Abstract
The current experiments examined the effects of repeated nicotine prior to acquisition, extinction, and reinstatement of methamphetamine-induced conditioned place preference (CPP). Methamphetamine-induced (METH; 0.25, 0.5, or 1 mg/kg, s.c.) CPP was established using separate groups of adult male Sprague-Dawley rats with an unbiased conditioning procedure. Following extinction of METH CPP, drug-primed reinstatement (0, 0.25, 0.5 or 1 mg/kg, s.c.) of METH CPP was assessed in order to determine whether METH-induced reinstatement depends on the METH dose used to induce CPP. In a second experiment, separate groups of rats received nicotine (NIC; 0 or 0.2 mg/kg, s.c.) for 7 days prior to undergoing METH (0 or 0.5 mg/kg, s.c.) conditioning, extinction, and drug-primed reinstatement. Results indicate that METH-primed reinstatement varied as a function of dose such that priming with the conditioning dose did not reinstate CPP, but reinstatement was observed following priming doses of METH that were either lower or higher than the conditioning dose. Prior NIC exposure had no effect on METH CPP, extinction, or reinstatement. Interestingly, at a METH dose (0.5 mg/kg) that did not induce reinstatement alone, acute NIC (0.2 mg/kg) in combination with METH induced reinstatement, suggesting that NIC produced a leftward shift in the dose-response effect of METH to reinstate CPP. These studies indicate that prior NIC exposure may not be necessary for enhancement of the rewarding effects of METH, in contrast to previous self-administration reports.
Keywords: nicotine, methamphetamine, conditioned place preference, reinstatement
A vast majority of illicit drug users also smoke tobacco cigarettes. It has been reported that as many as 97% of methamphetamine (METH) users also use tobacco [1], with 88% also reporting regular past-week tobacco use [2]. Although no study to date has investigated the effects of METH on tobacco cigarette smoking, studies have shown that administration of either amphetamine or cocaine increases ad libitum cigarette smoking [3–5]. A recent review also found evidence for more severe health problems, increased stimulant dependence, and poorer treatment outcomes in psychostimulant users who also smoke tobacco cigarettes compared to those who do not [6].
Preclinical studies suggest possible interactions between METH and nicotine (NIC), a major addictive alkaloid in tobacco cigarettes. NIC substitutes fully for METH in rats trained to discriminate METH from saline (SAL), suggesting that METH and NIC share some discriminative stimulus properties [7, 8]. In addition, repeated NIC exposure results in subsequent locomotor cross-sensitization in response to a METH challenge in mice [9]. Further, pretreatment with METH increases NIC self-administration in a biphasic manner [10] and nicotine reinstates METH-seeking behavior in rats previously exposed to NIC [11]. Thus, both clinical and preclinical studies suggest an interaction between NIC dependence and METH abuse. Further research on this possible interaction could lead to more efficacious treatments for METH abuse and dependence.
With the high rate of comorbidity between NIC and METH abuse, it is important to examine the effects of previous nicotine exposure on subsequent METH abuse. Several studies indicate that NIC increases both METH self-administration and METH-induced locomotor activity [9, 11, 12]. However, little is known about the role of conditioned cues on NIC-METH interactions. This is an important area of investigation for cue-elicited craving and relapse prevention, as exposure to NIC may reinstate not only NIC seeking, but also METH seeking. The purpose of the current experiments was to determine the effects of NIC on the drug-primed reinstatement of METH-induced conditioned place preference (METH CPP). The first experiment investigated reinstatement of METH CPP by priming injections of varying doses of METH (0.25–1.0 mg/kg). A second experiment investigated the effects of prior NIC administration on subsequent METH CPP and reinstatement. A low dose of NIC (0.2 mg/kg, s.c.) was used in this latter experiment because it reliably produces locomotor sensitization in our laboratory, as well as others [11, 13, 14]. Given the findings from previous self-administration results [11], it was hypothesized that NIC would reinstate METH CPP in NIC-exposed rats, but not in NIC-naïve rats.
Material and Methods
Animals
Male Sprague-Dawley rats (n= 72) were acquired from Harlan Industries (Indianapolis, IN), weighing 250–275 grams at the beginning of the experiment and were maintained in accordance with the University’s Institutional Animal Care and Use Committee. Rats were double-housed in a temperature-controlled colony with a 12-h/12-h light/dark schedule (lights on at 0700). Animals had free access to food and water while in their home cages during the experiment. Rats were allowed to acclimate to housing for three days and were subsequently handled for approximately three minutes per day for a total of three days prior to experimental manipulations.
Drugs
(+)-METH HCl from the National Institute on Drug Abuse (Bethesda, MD) was prepared in SAL (0.9% NaCl). All METH doses are represented as salt weight. S(−)-NIC ditartrate (Sigma, St. Louis, MO) was prepared in SAL solution; a pH of 7.4 was obtained using NaOH. All NIC doses are represented as free-base weight. Both drugs were administered by subcutaneous injection in a volume of 1 ml/kg.
Apparatus
For assessment of CPP, a rectangular box (21 × 21 × 68 cm) divided into three chambers (ENV-013; MED Associates, St. Albans, VT) was used. The box had two large end chambers (21 × 28 cm) separated by removable guillotine doors from a middle chamber (21 × 12 cm). One end chamber had white walls and stainless steel mesh flooring. The other end chamber had black walls with stainless steel rod flooring. The middle chamber had gray walls, as well as a solid floor, and wasa “neutral” cha mber. Each end chamber contained six photobeams that were located 1.25 cm from the end wall and 5 cm apart. The middle gray chamber had three photobeams located 4.75 cm apart.
