Abstract
The olfactory system is a useful model for studying central nervous system recovery from damage due to its neuroplasticity. We recently developed a novel method of deafferentation by repeated exposure of Triton X-100 to the olfactory organ of adult zebrafish. This long-term, reversible method of deafferentation allows both degeneration and regeneration to be observed in the olfactory bulb. The aim of the present study is to examine olfactory bulb innervation, glomerular patterns, and olfactory-mediated behavior with repeated Triton X-100 treatment and the potential for recovery following cessation of treatment. Olfactory bulbs of control, chronic-treated, and recovery animals were examined for the presence or absence of glomeruli that have been identified in the zebrafish glomerular map. Following chronic treatment, the number of glomeruli was dramatically reduced; however, partial innervation remained in the lateral region of the bulb. When animals were given time to recover, complete glomerular distribution returned. A behavioral assay was developed to determine if innervation remaining correlated with behavior of the fish. Chronic-treated fish did not respond to odorants involved with social behavior but continued to react to odorants that mediate feeding behavior. Following recovery, responses to odorants involved with social behavior returned. The morphological and behavioral effects of chronic Triton X-100 treatment in the olfactory system suggest there may be differential susceptibility or resistance to external damage in a subset of sensory neurons. The results of this study demonstrate the remarkable regenerative ability of the olfactory system following extensive and long-term injury.
Keywords: Chemical lesion, Triton X-100, Anti-Keyhole Limpet Hemocyanin, Amino acid, Bile salts, Regeneration
1. Introduction
The olfactory system is unique among sensory systems due to its direct contact with the environment and continuous neuronal turnover. This provides the olfactory system with the remarkable capacity for recovery after injury to help maintain critical sensory function. Damage to the peripheral olfactory organ can result from a variety of factors including physical injury, chemicals found in the environment, and disease. In addition, neurons are added to the olfactory bulb, the part of the brain that responds to olfactory signals, throughout life [1–5]. This continuous degeneration and regeneration makes the olfactory system a good model for understanding the molecular mechanisms required for peripheral and central nervous system recovery.
To study the importance of sensory input to a central nervous system structure, various methods of deafferentation are commonly used. Cautery ablation of the olfactory organ in fish results in complete and permanent removal of afferent fibers [6, 7]. Following ablation, it takes several weeks before deafferentation effects occur in the olfactory bulb. These changes include decreased olfactory bulb volume, removal of olfactory sensory neuron (OSN) axons, and reduced sensory input, as indicated by decreased tyrosine hydroxylase-like immunoreactivity [6]. Chemical lesioning of the peripheral olfactory organ is another method of deafferentation used to examine degeneration and subsequent regeneration of the olfactory bulb. Exposure of the olfactory organ to chemicals such as copper [8, 9], methyl bromide [10], zinc sulfate [11–15], or the detergent Triton X-100 [16–20] destroys OSNs and their axons, which reduces olfactory bulb weight and activity. The Triton X-100 technique causes only temporary removal of afferent input and, when given time to recover, there is reconstitution of the olfactory epithelium and subsequent olfactory bulb reinnervation as axons regenerate.
Previous studies from our laboratory have shown that one day following Triton X-100 application to the zebrafish nasal cavity, the number of OSNs is dramatically reduced and other morphological abnormalities are observed in the olfactory epithelium [19]. However, 5 days after treatment these changes are reversed and there is restoration of olfactory organ morphology and neuronal population. This rapid regeneration does not allow significant morphological and functional changes to occur in the olfactory bulb, since it takes several weeks for deafferentation effects to be observed following the removal of afferent input. Therefore, to examine degeneration and regeneration in the olfactory bulb, we used a method of deafferentation involving repeated exposure of the olfactory organ to Triton X-100 for several weeks. Following treatment, there is severe morphological disruption of the olfactory organ, decreased neuronal labeling in the olfactory epithelium, a reduction in olfactory bulb volume, and decreased tyrosine hydroxylase-like immunoreactivity in the bulb [20]. This technique also allows for regeneration; when the animals are given a survival period following the cessation of treatment, the olfactory organ recovers and olfactory bulb volume and tyrosine hydroxylase-like immunoreactivity return [20].
Teleosts possess three morphologically distinct types of OSNs: ciliated OSNs with long dendrites and somata located deep in the epithelium, near the basal lamina; microvillous OSNs with intermediate-length dendrites of variable thickness and spindle-shaped cell bodies found in the middle of the olfactory epithelium; and crypt OSNs with egg-shaped somata located in the upper third of the epithelium and no apparent dendrites [21]. In the olfactory organ, each OSN type expresses a specific chemosensory receptor, projects to a distinct region of the olfactory bulb, and produces a different physiological and behavioral response. In zebrafish, microvillous OSNs respond physiologically and behaviorally to amino acids and nucleotides, which mediate their feeding behavior, while ciliated OSNs are stimulated by bile salts and pheromones, which mediate social and reproductive behavior [22–24]. Although the receptor molecules and specific function of crypt neurons is not known, it has been suggested in the crucian carp that these OSNs may have a role in reproductive behavior by responding to sex pheromones [25]. An interesting observation seen after repeated Triton X-100 treatment is a differential effect on OSN subtypes. Following treatment, very few OSNs with long dendrites were observed and the majority of neurons remaining had ovoid-shaped somata that lacked dendrites or spindle-shaped somata with intermediate-length dendrites [20].
