Abstract
A first-line approach to treat anxiety disorders is exposure-based therapy, which relies on extinction processes such as repeatedly exposing the patient to stimuli (conditioned stimuli; CS) associated with the traumatic, fear-related memory. However, a significant number of patients fail to maintain their gains, partly attributed to the fact that this inhibitory learning and its maintenance is temporary and conditioned fear responses can return. Animal studies have shown that activation of the cannabinoid system during extinction learning enhances fear extinction and its retention. Specifically, CB1 receptor agonists, such as Δ9-tetrahydrocannibinol (THC), can facilitate extinction recall by preventing recovery of extinguished fear in rats. However, this phenomenon has not been investigated in humans. We conducted a study using a randomized, double-blind, placebo-controlled, between-subjects design, coupling a standard Pavlovian fear extinction paradigm and simultaneous skin conductance response (SCR) recording with an acute pharmacological challenge with oral dronabinol (synthetic THC) or placebo (PBO) 2 hours prior to extinction learning in 29 healthy adult volunteers (THC = 14; PBO = 15) and tested extinction retention 24 hours after extinction learning. Compared to subjects that received PBO, subjects that received THC showed low SCR to a previously extinguished CS when extinction memory recall was tested 24 hours after extinction learning, suggesting that THC prevented the recovery of fear. These results provide the first evidence that pharmacological enhancement of extinction learning is feasible in humans using cannabinoid system modulators, which may thus warrant further development and clinical testing.
1. Introduction
The inability to suppress inappropriate fear responses is the hallmark of anxiety disorders, such as post-traumatic stress (PTSD), panic, and phobic disorders (Rauch et al., 2006; Rosen and Schulkin, 1998). A common, empirically-validated approach to treat these disorders is Cognitive Behavioral Therapy (CBT) (Norton and Price, 2007), one component of which involves repeated exposure to fear-linked cues to produce “extinction” (clinically referred to as exposure therapy leading to desensitization) of fear and avoidance responses to these cues (Hofmann, 2008). After repeated presentations, the patient learns that the previously feared stimulus does not actually predict a negative outcome and anxiety is reduced. This exposure-based learning can be modeled in the laboratory, in both animals and humans, using Pavlovian fear conditioning models in which fear is first linked to a previously innocuous cue (conditioned stimulus; CS) and then decreased by presenting the CS alone (producing extinction).
Unfortunately, a major limitation of extinction is that it is a temporary phenomenon and extinguished fear can re-emerge simply with the passage of time (spontaneous recovery) (Hermans et al., 2006; Myers and Davis, 2007; Robbins, 1990). This phenomenon demonstrates that original fear memory remains within the brain and ready to re-emerge even after extinction, suggesting that extinction is a new learning process that “overlays” the original fear memory (Bouton, 2002). The vulnerability of fear memory to recovery creates significant limitations to the durability and effectiveness of exposure-based therapies (Arch and Craske, 2009; Craske et al., 2008), and this has become a topic of intense translational science efforts to improve treatments for PTSD and other anxiety disorders (Graham and Milad, 2011; Jovanovic and Ressler, 2010; Milad and Quirk, 2012). One approach to overcoming the limitations of exposure therapy may be to enhance the strength of fear inhibitory learning through understanding of its neural and neurochemical substrates (Graham and Milad, 2011; Jovanovic and Ressler, 2010; Milad and Quirk, 2012).
Exciting new evidence has shown that pharmacological agents known as “cognitive enhancers” can increase fear extinction in animals and facilitate exposure-based therapy in humans. Supported by animal evidence, clinical studies have shown that D-cycloserine (DCS), a N-methyl-D-aspartic acid (NMDA) receptor partial agonist, facilitates the retention (and maintenance when tested months later) of extinction memory from CBT in a number of anxiety disorders (Davis et al., 2006; Guastella et al., 2008; Hofmann, 2007, 2008; Ledgerwood et al., 2003, 2004, 2005; Norberg et al., 2008; Ressler et al., 2004; Walker et al., 2002). These studies demonstrate the clinical impact of translational neuroscience by coupling the basic science of fear extinction learning and human neuropsychopharmacology. However, other studies have failed to find any evidence that DCS facilitates fear extinction or exposure therapy (Guastella et al., 2007a; Guastella et al., 2007b; Norberg et al., 2008; Parnas et al., 2005; Storch et al., 2007), so while DCS is a promising cognitive enhancing agent for extinction and exposure therapy there is a need to investigate additional pharmacological targets.
