Abstract
New neurons are produced each day in the hippocampus through the process of neurogenesis. Both mental and physical training can modify this process by increasing the number of new cells that mature into functional neurons in the adult brain. However, the mechanisms whereby these increases occur are not necessarily the same. Physical activity, especially aerobic exercise greatly increases the number of new neurons that are produced in the hippocamal formation. In contrast, mental training via skill learning increases the numbers that survive, particularly when the training goals are challenging. Both manipulations can increase cognitive performance in the future, some of which are reportedly mediated by the presence of new neurons in the adult hippocampus. Based on these data, we suggest that a combination of mental and physical training, referred to here as MAP training, is more beneficial for neuronal recruitment and overall mental health than either activity alone.
Keywords: Neurogenesis, Learning, Hippocampus, Exercise, Mental Training, Physical Training, Cognitive Enhancement, Fluid Intelligence
The search for a better brain
Over the last decade, a large body of literature has revealed that both mental and physical training can improve a variety of cognitive abilities, including those related to learning and memory. Here, we review training procedures which facilitate learning and memory through mechanisms that coincidentally involve the incorporation of new neurons into the adult brain. Every day, thousands of new neurons are produced in the adult brain through a process known as adult neurogenesis (Cameron & McKay, 2001). Many of the new cells are generated in the hippocampal formation, a brain structure necessary for many types of new learning, and one that is highly responsive to the effects of mental and physical training.
New neurons in the hippocampus are extremely responsive to the external environment. Aerobic exercise is the most well-characterized of these influences, because it causes a large increase in the number of cells that are produced. The first study to demonstrate this effect was reported by van Praag and colleagues in 1999. In this study, two weeks of daily voluntary exercise resulted in an approximately fifty percent increase in the number of new cells generated in the dentate gyrus (van Praag, Kempermann, & Gage, 1999). Since then, numerous studies have revealed similar effects (Fabel et al., 2003; Kronenberg et al., 2006; Trejo, Carro, & Torres-Aleman, 2001), including a report that just one day of exercise can significantly increase the number of cells produced (Steiner, Zurborg, Hörster, Fabel, & Kempermann, 2008).
Regardless of how many cells are generated, approximately half undergo programmed cell death one-to-two weeks after their birth. In other words, even though many new cells might be produced in response to some environmental manipulations, a great number of those do not survive beyond a few weeks, and they typically die before they have become functionally connected with other neurons in the adult brain. However, in a series of studies, we have reported that most of these new cells can be rescued from death by mental training. Although the term “mental training” has come to encompass a number of different learning phenomenon and/or procedures, we use it here to refer to the direct manipulations of training procedures, the goal of which is to induce skill learning. In order to learn the skill, animals often require many trials each day, often over multiple days and they must exert sustained effort and/or concentration during the training manipulation. These types of mental training procedures keep the new cells alive, whereas less intensive training procedures do not. The types of training that we know to be effective involve processes related to associative learning, spatial learning, and even physical skill learning (Curlik, Agarwal, Maeng & Shors 2012; Gould, Beylin, Tanapat, Reeves, & Shors, 1999; Shors, Anderson, Curlik, & Nokia, 2011; Wurm, Keiner, Kunze, Witte, & Redecker, 2007). After learning, the surviving neurons remain in the dentate gyrus for several months (Leuner et al., 2004). By that time, these new cells will have formed functional monosynaptic connections to area CA3 of the hippocampus and polysynaptic connections with efferent sites elsewhere (van Praag et al., 2002). It is presumed that these cells have become integral units in circuitries used by the brain for learning and memory. Minimally, it can be concluded that the adult brain is supplemented with more neurons simply as a result of new learning.
With that said, rescuing new neurons from death is not so simple. Training will only rescue these cells when successful learning occurs during that training. Animals that fail to learn, or those that learn very poorly, do not retain any more of the new neurons than animals that are not trained at all (Curlik & Shors 2011). These relationships produce strong positive correlations between how well an animal learns the skill and the number of surviving cells in that animal’s dentate gyrus (Curlik & Shors, 2011; Dalla, Bangasser, Edgecomb, & Shors, 2007; Sisti, Glass, & Shors, 2007; Fig. 1a). Furthermore, when learning is pharmacologically prevented during training, the cells do not survive (Curlik & Shors, 2011). Thus, training by itself does not rescue these cells from death. Instead, learning must occur during the training process. However, not all forms of learning rescue these cells. In general, learning only rescues new neurons when the learning is difficult to master. For example, learning to associate two stimuli that overlap in time does not rescue new cells from death. This type of association is easy to learn and occurs with minimal if any conscious awareness (Beylin et al., 2001; Clark & Squire, 1998). When the associations are more difficult to acquire, the cells respond and survive. For example, learning to associate two stimuli that overlap but are separated by a long temporal window requires many more trials to learn; learning this association increases the number of surviving neurons (Leuner, Waddell, Gould & Shors, 2006). Similarly, learning to associate two stimuli that do not occur together in time increases the number of surviving neurons -- but only if the temporal gap is sufficiently long (Waddell, Anderson, & Shors, 2011). This relationship between task difficulty and neuronal survival also extends into spatial learning. For example, learning to navigate to a visible platform in a water maze task does not increase the number of cells that survive, but learning to find the platform using only spatial cues outside the maze does rescue new neurons (Gould et al., 1999). Of course, individual animals (including humans) tend to learn at different rates. To account for individual differences in learning ability, we typically assess how many trials are necessary for any given individual to learn, and have repeatedly observed strong positive correlations between the number of trials necessary for an individual animal to learn and the number of surviving cells in that animal’s dentate gyrus (Curlik & Shors, 2011; Waddell & Shors; 2008 Fig. 1). In other words, animals that learn the skill but require more trials of training to do so tend to retain more cells than animals that learn with less effort. Thus, learning appears to have the greatest impact on neurogenesis when the training task itself is challenging, and when the individual animal requires many trials and/or days of training to master the skill.