For assessment of locomotor activity, a square box (42 × 42 × 30 cm) consisting of clear acrylic walls and floor was used. Activity was recorded using an animal activity monitoring system with Versamax System software (AccuScan Instruments Inc., Columbus, OH). Inside the box, a horizontal 16 × 16 grid of photobeams with each beam 2.5 cm apart and 7.0 cm above the floor measured locomotor activity, expressed as distance (cm) traveled.
Experimental Procedures
Experiment 1: Reinstatement of extinguished METH CPP
This aim of this experiment was to determine if METH-induced reinstatement is dependent upon the METH dose used to induce CPP. Rats were given one session of habituation to the CPP chamber (day 1) during which they received SAL immediately before being placed into the neutral (gray) chamber and were allowed to explore all three chambers of the CPP apparatus for 15 min. The following day (day 2), the rats were given a pre-conditioning test to determine a baseline chamber preference. On this pre-conditioning test day, rats were administered SAL immediately before being placed into the neutral chamber and had access to all three chambers. The time spent in each chamber during this 15 min test was assessed and any animal which spent ≥80% of the total test time [after 15, 16] in either end chamber was considered to have an initial bias for one chamber and was excluded from further testing. On days 3–10, rats were randomly assigned to treatment groups and underwent conditioning sessions in a counterbalanced, unbiased fashion (i.e. regardless of initial preference, one half of the rats received METH in the black chamber and SAL in the white chamber; the other half received METH in the white chamber and SAL in the black chamber). During these conditioning sessions, the guillotine doors were closed and rats were confined to one of the end chambers. On alternating days, separate groups (n=13–14 per group) received METH (0.25, 0.5, or 1.0 mg/kg, s.c.) injections paired with one of the end chambers and SAL administration paired with the opposite end chamber. Rats were placed into the appropriate chamber immediately following METH or SAL administration for a 30-min conditioning session for at total of eight sessions (i.e. four METH-paired and four SAL-paired). The end chamber that was paired with METH and the order of METH and SAL sessions was counterbalanced across rats. Following the last conditioning day, a post-conditioning test was used to assess each animal’s preference. On this day (day 11), all rats were administered SAL immediately before being placed into the neutral chamber and were given free access to all three chambers. The time spent in each chamber during the 15-min test was assessed.
Days 12–19 were extinction sessions, during which SAL was paired with both chambers on alternating days for a total of eight, 30-min counterbalanced sessions (4 in the black chamber and 4 in the white chamber). Following the last extinction day, a post-extinction test was conducted to determine if each animal’s preference for the METH-paired chamber was successfully extinguished, defined by <80% of the total test time in the METH-paired chamber (after the initial chamber preference criteria; i.e., time in METH-paired chamber divided by time in both METH- and SAL-paired chambers × 100). SAL was administered immediately before placing the animal into the neutral chamber and the time spent in each of the 3 chambers during the 15-min period was assessed. Any animal which spent ≥80% of the total test time in the METH-paired chamber was considered not extinguished and was excluded from reinstatement testing until the extinction criterion described above was met.
Days 20–35 were reinstatement test sessions. For each reinstatement test, rats were administered either SAL or METH (0.25, 0.5, or 1.0 mg/kg, s.c.) in a randomized order immediately before being placed into the neutral gray chamber. Each rat received each dose on separate reinstatement tests. During the test, animals were given free access to the entire apparatus and the time spent in each chamber was assessed. To ensure that the animals did not display a significant preference following the reinstatement session, each reinstatement test was separated by two extinction sessions during which rats were injected with SAL prior to placement in one of the end chambers on alternate days (i.e. each end chamber was paired with SAL once) and then underwent an additional baseline test following a SAL injection (no reinstatement). The experimental procedures for Experiment 1 are outlined in Table 1.
TABLE 1.
Experimental timeline for Experiment 1. Rats were randomly assigned to one conditioning dose of METH (0.25, 0.5, or 1 mg/kg, s.c.). Following extinction by repeated SAL pairings, rats were challenged with METH (0.25, 0.5, or 1 mg/kg, s.c.) or SAL for reinstatement of METH CPP.