Emerging studies in rodents suggest that activation of the cannabinoid (CB) system within the brain may also regulate extinction learning and retention, similar to the effects of DCS. For example, activation of type 1 CB receptors, via agonists like Δ9-tetrahydrocannabinol (THC), facilitates extinction learning, whereas fear extinction does not occur when these receptors are disabled by pharmacological blockade or genetic deletion (Bitencourt et al., 2008; Chhatwal et al., 2005; de Oliveira Alvares et al., 2008; Lafenetre et al., 2007; Lin et al., 2008; Lin et al., 2009; Lutz, 2007; Marsicano et al., 2002; Pamplona et al., 2008; Pamplona et al., 2006; Roche et al., 2007). Pharmacologically enhancing endogenous cannabinoid (eCB) levels during extinction can enhance extinction retention and block return of extinguished fear in rats (Chhatwal et al., 2005). These studies suggest that the efficacy of extinction learning and retention can be enhanced via increasing activity of CB1 receptors.
In humans, however, the role of cannabinoids on fear extinction learning remains unknown. The primary goal of this study was to test the hypotheses that administration of an exogenous CB1 agonist would enhance the memory and maintenance of fear extinction. In a randomized, double-blind, placebo-controlled, between-subjects design, we coupled a standard Pavlovian fear conditioning and extinction paradigm with an acute pharmacological challenge with oral dronabinol (synthetic THC) or placebo 2 hours prior to extinction learning in healthy adult volunteers and tested extinction retention 24 hours after extinction learning.
2. Materials and Methods
2.1 Subjects
Twenty-nine healthy, right-handed volunteers (twelve males; aged 21–45 years; Caucasian = 21; African American = 4; Asian = 2; More than one race = 2) participated in this study and were randomly assigned to the THC (n = 14) or placebo (n = 15) condition. Some participants (n = 13) had a minimal history of marijuana use (limited to < 10 lifetime exposures; mean: 1.2 ± 0.44); none had history or signs of neurological, psychiatric (including substance and alcohol abuse/dependence), or medical illness as confirmed by medical examination and a modified Structured Clinical Interview for DSM-IV (SCID-NP; (First et al., 2002)). All subjects had negative urine toxicology and alcohol breathalyzer screens at time of study.
All female subjects completed study sessions about 1 week prior to menses onset (based on self-reports of last period and cycle length), to insure that they were studied while estrogen levels were low. This restriction was based on evidence that high estradiol levels can facilitate fear extinction (Milad et al., 2010; Zeidan et al., 2011). All participants gave written informed consent after explanation of the experimental protocol, as approved by the University of Michigan Institutional Review Board.
2.2 Fear conditioning, extinction, and testing procedures
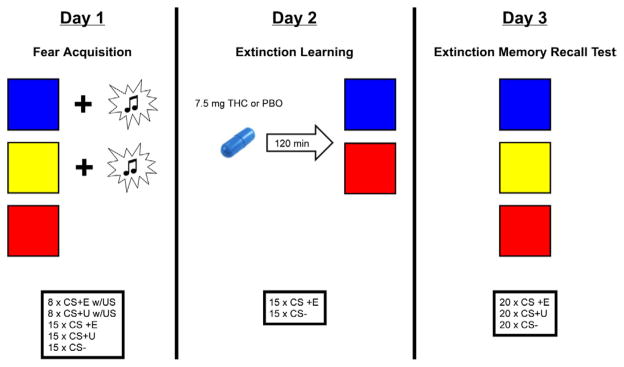
Participants were studied over 3 consecutive days (see Figure 1). On Day 1, all participants underwent partial discrimination fear conditioning, in which they were presented with two neutral visual conditioned stimuli on a computer screen (CS+s; e.g., blue and yellow squares) that co-terminated with an aversive white noise burst US through a pair of headphones (500 ms, 100dB; (Dunsmoor et al., 2008)) at a partial reinforcement rate of 35%. Fear acquisition consisted of 15 non-reinforced presentations of each of the CSs, intermixed with an additional 8 presentations of each CS+ that co-terminated with the US. A third CS (e.g. red square) was presented during fear conditioning but never paired with the US (CS−; adapted from (Schiller et al., 2010)).
Figure 1.
Schematic of the experimental protocol.