Figure 1.

Mental training can greatly increase the number of surviving cells in the adult dentate gyrus. A) Animals that learned a trace memory retrained more new cells than animals that failed to learn, resulting in a strong positive correlation between the number of conditioned responses emitted during training and the number of surviving cells in the dentate gyrus. B) Of those animals that learned, those that took longer to do so retained more new cells than those that learned quickly. Representative BrdU-labeled cells from C) an animal that successfully learned, and D) an animal that failed to learn the task (Adapted from Curlik & Shors, 2011).
Over the past decade, there has been increasing interest in the role that new neurons actually play in the instantiation of learning. The first study to report such a relationship took advantage of an antimitotic agent known as MAM, which significantly reduces the number of new cells that are produced across time. After several weeks of treatment (but not in the absence of treatment), we observed a select deficit in learning to associate events across time (Shors et al., 2001), an effect that has been replicated using another antimitotic known as TMZ (Nokia, Anderson, Shors, submitted). Since then, other techniques have been developed to reduce cell numbers, including focused irradiation or genetic manipulations (Reviewed in Shors et al., 2011). A summary of the many reports since then is beyond the scope of the current article. That said, many of the reported deficits in performance vary either as a function of the task parameters or the method used to reduce neurogenesis. Anti-mitotics tend to be the easiest to administer, being delivered via intraperitoneal injections, or osmotic pumps. As such, these compounds are not target specific, and can interfere with the proliferation of cells in other areas of the CNS and the periphery. Focused irradiation, on the other hand, can target precursor cells in the hippocampus, but it requires specialized equipment which is not readily available. Moreover, irradiation can lead to inflammation, especially in the immediate weeks after treatment. Genetic techniques can specifically target neuronal precursor cells, but they can have side effects, which impair aspects of performance unrelated to learning. Also, the genetic targets may have multiple functions or be involved in other physiological processes, independent of their role in regulating those involved in neurogenesis. Therefore, evaluating how these new neurons contribute to learning and memory is difficult and is probably best done by examining the learned responses following several different ablation techniques, in independent groups of animals and laboratories. These converging operations would help to ensure that any behavioral changes are due to a reduction in hippocampal neurogenesis, and not merely side effects of the chosen ablation technique.
Given these considerations, it appears that some forms of learning (such as trace conditioning and pattern separation) are especially sensitive to the loss of the new cells whereas others can still be learned in their near absence. For example, when neurogenesis was disrupted, with either antimitotics or whole brain irradiation, animals could readily learn to associate stimuli that occur close together and/or overlap in time (delay conditioning). They could also learn to associate one stimulus with a context in which a fearful event occurs (contextual fear conditioning), and to find a hidden platform using spatial cues in the environment (Morris water maze) (Achanta, Fuss, & Martinez, 2009; Shors et al., 2001; Shors, Townsend, Zhao, Kozorovitskiy, & Gould, 2002; Nokia, Anderson, Shors, submitted). However, animals with disrupted neurogenesis did have difficulty learning to associate events across longer periods of time as well as learning to distinguish between patterns that are closely related to one another in space (Achanta et al., 2009; Clelland et al., 2009). Overall, the data suggest that the new cells are most engaged when the task demands are high and mastering them depends on cognitively flexibility (Burghardt, Park, Hen, & Fenton, 2012; Shors et al., 2011).
Is More Better?
We often assume that more is better and this includes new neurons. Even though manipulations that effectively disrupt neurogenesis can impair learning, the number of proliferating cells in healthy animals (i.e. those with “normal” levels of neurogenesis) does not necessarily relate to how well those animals learn. For example, the number of proliferating cells in an individual animal’s dentate gyrus does not predict the amount of learning that will occur in the future in that animal (Anderson, Sisti, Curlik, & Shors, 2011; Bizon & Gallagher, 2005; Nokia, Sisti, Choksi, & Shors, 2012). Of course, it remains conceivable that some neurogenic manipulation could increase cell proliferation beyond the normal range of individual differences, and thereby influence the potential for learning in the future. Several experiments have examined this question, with conflicting results. In one study, animals increased their cell numbers by nearly 70% by living in an enriched environment. Afterwards, they were able to learn and remember a novel object for longer periods of time. The increase in memory was reportedly related to the increase in cell number because it did not occur when the new cells were not produced (Bruel-Jungerman, Laroche & Rampon 2005). In another study, neurogenesis was increased by allowing animals to exercise in activity wheels for approximately two months (Clark et al., 2008). Again, the animals learned a new task faster (the Morris water maze). However, when neurogenesis was disrupted the exercise no longer facilitated learning.