| Day(s) | Experimental Phase | Injection |
|---|---|---|
| 1 | Habituation | SAL |
|
| ||
| 2 | Pre-Conditioning Test | SAL |
|
| ||
| 3 – 10 | Conditioning | SAL |
| METH (0.25, 0.5 or 1 mg/kg) | ||
|
| ||
| 11 | Post-Conditioning Test | SAL |
|
| ||
| 12 – 19 | Extinction | SAL |
| SAL | ||
|
| ||
| 20 | Post-Extinction Test | SAL |
|
| ||
| 21 – 33 | Reinstatement | SAL or METH (0.25, 0.5 or 1 mg/kg) |
| Intra-Reinstatement Extinction | SAL | |
| SAL | ||
| Intra-Reinstatement Post-Extinction Test | SAL | |
| Conditioning (between-subject) | Reinstatement (within-subject) |
|---|---|
|
| |
|
| |
|
| |
Experiment 2: Reinstatement of METH-induced CPP in NIC-sensitized rats
This aim of this experiment was to determine if previous exposure to NIC would alter METH-seeking behavior. Throughout the first 7 days of this experiment, rats were administered either SAL (two groups of n=8 each) or NIC (0.2 mg/kg, s.c; two groups of n=8 each) immediately before being placed into a locomotor apparatus for 60-min sessions. Following the last day of locomotor activity monitoring, rats were given one day of habituation to the CPP apparatus followed by a pre-conditioning test as described in Experiment 1. Rats were then conditioned in a counterbalanced unbiased fashion in a similar manner to that described for Experiment 1 (days 10–17). The experimental design was a 2 X 2 (previous sensitization treatment X conditioning treatment) factorial that resulted in 4 experimental groups. Rats previously exposed to either NIC or SAL then underwent conditioning sessions with either METH (0.5 mg/kg, s.c.) or SAL (groups NIC-METH, NIC-SAL, SAL-METH, and SAL-SAL, respectively). For rats in the METH-conditioned groups (n=8/group), METH (0.5 mg/kg, s.c.) administration was paired with one of the end chambers and SAL administration was paired with the opposite end chamber on alternating days for total of 8 sessions. For rats in the SAL-conditioned groups (n=8/group), SAL administration was paired with one of the end chambers and SAL administration was also paired with the opposite end chamber on alternating days for a total of 8 sessions All conditioning sessions were 30-min in length. This intermediate dose of METH was chosen based on the results from Experiment 1 showing that METH (0.5 mg/kg, s.c.) resulted in significant CPP, but administration of this dose did not produce reinstatement. SAL-conditioned animals were randomly assigned a pseudo “METH-paired chamber” and “SAL-paired chamber” in order to be consistent with METH-conditioning groups. Following the last conditioning day (day 18), a post-conditioning test was conducted during which rats received a SAL injection and were immediately placed into the neutral chamber as described in the first experiment.
Days 19–26 were extinction sessions identical to those described in Experiment 1. Following the last extinction day, rats were given a baseline (post-extinction) test on day 27 and were tested for METH-induced reinstatement on days 28–40 (Table 2). During each reinstatement test, rats were pretreated with either SAL or NIC (0.2 mg/kg, s.c.) 30 min prior to SAL or METH (0.5 mg/kg, s.c.) in a counterbalanced order. This 30 min pretreatment time was chosen in order to avoid the well-established hypoactive effects of acute NIC administration, which would be likely to influence the amount of time spent in each chamber. Each rat received each combination (SAL+SAL, NIC+SAL, SAL+METH, NIC+METH) on separate reinstatement tests. Immediately following the second injection, rats were placed into the neutral chamber and the time spent in each of the three chambers was assessed for 15 min. As in the first experiment, each reinstatement test was separated by two extinction sessions (i.e. each chamber paired with SAL) followed by another baseline test following a SAL injection (no reinstatement). As described previously, reinstatement testing was not conducted whenever a rat exhibited a chamber preference (i.e. spent ≥80% of the test time in the METH-paired chamber) during the baseline test. The experimental procedures for all phases of Experiment 2 are outlined in Table 2.
Table 2.
Experimental timeline for Experiment 2. Following 7 days of exposure to either SAL or NIC (0.2 mg/kg, s.c.) in locomotor activity monitors, rats were randomly assigned to SAL or METH (0.5 mg/kg, s.c.) conditioning. Following extinction by repeated SAL pairings, rats were challenged with SAL or NIC (0.2 mg/kg, s.c.) 30 min prior to an injection of SAL or METH (0.5 mg/kg, s.c.).
| Day(s) | Experimental Phase | Injection |
|---|---|---|
| Sensitization in Locomotor Activity Monitor | ||
| 1–7 | Pretreatment | SAL or NIC (0.2 mg/kg) |
|
| ||
| CPP in 3-Chamber Apparatus | ||
| 8 | Habituation | SAL |
|
| ||
| 9 | Pre-Conditioning Test | SAL |
|
| ||
| 10 – 17 | Conditioning | SAL |
| SAL or METH (0.5 mg/kg) | ||
|
| ||
| 18 | Post-Conditioning Test | SAL |
|
| ||
| 19–26 | Extinction | SAL |
| SAL | ||
|
| ||
| 27 | Post-Extinction Test | SAL |
|
| ||
| 28 – 40 | Reinstatement | SAL or NIC (0.2 mg/kg) 30 min prior to SAL or METH (0.5 mg/kg) |
| Intra-Reinstatement Extinction (2 Sessions) | SAL | |
| SAL | ||
| Intra-Reinstatement Post-Extinction Test | SAL | |
| Exposure During Sensitization Phase (between-subject) | Conditioning (between-subject) | Reinstatement (within-subject) |
|---|---|---|
| ||
| ||
Statistical Analyses
Data were analyzed by separate mixed analyses of variance (ANOVA) using SPSS (Chicago, IL; version 18), with time spent in each chamber as the dependent variable. For Experiment 1, the conditioning dose of METH (0.25, 0.5, or 1.0 mg/kg) was the between-subject variable, while the paired chamber (SAL- or METH-paired) and reinstatement dose of METH (0, 0.25, 0.5, or 1.0 mg/kg) were within-subject variables. For Experiment 2, the sensitization pretreatment (SAL or NIC) and the conditioning treatment (SAL or METH) were between-subject variables and the paired chamber (SAL- or METH-paired) and reinstatement treatment (SAL, 0.5 mg/kg METH, 0.2 mg/kg NIC, or 0.2 mg/kg NIC + 0.5 mg/kg METH) were within-subject variables. Thus, for Experiment 1, a 3 (conditioning dose) X 2 (conditioning chamber) mixed ANOVA was used to analyze the conditioning and extinction phases and a 3 (conditioning dose) X 2 (conditioning chamber) X 4 (reinstatement dose) was used to analyze the reinstatement phase. For Experiment 2, a 2 (sensitization treatment) X 2 (conditioning treatment) X 2 (conditioning chamber) mixed ANOVA was used to analyze the conditioning and extinction phases. After the reinstatement phase, a 2 (sensitization treatment) X 2 (conditioning treatment) X 2 (conditioning chamber) X 4 (reinstatement dose) mixed ANOVA was analyzed. For Experiment 2, locomotor activity also was analyzed using a 2 (sensitization treatment) X 7 (locomotor session) mixed ANOVA. Planned paired samples t-tests with corrections for family wise error were performed to analyze group differences. Based on the criterion for extinction described above (<80% preference for METH-paired chamber), 8 reinstatement tests in Experiment 1 and 11 reinstatement tests in Experiment 2 were excluded from analysis.