The next day (Day 2) all participants underwent an extinction session in which one of the CS+s (e.g., blue square) was extinguished (CS+E) whereas the other CS+ (e.g., yellow square) was not (CS+U). Approximately 120 minutes prior to initiation of extinction training participants ingested an opaque gelatin capsule (size 00) with dextrose filler that contained either synthetic THC (Marinol; 7.5 mg; Solvay Pharmaceuticals, Marietta, GA) or dextrose alone (placebo; PBO) [PBO, 123.80 ± 3.19 min; THC, 119.36 ± 2.45 min]. The timing insured presence of peak subjective effects and plasma levels of THC (Wachtel et al., 2002) during the extinction training. The THC dose used is the lowest effective dose found to produce behavioral and subjective effects (Kirk and de Wit, 1999; Phan et al., 2008; Rabinak et al., 2011; Wachtel et al., 2002). During extinction learning the CS+E was presented in the absence of the US, whereas the CS+U was not presented. There were 15 CS+E and 15 CS− trials.
To assess extinction retention, we conducted an extinction memory recall test approximately 24 hours after the extinction learning session (Day 3). This consisted of 20 non-reinforced presentations of each of the CSs (CS+E, CS+U, and CS−). In each experimental session (fear acquisition, extinction learning, and extinction recall test) all CSs were presented for 4 sec each and the mean inter-trial interval (ITI) was 12 sec (range: 6–18 sec). The designation of colored squares (blue, yellow, or red) as CS+E, CS+U, or CS− was counterbalanced across the participants and the order of trials was pseudo-randomized, such that no more than 2 presentations of the same colored square (red, yellow, or blue) occurred in a row.
At the beginning of each session participants were told that they may or may not hear a loud noise burst and were instructed to pay attention to the computer screen and try to figure out the relationship between the colored squares and the noise burst. During each presentation of the colored square stimuli, participants were asked to rate their expectancy that the US would occur on a 5-point scale (“Will you hear a loud noise burst?”: 1 = Definitely not; 3 = Unsure; 5 = Definitely). US expectancy was scored as the first response within 3 sec of CS onset (adapted from (Dunsmoor et al., 2008)). Conditioned fear was indexed by changes in skin conductance responses (SCRs) for each CS trial. Electrodes and headphones remained in place during all sessions (removed only during breaks).
2.3 Psychophysiological measures and data analysis
SCRs were measured by two disposable carbon fiber electrodes attached between the first and second phalanges of the second and third digits of the left hand (EL509, BIOPAC Systems, Inc., Goleta, CA). The electrodes were connected to a BIOPAC Systems skin conductance module (GSR100C) and skin conductance was continuously sampled at a rate of 1000 samples per second, amplified, and stored on a Dell laptop computer for offline analysis using AcqKnowledge 4.1 software (BIOPAC Systems, Inc.). The recorded waveforms were low pass filtered using a Blackman window (cutoff frequency = 125 Hz) and mean value smoothed over 100 adjacent data points prior to scoring.
SCR for each CS trial was calculated by subtracting the mean skin conductance level during the 2 sec before stimulus onset from the highest skin conductance level that occurred in the 0.5 to 4.5 sec latency window after stimulus onset. SCRs below 0.02 μS were scored as zero (LaBar et al., 1998; Schiller et al., 2010). Raw SCRs were square root transformed to normalize the distributions and scaled according to each subject’s mean square root transformed US response (LaBar et al., 1998; Milad et al., 2005a; Orr et al., 2000; Schiller et al., 2010). To assess the level of conditioned responding in anticipation of the aversive US separate from unconditioned responses to the noise bursts, themselves, we included only non-reinforced trials of the CS+s in the analysis.
SCRs and US expectancy ratings were analyzed using analysis of variance (ANOVA). Post-hoc comparisons between and within drug groups, using independent and paired t tests, respectively, were performed after a significant F ratio was obtained. We used a significance threshold of p < 0.05 (two-tailed), corrected for multiple comparisons using Bonferroni correction. Unless otherwise stated all data are presented as means ± SEM.
3. Results
3.1 Fear Acquisition
An ANOVA of SCR during fear acquisition revealed a significant main effect of stimulus [F(1,27) = 16.12, p < 0.001], with greater SCR responses to the CS+ [THC = 0.38 ± 0.08; PBO = 0.27 ± 0.08] than to the CS− [THC = 0.10 ± 0.07; PBO = 0.18 ± 0.06] during the last trial of fear acquisition. There were no significant differences in SCR during fear acquisition between participants assigned to the THC and PBO groups, as evidence by the absence of a significant main effect of drug [F(1,27) = 0.05, p = 0.82] or drug by stimulus interaction [F(1,27) = 4.11, p = 0.53]. Similarly, participants rated the US as more likely to occur during the CS+ [THC = 3.39 ± 0.20; PBO = 3.40 ± 0.21] than the CS− [THC = 1.29 ± 0.19; PBO = 1.47 ± 0.24] during the last trial of fear acquisition [main effect of stimulus [F(1,27) = 80.76, p < 0.001]. There were no significant differences in US expectancy ratings during fear acquisition between participants assigned to the THC and PBO groups [absence of main effect of drug: F(1,27) = 0.23, p = 0.64; and drug by stimulus interaction: F(1,27) = 0.15, p = 0.70]. The two drug groups responded similarly to the US and displayed high levels of unconditioned SCRs [THC = 1.16 ± 0.11; PBO = 1.05 ± 0.10; t(27) = 0.75, p = 0.46].