What if a multitude of new neurons were produced in an otherwise healthy individual -- would this increase learning? A recent study addressed this question directly. A genetic manipulation was developed, which nearly doubled the number of surviving neurons in the adult dentate gyrus. The animals performed within the normal range on tests of spatial navigation learning, contextual fear conditioning, and novel object recognition but they were better able to distinguish between two highly similar overlapping contexts (Sahay, Scobie, et al., 2011). These data are consistent with the results of another study, which revealed that voluntary exercise could increase cell production, and also facilitate spatial pattern separation (Creer, Romberg, Saksida, van Praag, & Bussey, 2010). Together, it appears that interventions that increase neurogenesis can facilitate important processes related to learning and memory, including pattern separation (Sahay, Wilson, & Hen, 2011), memory resolution (Aimone, Deng, & Gage, 2011), timing and/or cognitive flexibility (Burghardt et al., 2012; Shors et al., 2011). Again, it would appear that tasks that require some degree of cognitive effort and/or discriminatory skill are the most likely to be dependent on and even facilitated by the presence of these new neurons.
Importantly, not all cognitive enhancements that occur as a result of physical exercise or mental training are due to the production or presence of new neurons. For example, increases in proliferation as a result of exercise did not critically contribute to increases in motor skill learning or contextual fear conditioning (Clark et al., 2008). Nor did the enhanced spatial learning after environmental enrichment depend on their presence (Meshi et al., 2006). More directly and as noted, genetically increasing the number of cells did not noticeably alter simple spatial, contextual, or novel object recognition learning; (Sahay, Scobie, et al., 2011). Overall, these results suggest that both mental and physical training may facilitate future learning, and some of these effects are potentially mediated by increases in adult neurogenesis. Because exercise and learning excerpt widespread changes on the brain’s structure and function, it is highly unlikely that changes to this one process, or any process for that matter, account for all the observed changes in cognition following training.
Does working out help the brain work?
For decades now, we have appreciated the fact that physical exercise can enhance select processes of learning (Clarkson-Smith & Hartley, 1989; Cotman & Berchtold, 2002; Dustman et al., 1990; Powell & Pohndorf, 1971). For example, older adults that engage in physical activity outperform their sedentary counterparts on tests of reasoning, working memory, and reaction time (Clarkson-Smith & Hartley, 1989). Likewise, retired individuals that remain physically active perform better on a series of cognitive tests than retired individuals who are physically inactive (Rogers, Meyer, & Mortel, 1990). Such increases in physical activity are also associated with increases in fluid intelligence, a term sometimes used to refer to an individual’s ability to solve novel problems (Powell & Pohndorf, 1971). However, most of these studies are retrospective and/or observational. Correlations could arise because individuals who are more active are also more mentally able and more likely to engage in other activities such as socializing, which thereby improves cognition.
To more directly address the relationship between physical exercise and cognition, randomized controlled trials have now been conducted. Meta-analyses of these trials indicate that physical activity positively influences many aspects of cognitive performance in healthy individuals, as well as subjects with minimal cognitive impairment and dementia (Colcombe & Kramer, 2003; Heyn, Abreu, & Ottenbacher, 2004; Smith et al., 2010). The effects of physical exercise tend to be most evident during tests involving executive function, which are those processes in the brain related to response inhibition, scheduling, planning, selective attention, and working memory (Colcombe & Kramer, 2003). Exercise also tends to facilitate performance on visuospatial tasks and tasks that require controlled processing (Colcombe & Kramer, 2003). Importantly, most of the published literature focuses on elderly individuals because of the increasing threats of cognitive decline and dementia in these populations. One meta-analysis focused on studies in children, reporting overall increases in perceptual skill, academic readiness, IQ, achievement, and performance on standardized math and verbal tests (Sibley & Etnier 2003). Combined with findings revealing that exercise can facilitate declarative learning in adults (Pereira et al., 2007) and executive function in the elderly (Reviewed in Colcombe & Kramer, 2003), these results suggest that exercise can act as a cognitive enhancer, facilitating learning seemingly across the lifespan.