Individual OSNs each express a single odorant receptor molecule and project their axons to very specific glomeruli in the olfactory bulb [26, 27]. The zebrafish olfactory bulb contains about 80 individual glomeruli, of which 22 have been identified and are highly stereotyped with similar morphology and distribution between animals, regardless of sex, and the rest residing in groupings such as the dorsal cluster, lateral chain, and anterior plexus [28]. In zebrafish, ciliated OSNs innervate glomeruli located in the dorsal and medial regions of the olfactory bulb while microvillous OSNs project to the lateral and ventrolateral regions of the bulb [23]. Physiological and behavioral studies have shown in several fish species, including zebrafish, the olfactory bulb is divided into functional zones with the medial regions processing social and reproductive behavior and the lateral regions processing feeding behavior [23, 29–33].
Repeated exposure of Triton X-100 to the olfactory organ is a useful technique for examining both degeneration and regeneration in the zebrafish olfactory system. This long-term, partial deafferentation method reduces afferent input to the olfactory bulb, without completely eliminating it, for an extended period of time. The current study further investigates the effects of detergent treatment and recovery by examining the glomerular distribution patterns in the olfactory bulb as well as the behavioral response to specific odorants. The hypothesis is that repeated Triton X-100 treatment will reduce innervation, alter glomerular patterns, and change olfactory-mediated behavior, but these effects will be reversed with cessation of treatment.
2. Materials and Methods
2.1. Glomerular Assay
2.1.1. Animals
Both male and female adult zebrafish, Danio rerio, aged 5–18 months and approximately 4–5 cm in length, were obtained from a commercial vendor and used for this study. Animals were fed flake food twice daily and maintained in aerated, conditioned freshwater tanks at 28.5°C. All experimental and animal care protocols were approved by the Institutional Animal Care and Use Committee.
2.1.2. Chemical lesioning
The olfactory organs of chronically treated fish were subjected to repeated chemical lesioning. Fish were anesthetized with tricaine methanesulfonate (0.03% MS222; Sigma) until they were no longer responsive to tail pinch then transferred to a clay dish. To prevent chemical contact with the contralateral olfactory organ, a thin strip of petroleum jelly was applied between the two nasal cavities. For detergent application, a Drummond wiretrol 50 μL micropipette (1.0 mm I.D.) was pulled with a WPI PUL-1 (World Precision Instruments) and the tip was broken to a diameter of about 30 μm. Using the pulled micropipette and wiretrol plunger, approximately 1 μL of a solution of 0.7% Triton X-100 and 0.005% methylene blue in 0.1M phosphate buffered saline (PBS) was applied to the right nasal cavity. The clay dish was put on ice for 2 minutes before the fish was returned to its housing tank to provide sufficient exposure to the detergent and ensure the fish remained anesthetized. The same procedure was repeated every 2–3 days for 3 weeks. The recovery fish were treated as described above but these animals were allowed 3 weeks of survival following the 3 weeks of repeated chemical lesioning. In both chronic and recovery groups, the left nasal cavity was not treated to serve as an internal control. Control fish did not receive any chemical exposure.
2.1.3. Tissue Processing
All fish were euthanized with 0.03% MS222, perfused transcardially with PBS, and fixed with 4% paraformaldehyde for 2 hours at room temperature or 24 hours at 4°C. Whole heads were dissected and decalcified with 0.5M ethylenediaminetetraacetic acid disodium salt dihydrate (pH 7.4, made soluble with sodium hydroxide pellets and heat) for 30 hours at 4°C. Heads were then dehydrated in an ascending ethanol series and xylenes, embedded in paraffin, and 10-μm semi-serial sections were mounted onto positively charged slides and left at 37°C overnight.
2.1.4. Immunocytochemistry
To identify OSN axonal projections in the olfactory bulb, an antibody to keyhole limpet hemocyanin (anti-KLH) was used. Mounted sections were dewaxed in xylenes and rehydrated in a descending ethanol series. Slides were subjected to antigen retrieval for 10 minutes in 10mM sodium citrate solution at 100°C. Slides were immersed for 1 hour at room temperature with 1% bovine serum albumin in PBS to block nonspecific binding and incubated overnight at 4°C with anti-KLH produced in rabbit (Sigma H0892; 1:1000) diluted in blocking buffer. Slides were then rinsed in PBS and incubated in biotinylated goat anti-rabbit antibody (Dako; 1:100) diluted in blocking solution for 1 hour at room temperature. Following buffer rinses, sections were treated with a fluorescent avidin (Alexafluor 488 avidin, 1:200, Molecular Probes) in PBS for 1 hour at room temperature, rinsed in PBS, coverslipped with glycerol and PBS (1:1), and viewed on a Nikon E600 microscope.
2.1.5. Analysis of Glomerular Patterns
The first group consisted of control fish (n=3) and was used to observe the staining patterns of anti-KLH. Glomerular distribution was determined by examining serial horizontal sections of the olfactory bulb and comparing the anti-KLH staining patterns to the zebrafish glomerular map [28]. Every section containing olfactory bulb was analyzed for the presence or absence of the 22 glomeruli that have been identified previously by the glomerular map. Glomeruli were scored as present (+), absent (−), or scattered but incomplete axons (s). The second group of fish (chronic) was treated repeatedly with Triton X-100 for 3 weeks (n=4) while the third group (recovery) was allowed 3 weeks of recovery following the 3 weeks of detergent treatment (n=4).
2.1.6. Retrograde Labeling with DiI
For OSN labeling in the olfactory epithelium, additional control animals (n=3) were euthanized and fixed in paraformaldehyde. Following fixation, the animals were pinned on a depression in a clay dish and the skull was carefully removed to expose the olfactory bulbs. A single crystal of 1,1′-dioctadocyl 3,3,3′,3′ tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes), was inserted into the lateral region of both olfactory bulbs using the tip of a pulled-glass wiretrol micropipette. Agar was placed on top of the brain to prevent unwanted diffusion of the dye. Fish were then returned to fixative and left at room temperature for 10–14 days to allow for diffusion. After incubation, olfactory organs were removed and whole mounts were viewed with a Zeiss LSM510 confocal microscope.