3.2 Extinction Learning
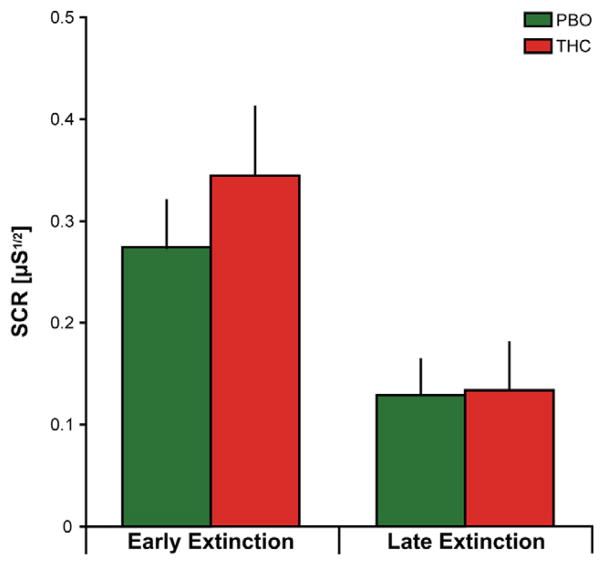
An ANOVA of SCR and of US expectancy ratings with three, two-level factors – drug (THC, PBO), stimulus (CS+E, CS−), and time (early extinction [average first 4 trials], late extinction [average last 4 trials]) – revealed a significant main effect of time [SCR: F(1,27) = 11.79, p = 0.002; US Expectancy: F(1,27) = 26.82, p < 0.001], main effect of stimulus [SCR: F(1,27) = 9.95, p = 0.004; US Expectancy: F(1,27) = 31.16, p < 0.001], and interaction between time and stimulus [SCR: F(1,27) = 17.67, p < 0.001; US Expectancy: F(1,27) = 30.58, p < 0.001]. Both drug groups displayed significantly greater SCRs and US expectancy ratings to the CS+E during early extinction [SCR: THC = 0.34 ± 0.07; PBO = 0.27 ± 0.05 (Figure 2); US Expectancy: THC = 3.48 ± 0.23; PBO = 3.13 ± 0.25 (not shown)] compared to late extinction [SCR: THC = 0.13 ± 0.05; PBO = 0.13 ± 0.04 (Figure 2); US Expectancy: THC = 1.91 ± 0.33; PBO = 1.90 ± 0.32 (not shown)]. There were no differences between drug groups in SCRs or US expectancy ratings to the CS+E [SCR: Early Extinction: t(27) = 0.84, p = 0.40; Late Extinction: t(27) = 0.06, p = 0.95; US Expectancy: Early Extinction: t(27) = 1.02, p = 0.32; Late Extinction: t(27) = 0.02, p =0.98].
Figure 2.
Skin conductance responses (SCRs) to the CS+E during extinction learning. Mean SCRs to the CS+E during early (average first four trials) and late (average last four trials) extinction learning. PBO (green bars) and THC (red bars).
Of note, elevated SCRs and greater US expectancy ratings to the CS+E during early extinction learning were comparable to SCRs and US expectancy ratings to the CS+E during late fear acquisition in both drug groups [SCR: THC: t(13) = 0.44, p = 0.67; PBO: t(14) = 0.07, p = 0.94; US Expectancy: THC: t(13) = −0.41, p = 0.69; PBO: t(14) = 0.97, p = 0.35], supporting successful acquisition and next day retention of conditioned fear. In addition, there were no significant differences in SCRs to the CS+E and CS− between the THC and PBO groups within or between early and late extinction, as evidence by the absence of a significant main effect of drug [SCR: F(1,27) = 0.04, p = 0.85; US Expectancy: F(1,27) = 0.34, p = 0.57], drug by stimulus interaction [SCR: F(1,27) = 2.15, p = 0.15; US Expectancy: F(1,27) = 0.07, p = 0.80], drug by time interaction [SCR: F(1,27) = 0.07, p = 0.80; US Expectancy: F(1,27) = 0.03, p = 0.85], and drug by stimulus by time interaction [SCR: F(1,27) = 1.44, p = 0.24; US Expectancy: F(1,27) = 1.80, p = 0.19].