In addition to these human studies, there are many studies about physical exercise and learning in laboratory animals, most of which are quite convincing. Either voluntary or forced exercise reportedly improves performance during training on a wide variety of learning tasks, including spatial navigation learning in a Morris water maze (Albeck, Sano, Prewitt, & Dalton, 2006; Kennard & Woodruff-Pak, 2012; van Praag, Shubert, Zhao, & Gage, 2005), a radial-arm maze (Anderson et al., 2000; van Praag, Christie, Sejnowski, & Gage, 1999), delay eyeblink conditioning (Green, Chess, Burns, Schachinger, & Thanellou, 2011), and contextual fear conditioning (Barrientos et al., 2011; Baruch, Swain, & Helmstetter, 2004). Importantly, many studies were conducted in healthy adult animals, indicating that exercise can have cognitive benefits in otherwise healthy individuals. Of course, too much exercise over too long a period of time can impose negative consequences, presumably due to the physical and emotional stress of this type of regime (Kennard & Woodruff-Pak, 2012). Moreover, certain forms of exercise facilitate learning in some individuals, whereas these same exercises impair learning in different individuals. With these caveats, there does appear to be a trend towards improved cognitive performance as a result of vigorous exercise. The neuronal mechanisms that reportedly mediate these effects include neurogenesis but they do not exclude angiogenesis, synaptogenesis, and the production of neurotrophins (Reviewed in Cotman, Berchtold, & Christie, 2007; van Praag, 2009)
Training the Brain
Over the last decade, the popularity of computerized “brain training” programs has skyrocketed. In 2005, American consumers spent an estimated $2 million on mental training programs (Aamodt & Wang 2007). That number rose to nearly $300 million just last year. Many of these programs claim to enhance the user’s ability to learn and retain information in the near future, and some claim to prevent more long-term age-related cognitive decline. The types of training vary from program to program, but most of them tend to rely on practicing simple computations, reading aloud, and memorizing words (Fuyuno, 2007).
Do mental training programs actually work? Is it really possible to train our brains to learn better? Over the course of mental training, individuals do tend to improve their ability to perform on the trained tasks. However, improvements on previously trained tasks do not necessarily translate into enhancements after training. Instead, they simply reveal what we already knew -- individuals get better at what they practice. If these training programs actually improve cognition, then they should also facilitate the acquisition of tasks that have never been attempted or practiced. Such verification is crucial, because this would indicate that the effects of mental training transfer from the training context to another novel context, and are accompanied by increases in learning, memory, executive function, selective attention, and/or fluid intelligence. Many of these training programs were only recently developed, with few scientific studies directly assessing their validity. One study has reported that training with the popular “Brain Age” computer software improved measures of executive functioning and processing speed in elderly individuals (Nouchi et al., 2012). Unfortunately, the sample size of this study was relatively low, and the research was performed by one of the creators of the Brain Age computer program. On the opposite end of the spectrum, one recent large-scale study failed to find any gains in attention, planning, visuospatial skills, or reasoning following six weeks of cognitive training (Owen et al., 2010). However, this study was criticized for using a very low dose of mental training: Individuals were instructed to perform a minimum of three ten-minute sessions of training per week over six weeks. Furthermore, subjects voluntarily signed up after watching a popular television program. Therefore the results of this study may not generalize to the overall population. Even with these concerns, one negative finding does not prove that all forms of “brain training” are ineffective.
A lot of attention was recently bestowed on a group of scientists who reported that sustained mental training increases fluid intelligence. Fluid intelligence loosely refers to an individual’s ability to solve novel problems or engage in reasoning. As such, increases in fluid intelligence should transfer to a wide variety of learning situations and real-life experiences. Furthermore, an individual’s fluid intelligence (as assessed by conventional psychometric tests) strongly correlates with their professional and educational success (te Nijenhuis, van Vianen, & van der Flier, 2007). For these reasons, researchers have spent decades attempting to improve fluid intelligence via mental training. Most of the results have been negative, thus supporting the general notion that fluid intelligence is not malleable. More recently, investigators have been attempting to increase fluid intelligence by training with working memory tasks. Working memory essentially refers to an individual’s ability to concurrently store and process information. When the processing demands are high, little information can be stored; however, when the processing demands are low, comparatively more information can be retained (Matzel & Kolata, 2010). Strong, positive correlations have been found between an individual’s working memory capacity and their individual fluid intelligence (Conway, Cowan, Bunting, Therriault, & Minkoff, 2002). The group of scientists to report this effect was led by Susanne Jaeggi (Jaeggi, Buschkuehl, Jonides, & Perrig, 2008; Jaeggi, Buschkuehl, Jonides, & Shah, 2011). In their study, participants were trained with a difficult working memory task, known as a dual N-back task. In this task, bits of information are presented at a fairly rapid pace. Individuals must retain numbers, letters or spatial locations in their working memory, and note when those bits of information reappear. The task can be rendered even more difficult by presenting information from two modalities simultaneously. After weeks of this working memory training (during which n-back performance improved), the subjects scored significantly higher on a standard measure of fluid intelligence (Bochumer Matrizen-Test). Furthermore, the effect was reported to be “dose-dependent”, with longer periods of training resulting in greater gains in measures of fluid intelligence. Upon publication, these results were hailed as evidence that fluid intelligence was malleable and susceptible to training (Sternberg, 2008). An additional study, conducted by the same group, has now revealed similar findings in children (Jaeggi et al., 2011). However, in this study measures of fluid intelligence only increased in those children that successfully learned the working memory task. Children that did not improve performance during working memory training also did not display improvements in measures of fluid intelligence. Furthermore, a positive correlation was observed between each child’s performance on the working memory task and their improvement on measures of fluid intelligence. Together, these results suggest that individual differences in learning may mediate this effect of mental training. It is noted that the validity of the N-back task as a verifiable measure of “working memory” has been questioned, because performance does not always correlate with other standard measures of working memory (Jaeggi, Buschkuehl, Perrig, & Meier, 2010). Since then and perhaps because of this issue, the initial claims have been tempered (Moody, 2009; Shipstead, Redick, & Engle, 2012). Regardless, these findings still suggest that mental training may enhance components of intelligent thought and behavior.