2.2. Behavioral Assay
2.2.1 Animals
To control for any sex-related differences in behavior that could confound analysis, only adult male zebrafish were used for the behavioral assay. Treatment groups for this part of the study included control, chronic, recovery, and anosmic animals. Control fish were left untreated, while chronic-treated and recovery animals were exposed to Triton X-100 as described in section 2.1.2.; however, both nasal cavities were treated with the detergent. Fish in the last group were rendered anosmic as follows: animals were lightly anesthetized then placed in a clay dish. Using a fine plastic tip, several layers of vetbond glue were applied to both nasal cavities. The glue hardened within minutes which occluded the nasal cavities and rendered the fish temporarily anosmic.
2.2.2. Behavioral Apparatus
A five-gallon white cylindrical bucket, filled to a depth of approximately 8 cm with water, was used as the experimental tank (Fig. 1). The bucket lid was mounted 1 meter above the top of the bucket with a small hole for the camera lens. A high-definition camera was secured to the mount and used to record zebrafish behavior. To minimize the effect of the experimenters’ presence, white cloth surrounded this 1 meter gap. Two surgical tubes, each approximately 1 meter in length, were mounted to the inside wall on opposite sides of the bucket. A syringe was inserted into the other end of each tube for odorant or water injection.
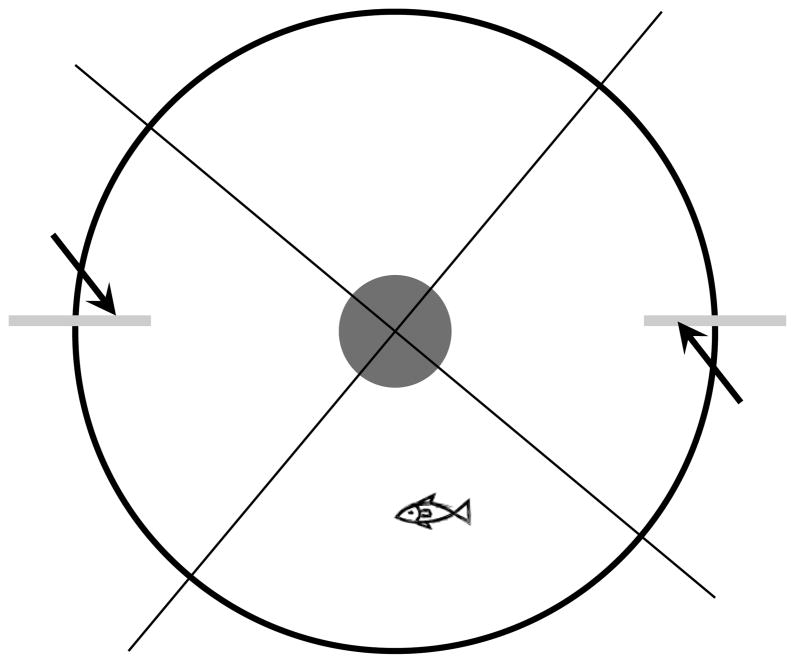
Figure 1.
Diagram of experimental apparatus for behavioral assay. The behavioral apparatus was constructed using a five-gallon white bucket with a camera mounted 1 meter above. The center of the bucket contained a drain so that water could be replaced easily after each session. Two surgical tubes (arrows) were attached to the inside wall of the bucket for odorant delivery. During trial, an odorant was delivered into one tube while water was simultaneously injected into the other tube. Four quadrants, relative to the location of the tubes, were drawn on the screen of the recorded video and used for analysis of behavior.
2.2.3. Experimental Groups
Six experimental groups were used for this study. Group 1 consisted of control fish that were exposed to an amino acid mixture (n=5). Group 2 fish (n=5) were also presented with the amino acid mixture, however, these animals were rendered anosmic. Group 3 animals had been chronically treated with Triton X-100 before being exposed to a mixture of amino acids (n=8). Fish in group 4 were control animals exposed to the bile salt taurocholic acid (TCA) (n=5). Group 5 animals were chronic-treated fish tested for their response to TCA (n=5), while group 6 was given a recovery period before being exposed to TCA (n=4).
2.2.4. Odorant Delivery
The amino acid mixture consisted of 5 amino acids: alanine, cysteine, histidine, methionine, and valine. A 10 mM stock solution of each amino acid and TCA was made and stored for up to 2 weeks at 4°C. Immediately prior to the experiment, each amino acid was diluted to 100 μM, then equal volumes of each amino acid were combined to make the mixture. TCA was diluted to the same concentration, 100 μM, before use.
Fish were deprived of food for 48 hours prior to the experiment. A single fish was transferred to the experimental tank at a time. After the fish was allowed at least 30 minutes to acclimate to the environment, 3 mL of either the amino acid mix or TCA was injected into one tube while 3 mL of water was delivered into the opposite tube. Whenever a stimulus was injected into one side of the tank, the odorant quadrant, the other side simultaneously received water to control for behavioral changes that could occur from mechanical stimulation. The side of the tank that received the odorant was altered between animals.
2.2.5. Quantitative Analysis of Behavior
Swimming behavior was recorded and analyzed by viewing the location of the animal at each second. Four quadrants were drawn on the video screen relative to the location of the surgical tubing (Fig. 1). The percentage of time spent in the odorant quadrant 30 seconds prior to odorant delivery was determined for each fish. After 30 seconds, the stimulant was injected and a 5 second delay was given to account for delivery time. The percentage of time spent in the odorant quadrant 30 seconds post-odorant addition was then calculated for each fish. For each experimental group, the raw data from each fish was averaged, and comparisons of pre- and post-trial behavior between groups were made using the two-factor ANOVA with the Bonferroni correction. P value < 0.05 was considered significant.