Results
Experiment 1: Reinstatement of extinguished METH CPP
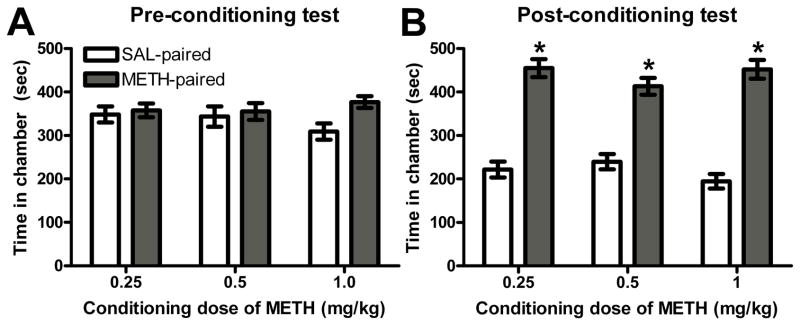
None of the conditioning groups showed a significant initial chamber preference during baseline testing (Figure 1A). A 2-way ANOVA with repeated measures revealed a significant main effect of chamber for the post-conditioning test (F(1, 37) = 119.2, p<0.001). Following conditioning with METH (0.25, 0.5, or 1 mg/kg), all groups showed a significant preference for the METH-paired chamber compared to the SAL-paired chamber (Figure 1B). METH-induced CPP was not dose-dependent, as no significant main effect of dose or dose X chamber interaction was found. Further, paired samples t-tests revealed that animals spent a significantly greater amount of time in the METH-paired chamber than in the SAL-paired chamber at each conditioning dose (p<0.001). Following extinction sessions, no significant preference for either chamber was found (results not shown).
Figure 1.
Experiment 1 – All conditioning doses of METH resulted in significant preference for the METH-paired chamber over the SAL-paired chamber (* p<0.05 vs. SAL-paired chamber). Bars represent mean (± S.E.M.) time spent in SAL- and METH-paired chambers before conditioning (Panel A) and after conditioning (Panel B).
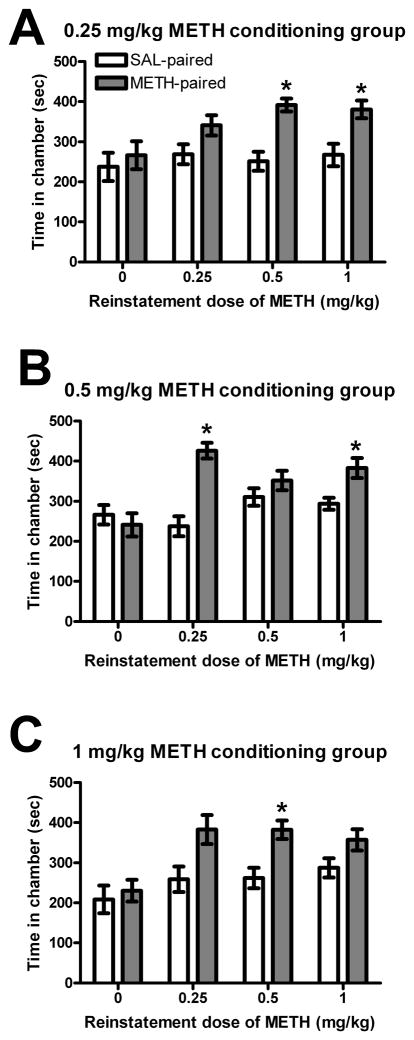
Reinstatement of METH CPP in Experiment 1 varied as a function of dose (Figure 2). A 3-way repeated measures ANOVA across reinstatement tests indicated significant main effects of reinstatement dose (F(3, 99) = 57.5, p<0.001) and chamber (SAL-paired vs. METH-paired; F(1, 33) = 12.5, p<0.01). In addition, a significant interaction of reinstatement dose X chamber (F(3, 99) = 6.98, p<0.001) was found, such that animals collapsed across conditioning groups preferred the METH-paired chamber when challenged with METH (0.25, 0.5, or 1 mg/kg, p<0.01), but not when challenged with SAL. In rats conditioned with 0.25 mg/kg METH (Figure 2A), preference for the METH-paired chamber was reinstated following a challenge of either 0.5 or 1 mg/kg METH (p<0.05), but not following SAL or 0.25 mg/kg METH. In rats conditioned with 0.5 mg/kg METH (Figure 2B), preference for the METH-paired chamber was reinstated following either 0.25 or 1 mg/kg METH (p<0.05), but not following SAL or 0.5 mg/kg METH. In rats conditioned with 1.0 mg/kg METH (Figure 2C), preference for the METH-paired chamber was reinstated only after administering a challenge of 0.5 mg/kg METH (p<0.05).