3.3 Extinction Memory Recall Test
An ANOVA of SCR during the extinction memory recall test (first two CS+E vs. first two CS+U trials) revealed a significant drug by stimulus interaction [F(1,27) = 4.80, p < 0.05]. The THC group exhibited smaller SCRs to the stimulus that had been previously extinguished during the previous extinction learning phase compared with the stimulus that had not been extinguished [0.22 ± 0.08 for CS+E vs. 0.35 ± 0.09 for CS+U], whereas the PBO group did not [0.37 ± 0.11 for the CS+E vs. 0.23 ± 0.09 for the CS+U]. Moreover there was a significant difference in the mean differential SCR to the CS+E minus the CS+U between the THC and PBO groups during the extinction memory recall test [Figure 3; t(27) = −2.29, p < 0.05]. Consistent with these findings the PBO group displayed spontaneous recovery of conditioned fear responses to the CS+E during extinction recall, as evidenced by significantly greater SCRs to the CS+E during extinction recall than during late extinction learning [Late Extinction learning: 0.13 ± 0.04 vs. Extinction Recall Test: 0.37 ± 0.11; t(14) = −2.45, p < 0.05], whereas THC during extinction learning prevented spontaneous recovery of conditioned fear responding to the CS+E [Late Extinction learning: 0.13 ± 0.05 vs. Extinction Recall Test: 0.22 ± 0.08; t(13) = −0.94, p = 0.37]. As expected the THC group reported low subjective US expectancy ratings to the CS+E during the extinction recall test [2.07 ± 0.35] that were not significantly different from late extinction learning [1.91 ± 0.33; t(13) = −1.80, p = 0.10], further suggesting good retention of extinction memory. Interestingly, the PBO group also reported low subjective US expectancy ratings to the CS+E during the extinction recall test [2.07 ± 0.32] that were no different from the THC group [t (27) = −0.48, p = 0.64] or from late extinction learning [1.90 ± 0.32; t(14) = −0.68, p = 0.51]. These results suggest that the PBO group was able to maintain subjective knowledge that the CS+E no longer predicted the occurrence of the US despite exhibiting a recovery of physiological fear responding (SCR) to the CS+E following extinction learning.
Figure 3.
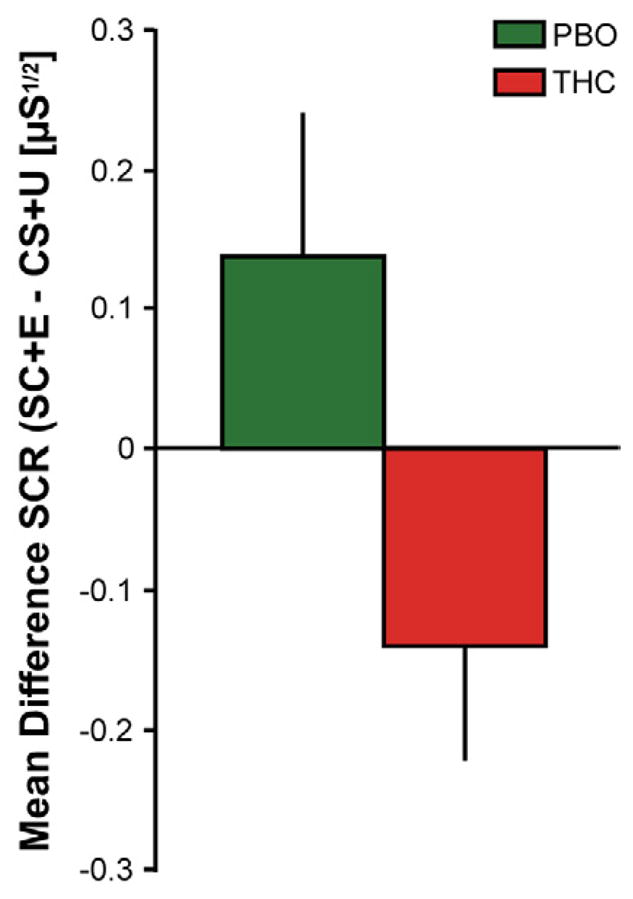
Skin conductance responses (SCRs) during the extinction memory recall test. Mean differential SCRs during the first two trials of the CS+E minus the first two trials of the CS+U during the extinction memory recall test. Difference scores greater than zero reflect greater responding to the CS+E, difference scores equal to zero reflect no difference in responding between the CS+E and CS+U, and scores less than zero reflect greater responding to the CS+U (LaBar et al., 1998; LaBar et al., 1995). PBO (green bars) and THC (red bars).