3. Results
3.1. Glomerular Innervation Following Chronic Detergent Treatment and Recovery
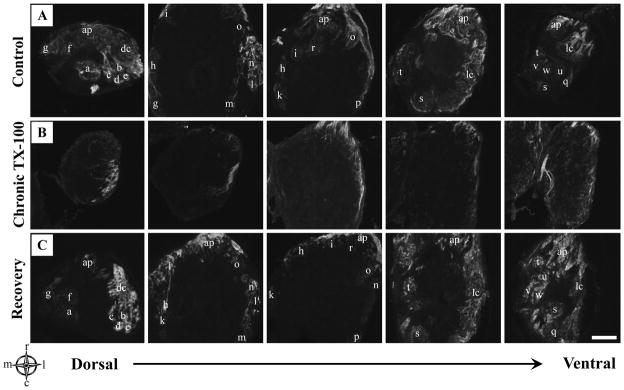
Glomerular distribution of OSN axons in the zebrafish olfactory bulb was initially observed in control animals using anti-KLH. KLH immunoreactivity has been observed reliably in the olfactory nerve and glomeruli in teleost fish [34–36]. Antibodies to KLH recognized an antigen on most or all of the olfactory axons in control zebrafish, and labeling within the olfactory bulb showed all 22 identifiable glomeruli were KLH-positive (Fig. 2A).
Figure 2.
Anti-KLH immunoreactivity in the olfactory bulb following chronic Triton X-100 treatment and recovery. A) Representative horizontal sections from the dorsal to the ventral regions of control olfactory bulbs stained with anti-KLH showed labeling of all identifiable glomeruli with each glomerulus letter-coded. B) Olfactory bulbs of animals following repeated detergent treatment had noticeably less anti-KLH labeling. The innervation that did remain was most obvious in the lateral regions while the medial region appeared to be affected the greatest. C) Following 3 weeks of recovery, KLH immunoreactivity seemed to return to control levels, with glomeruli present in all regions of the olfactory bulb. Glomeruli were letter-coded in control olfactory bulbs as follows: a=dcaG1 (dorsal-cluster-associated glomeruli), b=dcaG2, c=dcaG3, d=dcaG4, e=dcaG5, f=mdpG1 (mediodorsal posterior glomeruli), g=mdpG2, h=mdG (mediodorsal glomerulus), i=maG (medioanterior glomerulus), k=meG (medial elongated glomerulus), l=lcG1 (glomeruli of the lateral chain), m=lcG2, n=lcG3, o=lcG4, p=lcG5, q=lvpG (lateroventral posterior glomerulus), r=vaG (ventroanterior glomerulus), s=vpG (ventroposterior glomerulus), t=vmG (ventromedial glomerulus), u=vtG1 (glomeruli of the ventral triplet), v=vtG2, w=vtG3, dc=dorsal cluster glomeruli, ap=anterior plexus. For compass, m=medial, l=lateral, r=rostral, c=caudal. Scale bar = 100μm for all.
Chronic exposure to Triton X-100 resulted in markedly altered patterns of olfactory bulb innervation (Fig. 2B). Although there was some variation between animals in the extent of overall innervation remaining and specific glomeruli still receiving KLH-positive axons, there were several consistent trends observed. Anti-KLH labeling was dramatically decreased and many of the remaining axons did not form complete glomeruli while several others appeared fragmented. However, there was at least partial innervation of KLH-immunoreactive axons that remained in all animals. There were even a few complete glomeruli that were observed, with the typical spherical shape densely packed with axons. There also seemed to be regional selectively of the remaining glomeruli, or scattered axons, in the olfactory bulb of chronic animals. This was most apparent when comparing the medial and lateral regions, where more anti-KLH labeling was observed in the lateral region than the medial. Three weeks following cessation of treatment, KLH immunoreactivity and glomerular distribution appeared to return to normal with extensive staining in all regions of the olfactory bulb (Fig. 2C).
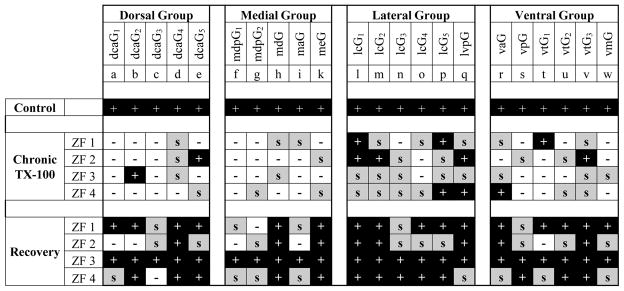
To investigate innervation patterns following chronic treatment and recovery more closely, the presence of individual glomeruli was determined based on the zebrafish glomerular map. Each glomerulus was scored as present (+), absent (−), or having incomplete or scattered (s) axons (Fig. 3). In chronic-treated animals, the medial and dorsal regions of the olfactory bulb appeared to be affected the most as several glomeruli located in these areas were absent. The ventral region also showed a decrease in the number of KLH-immunoreactive axons; however, several glomeruli were still present or possessed at least scattered axons. The most consistently and extensively innervated glomeruli were located in the lateral olfactory bulb. In all chronically treated animals examined, nearly every glomerulus in the lateral region was either present or maintained some axonal innervation. The 22 glomeruli that were examined do not account for the entire population of glomeruli in the olfactory bulb of zebrafish. There is a large dorsal cluster of glomeruli that have not been identified specifically but it is worthy to note that a few scattered axons in the dorsal cluster remained following repeated detergent treatment (Fig. 2B, Fig. 3). Another large cluster of glomeruli, termed the anterior plexus, makes up a significant portion of the anterior and medial olfactory bulb in control zebrafish. Although the individual glomeruli of the anterior plexus have not been identified, this cluster is extensive. The anterior plexus was severely affected by chronic detergent treatment and glomeruli typically located here, especially in the medial regions, were almost always absent (Fig. 2B). When animals were given a recovery period, all regions of the olfactory bulb had significant anti-KLH staining (Fig. 2C). Furthermore, glomerular distribution patterns returned to near control (Fig. 3). Only a few random glomeruli in different animals were absent, but they were not localized in any specific region. The majority of glomeruli were present following recovery; however, some glomerular regions clearly showed KLH-positive axons but were not the typical glomerular morphology. Although these glomeruli had anti-KLH axonal innervation, these glomeruli were still scored as (s) due to their incomplete nature.