Figure 2.
Experiment 1 - (A) 0.5 and 1 mg/kg METH challenge reinstated preference for the METH-paired chamber in the 0.25 mg/kg METH conditioning group. (B) 0.25 and 1 mg/kg METH challenge reinstated preference for the METH-paired chamber in the 0.5 mg/kg METH conditioning group. (C) 0.5 mg/kg METH challenge reinstated preference for the METH-paired chamber in the 1 mg/kg METH conditioning group. * p<0.05 vs. SAL-paired chamber; bars represent mean (± S.E.M.) time spent in SAL- and METH-paired chambers.
Experiment 2: Reinstatement of METH-induced CPP in NIC-sensitized rats
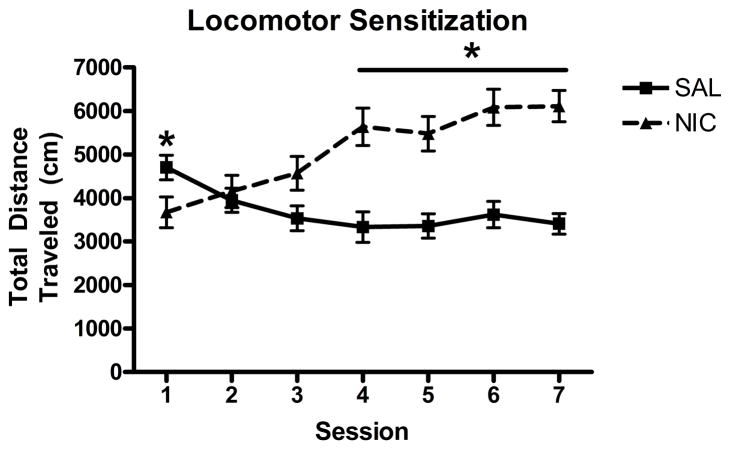
Animals exhibited a significant increase in total distance traveled across sessions following repeated administration of NIC (0.2 mg/kg, SC). The overall ANOVA revealed significant main effects of session (F(6, 180) = 5.81, p<0.001) and group (F(1, 30) = 11.3, p<0.01), as well as a significant group X session interaction (F(6, 180) = 26.9, p<0.001; Figure 3). Independent samples t-tests revealed that NIC animals had significantly lower levels of locomotor activity on session 1 and greater locomotor activity on sessions 4–7 compared to SAL animals (p<0.05).
Figure 3.
Experiment 2 - Effect of repeated NIC (0.2 mg/kg) on locomotor activity, expressed as mean (± S.E.M.) distance traveled. * p<0.05 vs. SAL-treated animals.
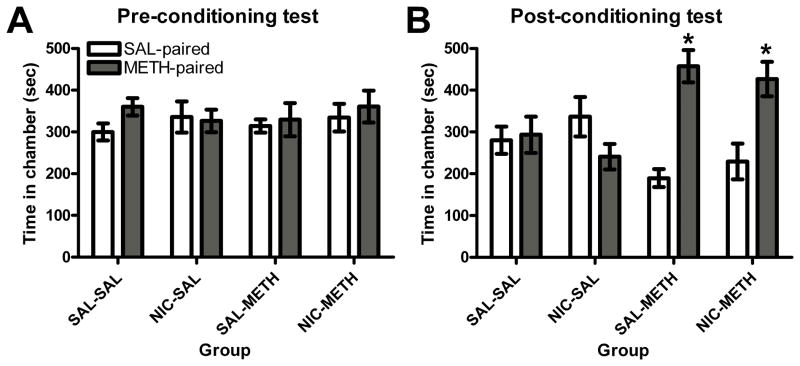
While none of the conditioning groups showed a significant chamber preference during baseline testing (Figure 4A), METH conditioned groups preferred the METH-paired chamber during the post-conditioning test (Figure 4B). A 3-way ANOVA revealed significant main effects of chamber (F(1, 28) = 7.646, p<0.05) and conditioning group (F(1, 28) = 5.68, p<0.05), as well as a significant conditioning group X chamber interaction (F(1, 28) = 15.7, p<0.001). Planned paired samples t-tests confirmed that this interaction was attributable to a significantly greater amount of time spent in the METH-paired chamber than in the SAL-paired chamber only in groups conditioned with METH (SAL-METH and NIC-METH, p<0.05). Neither the SAL-SAL nor NIC-SAL group showed a significant difference in time spent in either end chamber. A repeated measures ANOVA revealed no significant differences in the time spent in either end chamber as assessed by the post-conditioning test following extinction sessions (results not shown), thus indicating that the preference exhibited by METH conditioned groups was extinguished.
Figure 4.
Experiment 2 – METH conditioning resulted in a significant preference for the METH-paired chamber, regardless of prior NIC exposure. * p<0.05 vs. SAL-paired chamber. Bars represent mean (± S.E.M.) time spent in SAL- and METH-paired chambers before conditioning (Panel A) and after conditioning (Panel B).