4. Discussion
The present findings provide the first evidence that pre-extinction administration of THC facilitates extinction of conditioned fear in humans. In particular, participants that had received PBO during extinction learning exhibited spontaneous recovery of fear to a CS+ that was previously extinguished, whereas THC attenuated spontaneous recovery of fear. Of note, THC did not affect within-session extinction learning, but only influenced the ability to successfully recall extinction memory when compared to PBO, suggesting that THC affects the ability to maintain and/or successfully retrieve extinction memory. These findings are consistent with pre-clinical studies in rats in which cannabinoid activation and/or enhancement can lead to facilitation of extinction memory recall (Bitencourt et al., 2008; Chhatwal et al., 2005; Lafenetre et al., 2007; Lin et al., 2009; Lutz, 2007; Pamplona et al., 2008; Pamplona et al., 2006).
In rats, acute systemic administration of CB1 agonists, (e.g. WIN 55,212-2 and HU210) prior to extinction learning have been shown to facilitate fear extinction (Lin et al., 2008; Lin et al., 2006; Lin et al., 2009; Pamplona et al., 2008; Pamplona et al., 2006); but see (Chhatwal et al., 2005)) and prevent spontaneous recovery of extinguished conditioned fear responses (Lin et al., 2006). In addition, cannabidiol, a non-psychoactive phytocannabinoid, and pharmacological agents that enhance levels of released eCBs, such as AM404, an eCB reuptake inhibitor, and URB597, a fatty acid amide hydrolase (FAAH) inhibitor that blocks hydrolysis of anandamide, have been shown to facilitate within-session extinction learning (Bitencourt et al., 2008; Varvel et al., 2007), enhance the retention of extinction (Bitencourt et al., 2008; Chhatwal et al., 2005; de Oliveira Alvares et al., 2008; Lin et al., 2009; Varvel et al., 2007) and also decrease the recovery of conditioned fear responses in rats (Chhatwal et al., 2005) if given prior to extinction learning. Conversely, co-administration of CB1 antagonists, such as AM251 and rimonabant [SR141716], block the extinction enhancing effects of these CB1 agonists and reuptake/metabolism inhibitors (Chhatwal et al., 2005; de Oliveira Alvares et al., 2008; Varvel et al., 2007). When administered alone, CB1 antagonists lead to a profound disruption of extinction retention when given either prior to extinction learning or immediately following extinction learning, suggesting that CB1 receptor activation is necessary during extinction learning and for consolidation of extinction memories in order to successfully retrieve these memories at a later time (Chhatwal et al., 2005; de Oliveira Alvares et al., 2008; Lin et al., 2009; Marsicano et al., 2002; Pamplona et al., 2008; Pamplona et al., 2006; Suzuki et al., 2004). Consistent with these results are findings that extinction of fear is impaired in CB1 receptor knockout mice when compared to wild-type mice (Kamprath et al., 2006; Marsicano et al., 2002; Varvel et al., 2005).
Two of the most studied cognitive enhancers of extinction learning are DCS and yohimbine, an alpha2-receptor antagonist that promotes norepinephrine release (Kaplan and Moore, 2011). As mentioned previously, DCS has been shown to enhance extinction learning in rats and humans and facilitate exposure therapy in patients with anxiety (Davis et al., 2006; Hofmann, 2007, 2008; Ledgerwood et al., 2003, 2004, 2005; Norberg et al., 2008; Ressler et al., 2004; Walker et al., 2002). Moreover, DCS given immediately after extinction learning prevents recovery of fear (Ledgerwood et al., 2004) and enhances extinction recall in rats, suggesting that DCS also mediates consolidation of extinction memories (Ledgerwood et al., 2003). Likewise, yohimbine also enhances fear extinction in rats and humans (Holmes and Quirk, 2010) and has been shown to facilitate exposure therapy in patients with claustrophobia (Powers et al., 2009). However, there are concerns about the efficacy of these agents over repeated sessions (i.e. with chronic use), which produces behavioral desensitization (Parnas et al., 2005; Storch et al., 2007) and in preventing relapse in translational applications (Kaplan and Moore, 2011).