Figure 3.
Glomerular distribution following chronic Triton X-100 treatment and recovery. Using the zebrafish glomerular map to identify individual glomeruli, each glomerulus was designated as present (+), absent (−), or scattered, but incomplete (s) in 4 zebrafish from each group. In controls, all 22 glomeruli were observed. Chronic treated fish showed a dramatic decrease in glomerular innervation. Glomeruli located in the medial and dorsal regions were lacking more glomeruli than the other regions while the lateral region maintained the most innervation. Although the degree of innervation and recovery varied slightly between fish, following cessation of treatment, the majority of glomeruli were again observed in all regions of the olfactory bulb.
3.2. Retrograde Labeling with DiI
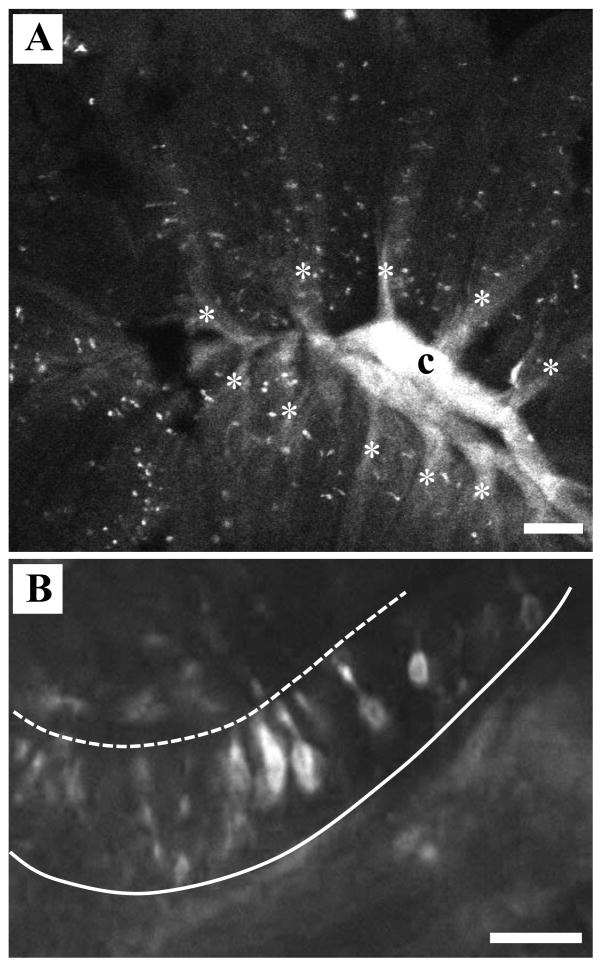
Following chronic Triton X-100 exposure, the olfactory bulb maintained KLH-immunoreactive axons localized predominantly in the lateral region. The peripheral olfactory organ of zebrafish is a multi-lamellar rosette that has a three-dimensional shape of a cup. A prediction could be made that, following detergent exposure, the regional selectively seen in the olfactory bulb is due to Triton X-100 pooling near the central raphe resulting in the removal of a specific subset of OSNs located deep in the olfactory epithelium. We tested this by inserting a small crystal of the lipophilic retrograde marker DiI into the lateral region of the olfactory bulb where axons typically remained. DiI-labeled OSNs were seen randomly distributed throughout the sensory region of the olfactory rosette (Fig. 4A). Since the laterally projecting sensory neurons are not from a particular region of the olfactory rosette, this indicates that the lateral innervation that persists in chronic Triton X-100-treated olfactory bulbs likely arises from sensory neurons found throughout the olfactory epithelium and is not likely to be a function of the detergent application procedure. Higher magnification analysis of DiI-labeled rosettes allowed examination of the morphology of the OSNs that project to the lateral olfactory bulb. The majority of these labeled neurons possessed medium length dendrites with cell bodies located in the middle of the olfactory epithelium (Fig. 4B). This morphology is typical of microvillous OSNs.
Figure 4.
DiI labeling of OSNs from lateral olfactory bulb. A) When the lipophilic tracer DiI was inserted into the lateral region of the olfactory bulb, the retrogradely labeled axons coalesced in the central raphe (c). Labeled profiles representing olfactory sensory neurons were seen randomly distributed throughout the sensory region of the olfactory rosette along the lamellae (*). Scale bar=100 μm. B) Higher magnification analysis of DiI-labeled rosettes allowed examination of the morphology of the olfactory sensory neurons that project to the lateral olfactory bulb. The majority of OSNs observed in the olfactory epithelium by retrograde labeling had cell bodies in the middle of the epithelium and intermediate-length dendrites. Dashed line=apical surface, solid line=basement membrane. Scale bar=20 μm.