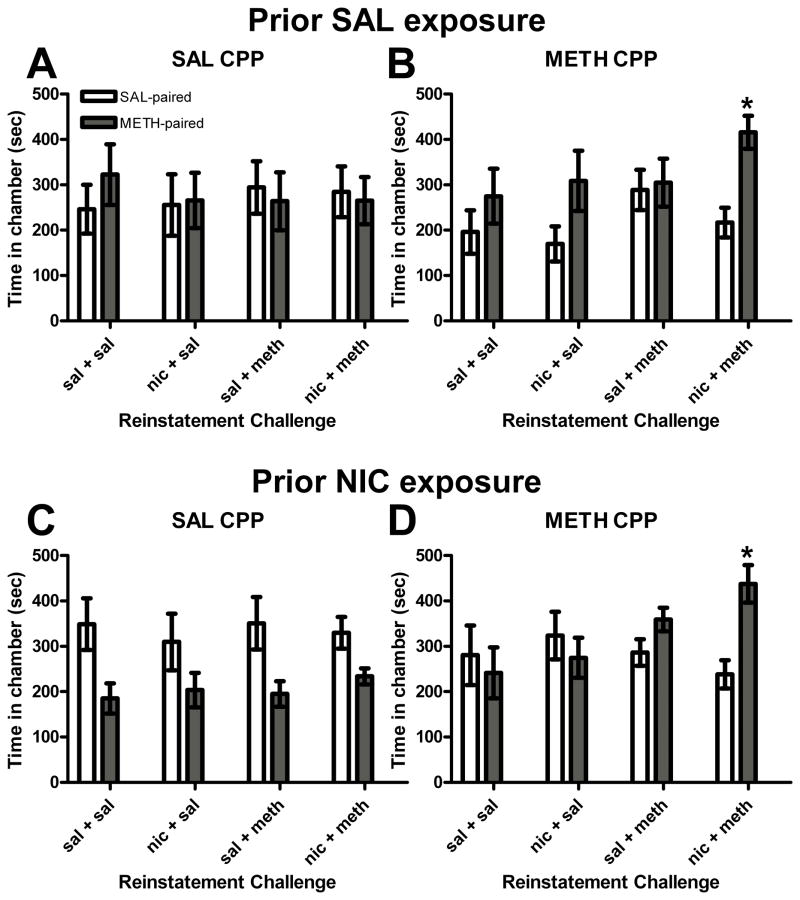
Reinstatement of METH CPP across conditioning groups was not altered by previous NIC exposure; however, NIC given in combination with METH reinstated CPP in METH conditioned groups regardless of previous NIC exposure (Figure 5). A repeated measures 4-way ANOVA revealed a significant main effect of reinstatement dose (F(3, 60) = 10.1, p<0.001) and a reinstatement dose X conditioning group interaction (F(3, 60) = 3.00, p<0.05), as well as a reinstatement dose X chamber interaction (F(3, 60) = 6.71, p<0.01) and a significant reinstatement dose X chamber X conditioning group interaction (F(3, 60) = 3.06, p<0.05). In rats conditioned with SAL (Figures 5A and 5B), no significant reinstatement was observed under any condition. In addition, rats conditioned with METH (Figures 5C and 5D) and challenged with SAL, NIC or METH did not reinstate the preference for the METH-paired chamber. Although this dose of METH alone did not induce reinstatement, a NIC+METH challenge significantly reinstated this preference (p<0.05).
Figure 5.
Experiment 2 – (A) No preference for the METH-paired chamber was found following any reinstatement challenge in animals conditioned with SAL and treated previously with SAL. (B) NIC + METH reinstated the preference for the METH-paired chamber in animals conditioned with METH and treated previously with SAL. (C) No preference for the METH-paired chamber was found following any reinstatement challenge in animals conditioned with SAL and treated previously with NIC. (D) NIC + METH reinstated the preference for the METH-paired chamber in animals conditioned with METH and treated previously with NIC. * p<0.05 vs. SAL-paired chamber; bars represent mean (± S.E.M.) time spent in SAL- and METH-paired chambers.
Discussion
The current studies sought to determine the effect of NIC exposure on subsequent METH CPP and reinstatement. The first experiment revealed that the current conditioning procedure produced significant METH CPP at all tested doses and that the CPP was extinguished by exposing rats to the previously drug-paired chamber for 4 sessions in the absence of METH. It is possible that the dose-dependent nature of METH CPP requires a longer period of training [17]; however, the findings of the current study are consistent with those observed by DeMarco et al. [16] in which dose-dependency was not observed following 10 METH pairings. It should be noted that SAL was administered prior to each test session in the current set of experiments (i.e. pre- and post-conditioning tests) in order to be consistent with subsequent reinstatement tests. Although this procedure has been used before [16, 18], it is possible, albeit unlikely, that the SAL injection altered the results of the test sessions. The first experiment also showed that a priming injection of METH reinstated the previously extinguished preference for the METH-paired chamber. The reinstatement of CPP by a challenge dose of METH is in agreement with previous self-administration studies in which, following extinction, a priming injection increases responding on the lever associated with administration of drug [11, 19]. However, the reinstatement effect did not generalize to all challenge doses of METH. Specifically, the group conditioned with the lowest tested dose of METH (0.25 mg/kg. s.c.) did not reinstate to the conditioning dose, but did reinstate to a higher dose of METH (0.5 mg/kg, SC). In contrast, however, the group conditioned with the intermediate dose of METH (0.5 mg/kg, s.c.) reinstated to a priming dose (0.25 and 1 mg/kg) either lower or higher than the METH conditioning dose, but not to the conditioning dose. Further, the group conditioned with the highest dose of METH (1.0 mg/kg, s.c.) reinstated to a lower challenge dose of METH (0.5 mg/kg), but not to the conditioning dose. Although these results may suggest development of tolerance to the low conditioning dose (0.25 mg/kg METH), no tolerance was apparent at higher conditioning doses. While we did not directly examine the development of behavioral tolerance to the training doses in the current experiment, this interesting possibility should be addressed in future investigations.