In the brain, CB1 receptors are densely localized within brain structures that are known to be critical for extinction learning, retention and successful retrieval of extinction memories (ventromedial prefrontal cortex [vmPFC] and hippocampus [HPC]) (Bouton et al., 2006; Corcoran et al., 2005; Kalisch et al., 2006; Mackie, 2005; Milad and Quirk, 2002; Milad et al., 2006; Milad et al., 2007; Myers and Davis, 2007; Ochsner and Gross, 2005; Phelps et al., 2004; Quirk and Beer, 2006; Quirk et al., 2006; Quirk et al., 2003; Quirk and Mueller, 2008). In humans, vmPFC activation during extinction recall and vmPFC thickness both correlate with magnitude of extinction retention (Hartley et al., 2011; Milad et al., 2005b; Milad et al., 2007; Phelps et al., 2004). In rats, cells within the infralimbic cortex (IL), a homologous structure to the human vmPFC, display robust CS-elicited activity during extinction recall which is inversely correlated with spontaneous recovery of fear CRs (Milad and Quirk, 2002). Similarly, the hippocampus (HPC) is associated with successful retrieval of extinction memory and is positively correlated with vmPFC activation during extinction recall in humans (Kalisch et al., 2006; Milad et al., 2007).
When eCBs are released from the postsynaptic cell they diffuse in a retrograde fashion to activate presynaptic CB1 receptors, which in turn inhibits presynaptic release of neurotransmitters (Chhatwal and Ressler, 2007; Howlett, 2005; Lafenetre et al., 2007; Pertwee, 2005). In rodents it has been suggested that during extinction learning activation of vmPFC CB1 receptors induces neuronal plasticity, which subsequently increases inhibition on brain areas involved in the expression of conditioned fear responses (e.g. amygdala) (Lin et al., 2009) and HPC CB1 receptor activation enhances glutamatergic neurotransmission, which may support long-term extinction memory formation (consolidation) (de Oliveira Alvares et al., 2008). We are currently investigating the role of cannabinoids on the retention of extinction memory and its effect on the underlying neural circuits in humans using a similar Pavlovian fear conditioning-extinction paradigm coupled with fMRI.
Anecdotal reports of recreational use of cannabis or psychopharmacological studies of marijuana/THC suggests that CB1 activation decreases subjective anxiety (D’Souza et al., 2004; Sethi et al., 1986; Wachtel et al., 2002). An early placebo-controlled study showed that nabilone, a synthetic THC, dramatically reduced anxiety in anxious patients (Fabre and McLendon, 1981). Consistent with this and evidence from rodents, studies in humans using functional magnetic resonance imaging (fMRI) have found that oral THC (vs. PBO) attenuates amygdala reactivity to aversive/fear stimuli using a social-threat paradigm (Phan et al., 2008) and that the level of amygdala reactivity is inversely related to the level of cannabis use (Cornelius et al., 2010), consistent with the notion that THC and other cannabinoids may have an anxiolytic role via central fear mechanisms. The anxiolytic effects of eCB enhancers in rodents and humans have sparked interest in CB1 receptors as a pharmacological target for treating anxiety disorders (Gaetani et al., 2003; Gaetani et al., 2009; Hill and Gorzalka, 2009; Witkin et al., 2005). However, some important issues need to be addressed if CB1 agonists are to be used as a treatment for anxiety disorders. For instance, the subjective effects of THC tend to be biphasic; low doses generally promote anxiolytic-like effects, whereas higher doses may induce anxiogenic-like effects (D’Souza et al., 2004; Genn et al., 2004; Viveros et al., 2005). Moreover, we found THC administration facilitates extinction of conditioned fear in participants with minimal or no previous history of marijuana usage; however we cannot generalize these findings to chronic marijuana users. In fact, chronic heavy marijuana smokers experience a disruption or alteration in frontal-limbic network, in the absence of intoxication, which in turn could affect the efficacy of THC on extinction learning (Cornelius et al., 2010; Gruber et al., 2009). Also, because some 13 of the 29 participants had minimal prior exposure to marijuana (mean: 1.2 times over lifetime), these findings also require replication in a THC-naïve cohort. Future studies are needed to address these important issues.
Anxiety disorders, such as PTSD, are difficult to treat because many patients only partially respond to CBTs (Cloitre, 2009; Foa, 2000; van Minnen et al., 2002) and even fewer respond to first-line pharmacological treatments, such as selective serotonin reuptake inhibitors (SSRIs) (Stein et al., 2009; Stein et al., 2006). Interestingly, poor extinction retention and vmPFC-HPC dysfunction have been implicated in anxiety disorders such as PTSD and could undermine the maintenance of the therapeutic effects of exposure (Charney and Deutch, 1996; Foa, 2000; Milad et al., 2009; Orr et al., 2000; Pitman et al., 2001; van Minnen and Hagenaars, 2002). Given that enhancing cannabinoid transmission, via CB1 agonists, helps extinction recall, the cannabinoid system is a promising target for improving the learning that goes on in psychotherapy and improving the likelihood of success and its maintenance in patients with PTSD, and other difficult-to-treat anxiety disorders. Moreover, proof-of-concept studies such as this will provide the necessary bioassay of THC’s putative anxiolytic effects and enhance the pace of drug development of cannabinoid modulators (agonists such as THC, or FAAH inhibitors such as URB597). The present study is a critical translational first step towards the development of cannabinoid modulators as an adjunctive strategy to exposure-based therapies to augment extinction learning and prevent the return of fear memories in people suffering from anxiety disorders.