Interestingly enough, analogous measures of intelligence in humans can be assessed in rodent models of learning and memory. Matzel and colleagues report that working memory training can improve measures of fluid intelligence in mice (Light et al., 2010). In one study, mice were first trained with a working memory task. The animals were concurrently trained with two radial arm mazes, which shared extra maze cues. The animals were required to maintain and process information about both mazes during the course of training, which taxed the animal’s working memory. Following working memory training, the animals were then trained with a battery of different learning tasks, which included measures of spatial learning, associative learning, odor discrimination, and passive avoidance learning, as well as tests of selective attention. The behavioral results from these tests were used to compute a general learning score for each mouse. This learning score is in many ways analogous to measures of human intelligence (Blinkhorn, 2003; Matzel et al., 2003). For example, mice with high general learning scores typically perform better across all of the tests, whereas mice with low general learning scores perform worse. In agreement with human findings, mice trained with the working memory protocol were better able to learn under novel training conditions in the future, including tasks unrelated in terms of sensory or motor experience to the training tasks. Furthermore, when the working memory demands of the training task were reduced, by altering the working memory task so that the two mazes no longer shared any extra maze cues, the improvement in the general learning ability of the mice was likewise reduced (Light et al., 2010). Therefore, the more difficult the mental training, the more marked is the increase in general learning ability. Likewise, Matzel et al., (2011) reported that working memory training across the lifespan protected aged animals from typical age-related cognitive decline. Particularly relevant was their finding that animals of natively lower body weight exhibited less age-related cognitive decline than natively heavier animals (Matzel, Grossman, Light, Townsend, & Kolata, 2008).
Overall, these and other results, suggest that specific forms of working memory training (such as the N-back task) may improve fluid intelligence, but the more common forms of “brain training” do not necessarily elicit these gains in healthy subjects. Most importantly, we do not know how changes in fluid intelligence will translate into the day-to-day lives of individuals who partake in such practice. Moreover, since most of the studies are conducted in humans, there is no way currently to assess the effects of this training on neurogenesis or cell survival. That said, there are data to suggest that mental training on one task will increase performance on a related task in the future and this process has a positive impact on neuronal survival (Nokia et al., 2012; Fig. 2). Animals were trained to learn to associate two events together across a specific period of time. Some weeks later, they were trained on a similar task, except the stimuli were separated by a different period of time. Animals that were already trained were better able to associate the stimuli over different time periods. This is not necessarily surprising since the skills acquired during the initial learning experience are easily and quickly transferred during training on the second task. What is surprising is the fact that cells that were not yet born during training on the first task were more readily rescued from death by training on the second related task. Therefore, learning about the environment in one situation transfers into future learning opportunities and with it, brings an increased ability to incorporate new neurons into the hippocampus.
Figure 2.

Learning one task can facilitate the acquisition of a similar task, thereby increasing the number of surviving adult-born cells. A) Animals were trained with two different forms of eyeblink conditioning. Half of the animals were trained with Task 1 and then with Task 2. The other half was trained with Task 2 before training with Task 1. B) The first phase of training facilitated acquisition during the second phase. C) Animals that successfully learned both tasks retained more new cells than animals that failed learn both tasks (Adapted from Nokia et al., 2012).
Imaging blood flow during mental and physical training
What about humans? Are there established neuronal changes that accompany mental and/or physical training? There are a few. One study reported that aerobic exercise reversed age-related decreases in hippocampal volume, which correlated with an improvement in spatial skill (Erickson et al., 2011). Combined with animal studies, these results suggest that exercise may facilitate learning-related processes through architectural changes in the hippocampus. However, there were some conflicting results to consider. For example, the group that only engaged in stretching exercises also expressed the increase in spatial skill. Therefore, aerobic exercise per se, may not have facilitated learning, but rather something about engaging in the experiment was as effective (Coen, Lawlor, & Kenny, 2011). Furthermore, the results were limited to elderly adults, and thus may reflect neuroprotective effects of exercise, and not a generalized cognitive enhancement.