3.3. Behavioral Response to Amino Acids in Control Animals and Following Repeated Detergent Treatment
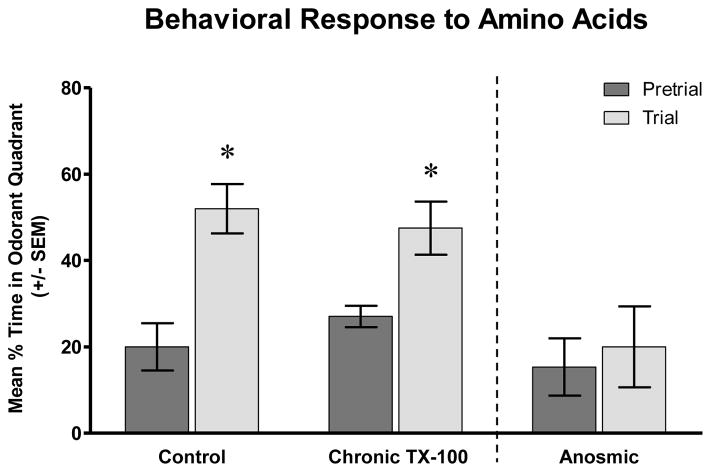
Pre-stimulatory behavior for all animals tested showed the swimming behavior in the experimental tank was random, and there was no preference for any particular quadrant (Fig. 5,6). Control fish tested for their response to amino acids spent approximately 20% ± 5.5% of the time swimming in the odorant quadrant before any stimulus was added. Following addition of the amino acid mixture, animals showed obvious appetitive behavior. They swam more aggressively, which was indicated by quicker movements and more turns, and they tested the water by swimming to the surface. The animals showed increased interest in the area of the testing tank where the amino acid mix was delivered with the average amount of time spent in the odorant quadrant increased significantly to 52% ± 5.7% (P<0.01) (Fig. 5).
Figure 5.
Behavioral response to amino acids following chronic Triton X-100 treatment. Prior to amino acid addition, all three groups spent about the same amount of time in each quadrant of the test tank, showing no preference for any particular quadrant. However, when an amino acid mixture was added to the tank, control fish spent significantly more time in the quadrant where the odorant was added. Chronic Triton X-100 treated fish showed similar behavior as control animals, with increased interest in the quadrant where the amino acid mixture was injected. In contrast, fish that had been rendered temporarily anosmic showed no quadrant preference or changes in behavior after the amino acid mixture was added to the tank. * = p<0.05.
Figure 6.
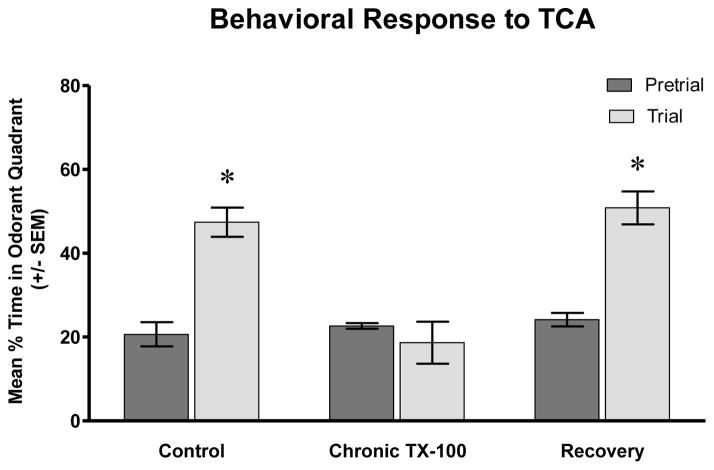
Behavioral response to TCA following chronic Triton X-100 treatment and recovery. Prior to the addition of an odorant, control fish spent approximately the same amount of time in all quadrants of the test tank, including the odorant quadrant. After the addition of the bile salt, TCA, control fish showed a significantly increased interest in the quadrant where the odorant was added. The pre-stimulant behavior of chronic detergent-treated animals was similar to control animals, with fish spending about the same amount of time in all quadrants. After TCA was added to the tank, these fish did not exhibit increased interest or show preference for the odorant quadrant and continued swimming around the tank as they had prior to TCA addition. However, when given a recovery period, the amount of time spent in the odorant quadrant after TCA injection increased significantly and behavior appeared similar to controls. * = p<0.05.
Chronic-treated animals that were exposed to amino acids showed similar behavior to control fish. Prior to the addition of the stimulus, the fish swam in the odorant quadrant about 27% ± 2.5% of the time. These animals responded to the presence of amino acids by swimming to the location of the stimulus and displaying the same appetitive behavior as control fish. The average amount of time spent in the odorant quadrant of chronic detergent treated fish increased significantly to 48% ± 6.2% following the addition of amino acids (P<0.01) (Fig. 5).
3.4. Behavioral Response of Anosmic Fish to Amino Acids
To determine whether the attractive response to amino acids was indeed based on olfaction, zebrafish were rendered temporarily anosmic by bilateral occlusion with vetbond glue. These fish showed no excitatory behavior or changes in swimming patterns when presented with an odor stimulant. These fish, on average, were observed in the odorant quadrant 15% ± 6.6% of the time pre-stimulation versus 20% ± 9.4% after odorant delivery (P>0.05) (Fig. 5). In fact, the general behavior of anosmic fish was noticeably different than control fish. Instead of intensely examining their environment by swimming throughout the experiment tank, a majority of these fish were less active overall and remained stationary during the testing period.
3.5. Behavioral Response to Taurocholic Acid Following Repeated Detergent Treatment and Recovery
We next examined the response of control, chronic-treated, and recovery fish to the bile salt, taurocholic acid (TCA). TCA is a potent olfactory stimulant for fish, both physiologically and behaviorally [30, 37–39]. Prior to the addition of the chemical stimulus, control fish were observed in the odorant quadrant 21% ± 2.9% of the time (Fig. 6). When TCA was introduced, animals showed a preference for that area of the tank, spending 47% ± 3.5% of the time in the odorant quadrant (P<0.001). Fish made more turns, surfaced more often, and spent a larger percentage of the time in the odorant quadrant than before the stimulus was added. One observation regarding the behavioral response to TCA that was different than the response to amino acids was that, in a few of the fish tested, immediately after the addition of TCA, they would become stationary for a few seconds before swimming in the direction of the chemical.
