.
These impairments can be targeted by both medications and behavioral approaches.
Cognitive enhancement to improve treatment outcomes is a novel strategy.
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC), K02-DA-021304, P50 DA09241, and K12-DA-031050 grants from the National Institutes of Health (NIH). MS serves as an expert witness on behalf of Pfizer in lawsuits related to varenicline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. International review of neurobiology. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Brooks AC, Nunes EV, Hasin DS. Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend. 2008;95:279–283. doi: 10.1016/j.drugalcdep.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biological psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Gold JICONBPA. How we say no: norepinephrine, inferior frontal gyrus, and response inhibition. Biological psychiatry. 2009;65:548–549. doi: 10.1016/j.biopsych.2009.01.022. Pmid, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MD, Bryson GJ, Greig TC, Fiszdon JM, Wexler BE. Neurocognitive enhancement therapy with work therapy: Productivity outcomes at 6- and 12-month follow-ups. J Rehabil Res Dev. 2005;42:829–838. doi: 10.1682/jrrd.2005.03.0061. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug and alcohol dependence. 2010;111:97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL, Pope HG, Jr, Yurgelun-Todd D, Fried PA, Watkinson B, Gray R, Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacology, biochemistry, and behavior. 2011;99:285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Combined treatments for substance dependence. In: Sammons MT, Schmidt NB, editors. Combined treatments for mental disorders: Pharmacological and psychotherapeutic strategies for intervention. Washington, DC: APA Press; 2001. pp. 215–238. [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, Ball SA, Martino S, Rounsaville BJ, Lejuez CW. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Substance use & misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clinic proceedings. Mayo Clinic. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science (New York, N.Y.) 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience and biobehavioral reviews. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers' success in changing. Psychopharmacology (Berl) 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Current opinion in psychiatry. 2008;21:14–18. doi: 10.1097/YCO.0b013e3282f32408. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, London ED, Sugar CA, Kitchen CM, Swanson AN, Heinzerling KG, Kalechstein AD, Shoptaw S. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug and alcohol dependence. 2009;105:48–55. doi: 10.1016/j.drugalcdep.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Sevak RJ, Monterosso JR, Hellemann G, Sugar CA, London ED. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72:943–953. doi: 10.15288/jsad.2011.72.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Osso B, Palazzo MC, Oldani L, Altamura AC. The noradrenergic action in antidepressant treatments: pharmacological and clinical aspects. CNS neuroscience & therapeutics. 2011;17:723–732. doi: 10.1111/j.1755-5949.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, Aitken MR, Sahakian BJ. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;202:531–539. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI) Journal of psychopharmacology (Oxford, England) 2007;21:171–178. doi: 10.1177/0269881107066855. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–111. doi: 10.1016/j.drugalcdep.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcoholism, clinical and experimental research. 2007;31:775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Michelson D, Adler L, Reimherr F, Seidman L. Atomoxetine and stroop task performance in adult attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15:664–670. doi: 10.1089/cap.2005.15.664. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perales JC, Moreno-Lopez L, Perez-Garcia M, Verdejo-Garcia A. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology (Berl) 2012;219:673–683. doi: 10.1007/s00213-011-2485-z. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress In Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriks VM, Stam CJ, Van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol. 2004;14:503–508. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana-- a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Adler DN, Temporini HD, Kemether E, Harvey PD, White L, Parrella M, Davis KL. Guanfacine treatment of cognitive impairment in schizophrenia. Neuropsychopharmacology. 2001;25:402–409. doi: 10.1016/S0893-133X(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of experimental psychology. General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Rivalan M, Le Moine C, Dellu-Hagedorn F. The synergy of working memory and inhibitory control: behavioral, pharmacological and neural functional evidences. Neurobiology of learning and memory. 2012;97:202–212. doi: 10.1016/j.nlm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Ernst M, Pine DS. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EECINNA. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. Pmid, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain research reviews. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and alcohol dependence. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18:489–497. doi: 10.1037/a0021791. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Hill KP, Sofuoglu M. Biological treatments for amfetamine dependence : recent progress. CNS Drugs. 2007;21:851–869. doi: 10.2165/00023210-200721100-00005. [DOI] [PubMed] [Google Scholar]
- Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 2006;185:358–368. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Cooney NL, Kabela E, Getter H. Prospective matching of alcoholic clients to cognitive-behavioral or interactional group therapy. J Stud Alcohol. 2001;62:359–369. doi: 10.15288/jsa.2001.62.359. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Newton TF. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am J Addict. 2010;19:340–344. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempel P, Lampe K, Parnefjord R, Hennig J, Kunert HJ. Auditory-evoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Babuscio T, Carroll KM. Quality versus quantity: acquisition of coping skills following computerized cognitive-behavioral therapy for substance use disorders. Addiction. 2010;105:2120–2127. doi: 10.1111/j.1360-0443.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Carroll KM. Relationship of cognitive function and the acquisition of coping skills in computer assisted treatment for substance use disorders. Drug Alcohol Depend. 2011;114:169–176. doi: 10.1016/j.drugalcdep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bushnell PJ, Rezvani AH. Attention-modulating effects of cognitive enhancers. Pharmacology, biochemistry, and behavior. 2011;99:146–154. doi: 10.1016/j.pbb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug and alcohol dependence. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL, Rounsaville BJ, Ding YS, Malison RT. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biological psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Lynch B. Historical review of computer-assisted cognitive retraining. J Head Trauma Rehabil. 2002;17:446–457. doi: 10.1097/00001199-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, George WH. Relapse prevention: introduction and overview of the model. Br J Addict. 1984;79:261–273. doi: 10.1111/j.1360-0443.1984.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Marquand AF, De Simoni S, O'Daly OG, Williams SC, Mourao-Miranda J, Mehta MA. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1237–1247. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller WR. Motivation for treatment: a review with special emphasis on alcoholism. Psychol Bull. 1985;98:84–107. doi: 10.1037/0033-2909.98.1.84. [DOI] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97:265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Phillips LH. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia. 2007;45:617–629. doi: 10.1016/j.neuropsychologia.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology. 2008;196:233–241. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Cummins TD, Garner K, Dean AJ, Kim BN, Nathan PJ, Mattingley JB, Bellgrove MA. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2011;69:902–904. doi: 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooyens AC, van Gelder BM, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health. 2008;98:2244–2250. doi: 10.2105/AJPH.2007.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A, Otte DM, Shah NJ, Maier W, Kendrick KM, Fink GR, Hurlemann R. The N-methyl-D-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biological psychiatry. 2010;67:1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Cohen RA, Williams LM, Niaura R, Pogun S, Clark CR, Gunstad J, Gordon E. Cognitive status of young and older cigarette smokers: data from the international brain database. J Clin Neurosci. 2006;13:457–465. doi: 10.1016/j.jocn.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency management treatments. Br J Psychiatry. 2006;189:97–98. doi: 10.1192/bjp.bp.106.022293. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: the current status of research. Drug & Alcohol Dependence. 1995;38:25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Jama. 1996;275:521–527. [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl) 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Price KL, DeSantis SM, Simpson AN, Tolliver BK, McRae-Clark AL, Saladin ME, Baker NL, Wagner MT, Brady KT. The impact of clinical and demographic variables on cognitive performance in methamphetamine-dependent individuals in rural South Carolina. Am J Addict. 2011;20:447–455. doi: 10.1111/j.1521-0391.2011.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, Thorsteinsson EB. Implicit cognition and substance use: a meta-analysis. Addict Behav. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MICINPNASUSAO. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. Pmid, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan F. Detected, selected, and sometimes neglected: cognitive processing of cues in addiction. Exp Clin Psychopharmacol. 2002;10:67–76. doi: 10.1037//1064-1297.10.2.67. [DOI] [PubMed] [Google Scholar]
- Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med. 2008;168:1165–1173. doi: 10.1001/archinte.168.11.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: national findings; 2011 [Google Scholar]
- Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Parikh V, Martinez V, Kozak R, Richards JB. Forebrain dopaminergic-cholinergic interactions, attentional effort, psychostimulant addiction and schizophrenia. EXS. 2006;98:65–86. doi: 10.1007/978-3-7643-7772-4_4. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Schooler JW, Reichle ED. Out for a smoke: the impact of cigarette craving on zoning out during reading. Psychological science. 2010;21:26–30. doi: 10.1177/0956797609354059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AF, Cohen DJ, Leckman JF. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32:43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend. 2010;109:30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak ACINAF. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. Pmid, [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Carroll KM. Effects of galantamine on cocaine use in chronic cocaine users. Am J Addict. 2011;20:302–303. doi: 10.1111/j.1521-0391.2011.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Herman A, Li Y, Waters A. Galantamine attenuates some of the subjective effects of intravenous nicotine and improves performance on a Go No-Go task in abstinent cigarette smokers (in submission) Neuroscience. 2012 doi: 10.1007/s00213-012-2763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am. 2004;27:627–648. doi: 10.1016/j.psc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin Emerg Drugs. 2006;11:91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M, Kosten T, Waters A, Hashimoto K. Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology. 2010a doi: 10.1007/s00213-010-2014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sugarman DE, Carroll KM. Cognitive function as an emerging treatment target for marijuana addiction. Exp Clin Psychopharmacol. 2010b;18:109–119. doi: 10.1037/a0019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Exp Clin Psychopharmacol. 2011;19:11–19. doi: 10.1037/a0022213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sciences. 1995;56:2119–2126. doi: 10.1016/0024-3205(95)00197-e. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biological Psychiatry. 1995;37:731–739. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J Marijuana Treatment Project Research, G. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [comment][erratum appears in JAMA2002 Apr 3;287(13):1651] [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology. 2006;50:755–760. doi: 10.1016/j.neuropharm.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Swartz BE, McDonald CR, Patel A, Torgersen D. The effects of guanfacine on working memory performance in patients with localization-related epilepsy and healthy controls. Clinical neuropharmacology. 2008b;31:251–260. doi: 10.1097/WNF.0b013e3181633461. [DOI] [PubMed] [Google Scholar]
- Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2005;178:286–295. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. Journal of substance abuse treatment. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, Gonzalez-Saiz F, Fernandez-Calderon F, Bilbao-Acedos I, Perez-Garcia M. Self-regulation and treatment retention in cocaine dependent individuals: A longitudinal study. Drug Alcohol Depend. 2012;122:142–148. doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Grunder G, Spreckelmeyer KN, Wienker T, Diaz-Lacava A, Mobascher A, Dahmen N, Clepce M, Thuerauf N, Kiefer F, de Millas JW, Gallinat J, Winterer G. Neurocognitive impairments in non-deprived smokers-results from a population-based multi-center study on smoking-related behavior. Addiction biology. 2012 doi: 10.1111/j.1369-1600.2011.00429.x. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Porter RH. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochemical pharmacology. 2011;82:891–903. doi: 10.1016/j.bcp.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate and desipramine. Drug Alcohol Depend. 2004;75:271–276. doi: 10.1016/j.drugalcdep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients' approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011;22:490–497. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Wiers RWHJ, Stacy AW. Handbook of implicit cognition and addiction. Thousand Oaks: Sage Publications; 2006. [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Yakir A, Rigbi A, Kanyas K, Pollak Y, Kahana G, Karni O, Eitan R, Kertzman S, Lerer B. Why do young women smoke? III. Attention and impulsivity as neurocognitive predisposing factors. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17:339–351. doi: 10.1016/j.euroneuro.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK. Statistics to tell the truth, the whole truth, and nothing but the truth: formulae, illustrative numerical examples, and heuristic interpretation of effect size analyses for neuropsychological researchers. Arch Clin Neuropsychol. 2001;16:653–667. [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Progress in neuro-psychopharmacology & biological psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]