And what about the effects of mental and physical training on neurogenesis in humans? It seems likely that exercise would increase the number of new neurons produced in the human hippocampus, as it greatly increases the number of neurons produced in laboratory animals, but this has not been established. The problem here is that it is not yet possible to precisely assess the number of newborn cells as they are produced within the living human hippocampus. One recent study attempted to sidestep this technological limitation by examining cerebral blood volume, a neural correlate of neurogenesis, in mice and humans (Pereira et al., 2007). They began the study by demonstrating a relationship between dentate gyrus cerebral blood volume and neurogenesis in mice, as well as an increase in both measures after exercise. When neurogenesis was impaired, via irradiation of the dentate gyrus, exercise no longer increased neurogenesis; nor did it increase cerebral blood volume. Based on these data, they suggested that exercise-induced increases in dentate gyrus cerebral blood volume can be considered a neural correlate of exercise-induced neurogenesis in humans. The researchers then went on to study these relationships in healthy humans, except for the fact that they were sedentary. The researchers then engaged these subjects in twelve weeks of physical training, with four one-hour sessions of aerobic exercise per week. This exercise program greatly increased cerebral blood volume in the dentate gyrus, where the new neurons are generated. Indeed, the effect was confined to the dentate gyrus, and individual blood volume correlated with the maximum oxygen consumption. The participants also expressed a facilitation in the initial acquisition of a declarative memory, suggesting that exercise improves declarative learning via an increase in neurogenesis in healthy human beings (Pereira et al., 2007). It is noted that these results do not demonstrate a precise relationship between exercise and neurogenesis, nor one between exercise and learning, but they do nonetheless demonstrate positive outcomes for brain health as a result of physical exercise and aerobic training.
Concluding with a Combination of Mental and Physical Skill Learning
Can we really train our brains to learn better? It appears that physical training with aerobic exercise can improve learning and memory in healthy individuals. It also appears that some regimes of mental skill training, when rigorous and sustained, can increase processes related to cognition and associative learning. But even if this were true on both counts, these activities are not necessarily acting via the same neuronal mechanisms, neurogenesis notwithstanding. Here we suggest that a combination of these two forms of training may result in greater cognitive gains than either form alone. To our knowledge, no such studies have examined the cognitive enhancing effects of combined mental and physical training in healthy humans. However, these two forms of training do appear to have an additive effect on neurogenesis. For example, a combination of exercise and environmental enrichment resulted in a greater increase in neurogenesis than either exercise or enrichment alone (Fabel et al., 2009; Fig. 3). Thus, as one might expect, a combination of mental and physical training can have additive effects on the structure of the adult brain, which may help to keep the brain fit for future learning (Kempermann, 2008; Shors, Anderson, Curlik, & Nokia, 2012).
Figure 3.
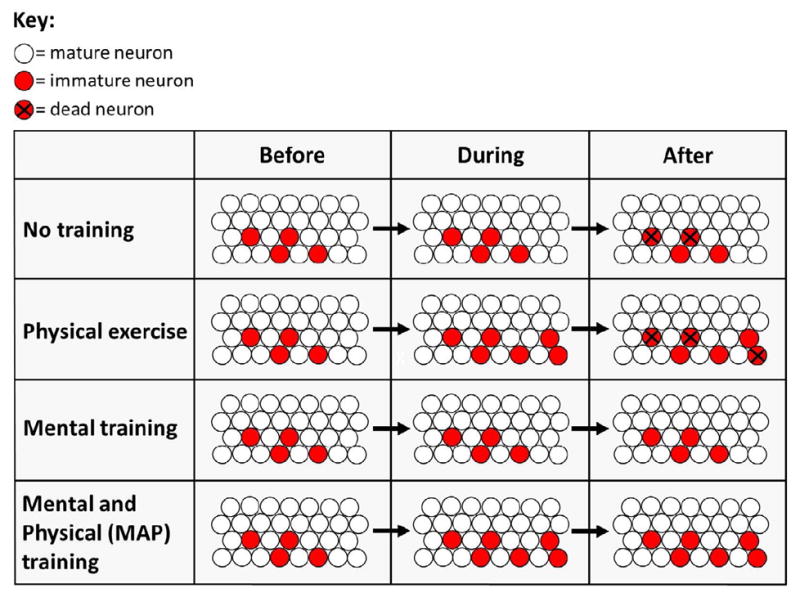
Physical exercise greatly increases the number of new neurons produced during training (van Praag, et al., 1999; van Praag, 2009), and mental training increases the numbers that survive after training (Gould et al., 1999; Shors et al., 2011). In principle, a combination of both mental and physical (MAP) training should be more effective than either training approach alone, increasing the overall number of neurons that survive to become mature functioning neurons in the adult brain (Fabel et al., 2009).