In chronic-treated animals, the response to TCA was much different than control. Pre-stimulatory behavior was consistent with the other animals tested, spending 23% ± 0.6% of the time in the odorant quadrant. However, after TCA was added, treated fish showed no behavioral response to the chemical and continued swimming around the tank as they had before the bile salt was added. The average amount of time spent in the odorant quadrant post-stimulus addition was 19% ± 5.0%, which was not significantly different than the pre-trial time (P>0.05) (Fig. 6). When animals were given a recovery period following chronic Triton X-100 treatment, the behavioral response to TCA appeared similar to control. Percent of time in the odorant quadrant pre-trial was 24% ± 1.6% and increased significantly to 51% ± 3.9% after the odorant was added (P<0.001) (Fig 6).
4. Discussion
The results of this study show that repeated exposure of the peripheral olfactory organ of zebrafish to the detergent Triton X-100 causes severe morphological, functional, and behavioral changes. The behavioral assay showed that chronic Triton X-100 treated fish are still capable of responding to amino acids but are not responsive to bile salts. These results are consistent with the OSN types that were observed in our previous study following chronic treatment. We previously showed that, in the olfactory epithelium, microvillous OSNs, which detect amino acids, remained following treatment while ciliated OSNs, which detect bile salts, were absent [20]. In the olfactory bulb, the lateral regions mediate feeding behavior in response to amino acid exposure. In the current study, we show that following repeated detergent treatment this area consistently maintained innervation, although it was reduced. In contrast, glomeruli in the medial and dorsal regions of the bulb, which mediate social and reproductive behavior in response to bile salts and pheromones, were largely eliminated after chronic treatment.
The DiI experiment confirmed that the selective response to detergent treatment was not due to the technique used, since the OSNs that project to the spared region of the olfactory bulb were seen distributed throughout the rosette in control fish. It has been shown in zebrafish that OSNs projecting to a single glomerulus are found evenly distributed within the sensory region of the rosette, without the topographical arrangement that is seen in other sensory systems [40]. Furthermore, the OSNs that project to the lateral olfactory bulb possess intermediate-length dendrites and spindle-shaped cell bodies, which is typical of microvillous OSNs. This confirms other studies of OSN type and projections in the fish, with microvillous OSNs projecting to the lateral region of the olfactory bulb and ciliated OSNs projecting to the medial and dorsal regions of the olfactory bulb [23,27,36].
One of the most impressive results from this study is the remarkable capacity for recovery. Three weeks following cessation of detergent treatment, olfactory organ morphology and neuronal population return to near control in the olfactory epithelium [20]. In the olfactory bulb, there is also recovery of bulb volume and morphology [20]. We show here that glomerular distribution in the olfactory bulb also recovers when given a survival period after repeated olfactory organ damage. Furthermore, the animals’ ability to detect TCA returns, which indicates recovery of olfactory-mediated behavior. These results demonstrate that even when the olfactory system is subjected to long-term, extensive damage, it is capable of morphological, functional, and behavioral recovery.
Other electrophysiological and behavioral studies have shown the remarkable ability for sustained food-mediated activity following deafferentation. When goldfish are exposed to copper, electroolfactogram responses show differences in the sensitivity of socially and reproductively important stimuli and food-related stimuli. Steroids, prostaglandins, and bile acids are affected the most, while amino acid sensitivity is less affected by copper exposure [41]. In axotomized goldfish, electroolfactogram recordings with various amino acids before and after olfactory nerve transection do not change, suggesting functional activity in the remaining OSNs [42]. Behaviorally, these animals were able to respond to food odors after two weeks, but detection of pheromones required twice the amount of recovery time [43]. In the rat, methyl bromide exposure destroys over 95% of OSNs with only sparse glomerular innervation remaining [44]. However, even with this mass destruction, animals are able to detect odor stimuli and exhibit food-finding behavior after only 3 days. In fact, one animal became more sensitive to the odorant following lesioning. Similarly, when rats are exposed to zinc sulfate, they continue to display essentially normal olfactory behavior [45]. Zinc sulfate appears to be more detrimental to mice than rats as normal food-finding behavior occurs 6 weeks after treatment, with less than 10% of functioning receptor cells remaining [46]. In a similar study, mice were not capable of odor detection or discrimination for 5–30 days following zinc sulfate nasal irrigation [47]. Thus, these studies suggest that animals are capable of detecting and locating food even with minimal innervation remaining.
Differences in degeneration and subsequent regeneration of the olfactory epithelium and olfactory bulb have been observed in previous reports following olfactory nerve transection in fish. In the trout, ciliated OSNs are removed more rapidly than microvillous OSNs in the degenerating epithelium; they also reappear sooner in the regenerating epithelium [48]. Another study in the trout showed almost all KLH-immunoreactive axons are removed from the olfactory bulb following axotomy, except for a few fibers in the dorsal and ventral-medial fields [34]. When these animals are given longer survival periods, the reinnervating fibers do not regenerate by proceeding anteriorly to posteriorly but instead axons in the dorsal and lateral regions return first, followed by the reinnervation of other remote sites, including the posterior areas of the bulb. Following nerve transection in the goldfish, ciliated OSNs remain at all times post-axotomy while the number of microvillous OSNs decreases dramatically and are completely eliminated after 7 days [42]. In contrast, when goldfish are exposed to copper, ciliated OSNs disappear and recover after 2 weeks, while microvillous OSNs appear unaffected at all times following treatment [41]. Differential glomerular innervation patterns have also been observed in rodents subjected to various methods of deafferentation. Following methyl bromide inhalation, the olfactory bulb maintains some innervation in the ventral and posterior regions [10, 44, 49]. Regional variations in axonal innervation have also been observed in the olfactory bulbs of mice following naris closure. Seven days post-closure, TH immunoreactivity was markedly diminished. This decrease is most prominent in the medial region of the olfactory bulb [50].