Highlights.
Δ9-tetrahydrocannabinol (THC) facilitates extinction of conditioned fear in humans.
THC attenuated spontaneous recovery of fear (vs. placebo).
THC did not affect within-session extinction learning.
Cannabinoids may facilitate exposure-based therapy in patients with anxiety.
Acknowledgments
This work was supported by a grant from the National Center for Research Resources (UL1RR024986) to C.A.R. and K.L.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors would like to thank Shoko Mori, Maryssa, Lyons, Christina Harrison, and Daanish Chawala for their assistance in recruitment, participant screening, data collection and analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arch JJ, Craske MG. First-line treatment: a critical appraisal of cognitive behavioral therapy developments and alternatives. Psychiatr Clin North Am. 2009;32:525–547. doi: 10.1016/j.psc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Ressler KJ. Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectr. 2007;12:211–220. doi: 10.1017/s1092852900020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloitre M. Effective psychotherapies for posttraumatic stress disorder: a review and critique. CNS Spectr. 2009;14:32–43. [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. 2010;35:644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience. 2008;154:1648–1655. doi: 10.1016/j.neuroscience.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol. 1981;21:377S–382S. doi: 10.1002/j.1552-4604.1981.tb02617.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzter RL, Gibbon M, Williams JB. Structured clinical interivew for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCID-I/NP) Biometrics Reserach Department, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 5):43–48. discussion 49–51. [PubMed] [Google Scholar]
- Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends Mol Med. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol Biochem Behav. 2004;77:567–573. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. American Journal of Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007a;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007b;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biological Psychiatry. 2006;60:361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Enhancing exposure-based therapy from a translational research perspective. Behav Res Ther. 2007;45:1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Quirk GJ. Pharmacological facilitation of fear extinction and the search for adjunct treatments for anxiety disorders--the case of yohimbine. Trends Pharmacol Sci. 2010;31:2–7. doi: 10.1016/j.tips.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. In: Cannabinoid Receptor Signaling Cannabinoids. Pertwee RG, editor. Springer; Berlin Heidelberg: 2005. pp. 53–79. [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. Journal of Neuroscience. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99:217–228. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learning & Memory. 2008;15:876–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learning & Memory. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Molecular Neurobiology. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Mackie K. In: Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System Cannabinoids. Pertwee RG, editor. Springer; Berlin Heidelberg: 2005. pp. 299–325. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005a;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005b;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Norton PJ, Price EC. A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. J Nerv Ment Dis. 2007;195:521–531. doi: 10.1097/01.nmd.0000253843.70149.9a. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiology of Learning and Memory. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiology of Learning and Memory. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. In: Pharmacological Actions of Cannabinoids Cannabinoids. Pertwee RG, editor. Springer; Berlin Heidelberg: 2005. pp. 1–51. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. Journal of Neuroscience. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- Powers MB, Smits JA, Otto MW, Sanders C, Emmelkamp PM. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. J Anxiety Disord. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Sripada CS, Angstadt M, de Wit H, Phan KL. Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. J Neural Transm. 2011 doi: 10.1007/s00702-011-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robbins S. Mechanisms underlying spontaneous recovery in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:235–249. [Google Scholar]
- Roche M, O’Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. European Journal of Neuroscience. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi BB, Trivedi JK, Kumar P, Gulati A, Agarwal AK, Sethi N. Antianxiety effect of cannabis: involvement of central benzodiazepine receptors. Biological Psychiatry. 1986;21:3–10. doi: 10.1016/0006-3223(86)90003-x. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Ipser J, McAnda N. Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectr. 2009;14:25–31. [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006:CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Minnen A, Arntz A, Keijsers GP. Prolonged exposure in patients with chronic PTSD: predictors of treatment outcome and dropout. Behav Res Ther. 2002;40:439–457. doi: 10.1016/s0005-7967(01)00024-9. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Hagenaars M. Fear activation and habituation patterns as early process predictors of response to prolonged exposure treatment in PTSD. J Trauma Stress. 2002;15:359–367. doi: 10.1023/A:1020177023209. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]