But we might also take this idea a bit farther by using training regimes that combine mental and physical skill training. After all, exercise and skill learning often coexist and interact while learning a new sport or learning to play a new instrument. For example, learning to perform a new dance routine or a new symphony engages a great many learning processes, including working memory and requires some significant degree of cognitive effort. Thus, it might be informative to explore the combined effects of mental and physical skill training on cognition and neural plasticity. To this end, we have been examining the effects of motor skill learning on neurogenesis and cell survival. In these experiments, we took advantage of a task known as the rotarod test, in which rodents learn to stay on a large rod as it rotates (Buitrago, Schulz, Dichgans, & Luft, 2004). To make it more difficult, we increased speed of the rod over time. As animals learned the motor skill, they were able to stay on the rod for longer and longer periods of time. Those that did learn retained many more neurons in their hippocampus than animals that were not trained. They also retained more than those that did not learn AND those that were trained on a simple version in which the rod rotated very slowly and predictably. Animals that exercised in an activity wheel for days, running twenty times the distance of the trained animals in the same time period, did not retain any more cells than those that were sedentary. These results suggest that, the increase in cell survival was not due to exercise but rather to learning the new motor skill (Fig. 4). It is noted that the new cells were already present during the training experience and about to undergo programmed cell death (Curlik, Maeng, Agarwal & Shors 2011). Thus, it would appear that the effort expended and expertise acquired during the motor skill learning was sufficient to keep neurons alive that would have otherwise died. Overall, these results indicate that the mental effort intrinsic to many athletic and sporting endeavors can produce long-lasting effects on structure of the adult brain. More generally, they concur with the adage: “There are many roads to Rome.” That is, training your brain may have less to do with the road you take and more to do with the effort you spend finding the right one.
Figure 4.

The acquisition of a difficult physical skill increases the number of surviving cells. Animals that learned the physical skills necessary to remain on top of an accelerating rotarod displayed a large increase in the number of surviving cells. This increase was only observed in animals that successfully acquired the skill. Those animals that failed to learn, or that learned poorly, displayed no such increase in cell survival. An additional group of animals were not trained on the rotoarod. Instead, they were allowed to exercise in activity wheels. This voluntary exercise did not rescue cells from death. Together, these results suggest that skill learning, and not merely exercise, increases the number of surviving neurons in the adult hippocampus. This increase in cell survival may result from sustained, or rhythmic, activation of the hippocampal formation that would occur during training (Curlik, Maeng, Agarwal & Shors 2011).
Mental and physical training increase hippocampal neurogenesis and facilitate learning.
Neurogenesis is required for some, but not all, cognitive enhancing effects of training.
Mental training has its greatest effect when difficult, but attainable.
Larger effects may be observed following a combination of both mental and physical (MAP) training.
Acknowledgments
The authors thank L. Matzel for his comments on the manuscript, and A. Curlik for her assistance with figure generation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aamodt S, Wang A. Exercise on the brain. The New York Times. 2007 http://www.nytimes.com/2007/11/08/opinion/08aamodt.html.
- Achanta P, Fuss M, Martinez JL. Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behavioral neuroscience. 2009;123(5):1036–45. doi: 10.1037/a0016870. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–96. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behavioural brain research. 2006;168(2):345–8. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiology & Behavior. 2000;70(5):425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Anderson ML, Sisti HM, Curlik DM, Shors TJ. Associative learning increases adult neurogenesis during a critical period. The European journal of neuroscience. 2011;33(1):175–81. doi: 10.1111/j.1460-9568.2010.07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HEW, Campeau S, et al. Little Exercise, Big Effects: Reversing Aging and Infection-Induced Memory Deficits, and Underlying Processes. Journal of Neuroscience. 2011;31(32):11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral neuroscience. 2004;118(5):1123–7. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiology of learning and memory. 2001;76(3):447–61. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. More is less: neurogenesis and age-related cognitive decline in Long-Evans rats. Science of aging knowledge environment. 2005;2005(7):re2. doi: 10.1126/sageke.2005.7.re2. [DOI] [PubMed] [Google Scholar]
- Blinkhorn S. Neuroscience: of mice and mentality. Nature. 2003;424(6952):1004–5. doi: 10.1038/4241004a. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. The European journal of neuroscience. 2005;21(2):513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiology of learning and memory. 2004;81(3):211–6. doi: 10.1016/j.nlm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012 doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of comparative neurology. 2001;435(4):406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280(5360):77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clarkson-Smith L, Hartley AA. Relationships between physical exercise and cognitive abilities in older adults. Psychology and aging. 1989;4(2):183–9. doi: 10.1037//0882-7974.4.2.183. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen RF, Lawlor BA, Kenny R. Failure to demonstrate that memory improvement is due either to aerobic exercise or increased hippocampal volume. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(18):E89. doi: 10.1073/pnas.1102593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Conway AR, Cowan N, Bunting MF, Therriault DJ, Minkoff SR. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30(2):163–183. [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30(9):464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2367–72. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik DM, 2nd, Maeng LY, Agarwal PR, Shors TJ. Gym rats are not as stupid as you think: Physical skill learning increases cell survival in the hippocampus. Program No. 595.10. 2011 Neuroscience Meeting Planner; Washington, DC. Society for Neuroscience; 2011. Online. [Google Scholar]