While previous findings from self-administration literature indicate that reinstatement of METH-seeking is dose-dependent [20, 21], the dose-dependent nature of the reinstatement phenomenon observed here was unexpected. Although most previous CPP reinstatement experiments using either psychostimulants or morphine have used a challenge dose lower than the conditioning dose [22–25], one recent study found evidence for METH-induced CPP reinstatement using a training and reinstatement dose of 2.5 mg/kg [26]. Even though significant methodological differences exist between the two studies, results from the current study suggest that the training dose and reinstatement dose may be important considerations for future reinstatement experiments with METH CPP. Consistent with this, rats conditioned with either 0.5 or 1.0 mg/kg METH showed reinstatement using doses lower than the conditioning dose.
In the second experiment, previous treatment with NIC had no effect on the acquisition or extinction of METH CPP, similar to that previously reported with METH self-administration [11]. Since NIC and METH share, at least in part, similar neurochemical and behavioral effects [27], the finding that prior exposure to NIC did not alter the acquisition of METH-induced CPP is surprising. Given the behavioral interactions between NIC and METH shown in previous research [11, 28, 29], it was predicted that cross-sensitization would occur between NIC and METH, resulting in enhanced expression of METH CPP on the post-conditioning test in the NIC sensitized group compared to SAL control. It is possible that the doses used to induce METH CPP resulted in a maximal effect such that no further increase from NIC treatment could be observed. Future studies are needed to determine the effects of NIC on lower conditioning doses of METH. An alternative possibility is that METH-induced CPP is relatively insensitive to dose adjustments, as has been suggested previously [30]. Therefore, the self-administration paradigm may be more useful to study the dose-dependent effects of cross-sensitization of reward-related behaviors. With self-administration, the incubation period between drug exposure and alterations in subsequent drug-related behaviors is a critical variable, and thus, it is possible that a longer incubation period in the current study would have resulted in altered conditioning to METH [for a review, see 32]. Consistent with this, Schoffelmeer et al. [27] found that repeated exposure to NIC significantly enhanced amphetamine-induced hyperactivity 3 weeks after NIC exposure.
The second experiment also replicated the finding from Experiment 1 showing that, with the current procedures, METH-induced reinstatement was not obtained when 0.5 mg/kg METH was used as both the conditioning dose and reinstatement dose. More important, the results of Experiment 2 also revealed that a priming dose of NIC given in combination with METH produced reinstatement of METH seeking. A priming dose of NIC alone had no effect in either METH-conditioned group, indicating that this finding was specific to the NIC+METH combination. Further, the effect of the NIC+METH reinstatement was obtained regardless whether rats were treated previously with NIC or SAL, suggesting that NIC enhanced the rewarding effect of METH-associated cues. This finding extends previous work showing that amphetamine- and cocaine-induced behavioral sensitization depends on nicotinic receptor signaling [27].
The current results contrast with previous self-administration results showing that NIC challenge alone reinstates METH-seeking behavior only when rats had a history of NIC exposure [11]. Since repeated NIC pretreatment produced acute hypoactivity, followed by sensitization, it may be that the lever-press response used to measure reinstatement in self-administration is more sensitive to the suppressant effect of acute NIC than the choice behavior used in CPP. With repeated nicotine pretreatment, however, reinstatement may be obtained with both paradigms. The discrepancy in results between studies could also reflect differences in doses used to induce reinstatement or differences in pretreatment interval times. In any case, direct comparison across studies requires caution, as self-administration may reflect primary drug reinforcement, whereas CPP is thought to indicate an independent process reflecting the conditioned rewarding effect of drug-associated cues [33, 34].
The ability of NIC co-exposure to enhance METH-induced rewarding effects may be due, at least in part, to an overlap in neurochemical effects within the mesolimbic reward system. This pathway, consisting of fibers from the ventral tegmental area (VTA) projecting to the nucleus accumbens and other limbic structures (i.e. hippocampus), is critical for all reinforcers, including METH [35, 36]. NIC has been shown to have facilitatory, direct effects on dopaminergic neurons in VTA [37] as well as increase glutamatergic excitation and decreased GABAergic inhibition of dopamine neurons in VTA [38]. Thus, NIC may act on VTA neurons to increase dopamine release within the nucleus accumbens, while METH administration may add to this effect [39], thus resulting in the combination producing greater reward than either stimulus alone.
In the current study, since NIC was administered 30 min prior to the reinstatement test, whereas METH was administered immediately prior to the reinstatement test, it is not clear if the optimal injection-test intervals were used. NIC (0.8 mg/kg, s.c.) has a half-life of 52 min in rat brain [40]; thus, the low dose of NIC (0.2 mg/kg) used in the current report may not have been sufficient to reinstate behavior when given alone. Additionally, the injection-test interval used may have resulted in desensitization of the nicotinic receptor in areas critical to the expression of reward-related behaviors [37]. Further studies are needed to determine if NIC administration potentiates the rewarding effects of METH and, if so, the temporal parameters under which this phenomenon may occur.