- Curlik DM, 2nd, Shors TJ. Learning increases the survival of newborn neurons provided that learning is difficult to achieve and successful. Journal of cognitive neuroscience. 2011;23(9):2159–70. doi: 10.1162/jocn.2010.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiology of learning and memory. 2007;88(1):143–8. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman RE, Emmerson RY, Ruhling RO, Shearer DE, Steinhaus LA, Johnson SC, Bonekat HW, et al. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiology of aging. 1990;11(3):193–200. doi: 10.1016/0197-4580(90)90545-b. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. The European journal of neuroscience. 2003;18(10):2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Frontiers in neuroscience. 2009;3(50) doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuyuno I. Brain craze. Nature. 2007;447(7140):18–20. doi: 10.1038/447018a. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature neuroscience. 1999;2(3):260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Green JT, Chess AC, Burns M, Schachinger KM, Thanellou A. The effects of two forms of physical activity on eyeblink classical conditioning. Behavioural brain research. 2011;219(1):165–74. doi: 10.1016/j.bbr.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Archives of physical medicine and rehabilitation. 2004;85(10):1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6829–33. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10081–6. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18(4):394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends in neurosciences. 2008;31(4):163–9. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kennard JA, Woodruff-Pak DS. A comparison of low- and high-impact forced exercise: Effects of training paradigm on learning and memory. Physiology & behavior. 2012 doi: 10.1016/j.physbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiology of aging. 2006;27(10):1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Leuner B. Learning Enhances the Survival of New Neurons beyond the Time when the Hippocampus Is Required for Memory. Journal of Neuroscience. 2004;24(34):7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. The Journal of neuroscience. 2006;26(52):13437–42. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KR, Kolata S, Wass C, Denman-Brice A, Zagalsky R, Matzel LD. Working memory training promotes general cognitive abilities in genetically heterogeneous mice. Current biology. 2010;20(8):777–82. doi: 10.1016/j.cub.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Grossman H, Light K, Townsend D, Kolata S. Age-related declines in general cognitive abilities of Balb/C mice are associated with disparities in working memory, body weight, and general activity. Learning & memory. 2008;15(10):733–46. doi: 10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, et al. Individual differences in the expression of a “general” learning ability in mice. The Journal of neuroscience. 2003;23(16):6423–33. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neuroscience and biobehavioral reviews. 2010;34(1):23–30. doi: 10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Light KR, Wass C, Colas-Zelin D, Denman-Brice A, Waddel AC, Kolata S. Longitudinal attentional engagement rescues mice from age-related cognitive declines and cognitive inflexibility. Learning & memory. 2011;18(5):345–56. doi: 10.1101/lm.2034711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nature neuroscience. 2006;9(6):729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Moody DE. Can intelligence be increased by training on a task of working memory? Intelligence. 2009;37(4):327–328. [Google Scholar]
- Nokia MS, Sisti HM, Choksi MR, Shors TJ. Learning to Learn: Theta Oscillations Predict New Learning, which Enhances Related Learning and Neurogenesis. PloS one. 2012;7(2):e31375. doi: 10.1371/journal.pone.0031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, Sekiguchi A, et al. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PloS one. 2012;7(1):e29676. doi: 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Howard RJ, et al. Putting brain training to the test. Nature. 2010;465(7299):775–8. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RR, Pohndorf RH. Comparison of adult exercisers and nonexercisers on fluid intelligence and selected physiological variables. Research quarterly. 1971;42(1):70–7. [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. Journal of the American Geriatrics Society. 1990;38(2):123–8. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70(4):582–8. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is Working Memory Training Effective? Psychological bulletin. 2012;138(4):628–54. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM, Nokia MS. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behavioural brain research. 2012;227(2):450–8. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM, Nokia MS. Use it or lose it: How neurogenesis keeps the brain fit for learning. Behavioural brain research. 2011 doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley B, Etnier J. The relationship between physical activity and cognition in children: A meta-analysis. Pediatric Exercise Science. 2003;15:243–256. [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learning & memory. 2007;14(5):368–75. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine. 2010;72(3):239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Zurborg S, Hörster H, Fabel K, Kempermann G. Differential 24h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154(2):521–9. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Sternberg RJ. Increasing fluid intelligence is possible after all. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6791–2. doi: 10.1073/pnas.0803396105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. The Journal of neuroscience. 2001;21(5):1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Anderson ML, Shors TJ. Changing the rate and hippocampal dependence of trace eyeblink conditioning: slow learning enhances survival of new neurons. Neurobiology of learning and memory. 2011;95(2):159–65. doi: 10.1016/j.nlm.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. The European journal of neuroscience. 2008;27(11):3020–8. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm F, Keiner S, Kunze A, Witte OW, Redecker C. Effects of skilled forelimb training on hippocampal neurogenesis and spatial learning after focal cortical infarcts in the adult rat brain. Stroke. 2007;38(10):2833–40. doi: 10.1161/STROKEAHA.107.485524. [DOI] [PubMed] [Google Scholar]
- te Nijenhuis J, van Vianen AEM, van der Flier H. Score gains on g-loaded tests: No g. Intelligence. 2007;35(3):283–300. [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends in neurosciences. 2009;32(5):283–90. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