From a clinical perspective, the preclinical knowledge gained through investigating novel pharmacotherapies for drug dependence could help to treat and prevent METH relapse in humans. The results of the present experiment suggest that clinical treatments aimed at preventing relapse to METH abuse should also consider the management of NIC-containing tobacco product use, as nicotine may enhance the ability of METH to reinstate drug-seeking.
Highlights.
Reinstatement of methamphetamine-induced CPP varies as a function of dose
Prior exposure to nicotine did not alter methamphetamine-induced CPP
Nicotine given in combination with methamphetamine reinstated the preference
Nicotine may alter the rewarding effects of methamphetamine
Acknowledgments
Supported by NIH grants P50 DA05312, R01 DA013519 and T32 DA016176.
Abbreviations
- ANOVA
analysis of variance
- CPP
conditioned place preference
- GABA
gamma-aminobutyric acid
- METH
methamphetamine
- NIC
nicotine
- SAL
saline (NaCl)
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addictive behaviors. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 2.Brecht ML, Greenwell L, Anglin MD. Substance use pathways to methamphetamine use among treated users. Addict Behav. 2007;32:24–38. doi: 10.1016/j.addbeh.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Henningfield JE, Griffiths RR. Cigarette smoking and subjective response: effects of d-amphetamine. Clin Pharmacol Ther. 1981;30:497–505. doi: 10.1038/clpt.1981.194. [DOI] [PubMed] [Google Scholar]
- 4.Schuster CR, Lucchesi BR, Emley GS. The effects of d-amphetamine, meprobamate, and lobeline on the cigarette smoking behavior of normal human subjects. NIDA Res Monogr. 1979:91–9. [PubMed] [Google Scholar]
- 5.Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol. 1997;5:263–8. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–7. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010;335:807–16. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuribara H. Does nicotine modify the psychotoxic effect of methamphetamine? Assessment in terms of locomotor sensitization in mice. J Toxicol Sci. 1999;24:55–62. doi: 10.2131/jts.24.55. [DOI] [PubMed] [Google Scholar]
- 10.Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- 11.Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106:72–8. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutkiewicz EM, Nicolazzo DM, Kim MN, Gnegy ME. Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharmacology (Berl) 2008;200:93–103. doi: 10.1007/s00213-008-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wooters TE, Neugebauer NM, Rush CR, Bardo MT. Methylphenidate enhances the abuse-related behavioral effects of nicotine in rats: intravenous self-administration, drug discrimination, and locomotor cross-sensitization. Neuropsychopharmacology. 2008;33:1137–48. doi: 10.1038/sj.npp.1301477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ericson M, Norrsjo G, Svensson AI. Behavioral sensitization to nicotine in female and male rats. J Neural Transm. 117:1033–9. doi: 10.1007/s00702-010-0449-9. [DOI] [PubMed] [Google Scholar]
- 15.German PW, Fields HL. How prior reward experience biases exploratory movements: a probabilistic model. J Neurophysiol. 2007;97:2083–93. doi: 10.1152/jn.00303.2006. [DOI] [PubMed] [Google Scholar]
- 16.DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, Hammel C, et al. Racemic gamma vinyl-GABA (R,S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse. 2009;63:87–94. doi: 10.1002/syn.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behavioural brain research. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:130–4. doi: 10.1016/S0893-133X(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 19.Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruzich PJ, Xi J. Differences in extinction responding and reinstatement of methamphetamine-seeking behavior between Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2006;83:391–5. doi: 10.1016/j.pbb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–80. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- 22.Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro Do Couto B, Aguilar MA, Rodriguez-Arias M, Minarro J. Long-lasting rewarding effects of morphine induced by drug primings. Brain Res. 2005;1050:53–63. doi: 10.1016/j.brainres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi RE, Lewis JR, Lattal KM, Berger SP. Modafinil reinstates a cocaine conditioned place preference following extinction in rats. Behav Brain Res. 2009;204:250–3. doi: 10.1016/j.bbr.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behav Brain Res. 2002;136:389–97. doi: 10.1016/s0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 26.Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain research. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–76. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birrell CE, Balfour DJ. The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to D-amphetamine. Psychopharmacology. 1998;140:142–9. doi: 10.1007/s002130050751. [DOI] [PubMed] [Google Scholar]
- 29.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004;153:175–87. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Brabant C, Quertemont E, Tirelli E. Influence of the dose and the number of drug-context pairings on the magnitude and the long-lasting retention of cocaine-induced conditioned place preference in C57BL/6J mice. Psychopharmacology. 2005;180:33–40. doi: 10.1007/s00213-004-2138-6. [DOI] [PubMed] [Google Scholar]
- 31.Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–62. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- 32.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 34.Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- 35.Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, et al. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1715–22. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- 36.Keleta YB, Martinez JL. Brain Circuits of Methamphetamine Place Reinforcement Learning: The Role of the Hippocampus-VTA Loop. Brain Behav. 2012;2:128–41. doi: 10.1002/brb3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–4. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 38.Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–9. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa T, Suzuki Y, Nagayasu K, Kitaichi M, Shirakawa H, Kaneko S. Repeated exposure to methamphetamine, cocaine or morphine induces augmentation of dopamine release in rat mesocorticolimbic slice co-cultures. PLoS One. 2011;6:e24865. doi: 10.1371/journal.pone.0024865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2′-(14)C]nicotine. Drug Metab Dispos. 1999;27:1448–55. [PubMed] [Google Scholar]