The peripheral olfactory organ is in direct contact with the environment and is constantly exposed to chemicals and other harmful substances. As a result, the olfactory epithelium has developed several defense mechanisms including rapid neuronal regeneration and detoxification of the immediate environment. In the rainbow trout, a family of detoxifying enzymes, glutathione S-transferase, is found in OSN dendrites and cell bodies [51]. These enzymes detoxify electrophilic substances and also participate in reactions that transform lipophilic xenobiotic compounds so they are more water soluble and available for further metabolism [52]. In the mouse main olfactory epithelium, a population of nonneuronal microvillous cells are capable of synthesizing and releasing acetylcholine which has been shown to modulate the activities of supporting cells and OSNs differently due to different receptor subtypes on these cells [53]. These nonneuronal microvillous cells appear to have nonspecific chemoreactivity and are activated in response to a variety of external chemicals and thermal stimuli. The functional consequences of acetylcholine-mediated effects are not known. However, it is hypothesized that supporting cell activation may modulate OSN activity and serve as a protective function [53]. Other cell-type specific features that have been identified and may aid in olfactory protection include proteins, called epsins, that are found in the dendrites of microvillous OSNs in the mouse vomeronasal organ [54]. These proteins are resistant to increased calcium concentrations and also trigger protective reflexes in the presence of chemical stimuli. Furthermore, a glycocalyx, which acts as a barrier between the plasma cell membrane and the external environment, has been identified on the cilia of OSNs in fish and salamanders [55, 56]. Any or all of these mechanisms could be involved in the neuroprotection of specific OSNs we observed after detergent treatment.
The results of the current study raise several intriguing questions about the defense mechanisms and regenerative abilities of the olfactory system. Although OSNs are continuously replaced, the olfactory epithelium is in direct contact with the external environment, making defense mechanisms necessary. The apparent sensitivity and resistance of specific OSNs to chemical exposure does, however, make evolutionary sense. In fish, olfaction is critical for locating food and it would seem plausible that OSNs mediating reproductive and social behavior may be more sensitive while OSNs that are required for food searching behavior are more resistant. This hypothesis is reinforced in the olfactory bulb, where the region that responds to amino acids and nucleotides, which are feeding cues for fish, maintains the most significant amount of innervation following chronic detergent treatment. Although this has not been specifically examined, there are several possible mechanisms that could allow this. It could be that certain OSN types are inherently more protected by a glycocalyx or may have receptors that can be activated by the release of ligands from supporting cells to produce some protective function when a harmful substance is detected. Another possibility is that specific OSN populations have a greater number of immature neurons that are ready to replace damaged OSNs immediately after elimination. Different OSN subtypes may also have faster turnover and axonal growth rates.
Few studies have examined and compared both the morphological and behavioral effects of deafferentation in the olfactory system. We have shown that repeated damage to the olfactory organ of zebrafish results in a decreased number of glomeruli and altered glomerular patterns as well as changes in olfactory-mediated behavior. Using this long-term partial deafferentation method, when animals are given a survival period after treatment, there also appears to be regeneration and recovery of glomerular organization and return of normal behavior. Our work shows the remarkable regenerative ability of the olfactory system and will allow further investigation into the adult brain’s ability to recover following injury or neurological disease.
Highlights.
Chronic intranasal detergent exposure causes reversible olfactory deafferentation.
Medial glomeruli are severely affected, but lateral glomeruli retain innervation.
Behavioral preference for bile salts is lost, but amino acid detection remains.
Glomerular distribution in the olfactory bulb returns with olfactory organ recovery.
Olfactory-mediated response to bile salts returns with glomerular reinnervation.
Acknowledgments
This project was supported by Western Michigan University FRACAA Award #09-019 and NIH-NIDCD #011137. We are grateful for the technical assistance of Evan White.
Abbreviations
- OSN
olfactory sensory neuron
- MS222
tricaine methanesulfonate
- KLH
keyhole limpet hemocyanin
- PBS
phosphate-buffered saline
- DiI
1,1′-dioctadocyl 3,3,3′,3′ tetramethylindocarbocyanine perchlorate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Taylor R. Paskin, Email: taylor.r.paskin@wmich.edu.
Christine A. Byrd-Jacobs, Email: christine.byrd@wmich.edu.
References
- 1.Altman J. Autoradiographic and histological studies of postnatal neurogenesis IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137(4):433–57. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 2.Thornhill RA. Cell division in the olfactory epithelium of the lamprey, Lampetra fluviatilis. Z. Zellforsch. 1970;109:147–57. doi: 10.1007/BF00365237. [DOI] [PubMed] [Google Scholar]
- 3.Monti Graziadei GA, Graziadei PPC. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- 4.Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec (New Anat) 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- 5.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 6.Byrd CA. Deafferentation-induced changes in the olfactory bulb of adult zebrafish. Brain Res. 2000;866:92–100. doi: 10.1016/s0006-8993(00)02252-6. [DOI] [PubMed] [Google Scholar]
- 7.Poling KR, Brunjes PC. Sensory deafferentation and olfactory bulb morphology in the zebrafish and related species. Brain Res. 2000;856:135–41. doi: 10.1016/s0006-8993(99)02412-9. [DOI] [PubMed] [Google Scholar]
- 8.Moran DT, Rowley JC, Aiken G. Trout olfactory receptors degenerate in response to water-borne ions – a potential bioassay for environmental neurotoxicology. Ann NY Acad Sci. 1987;510:509–11. [Google Scholar]
- 9.Bettini S, Ciani F, Franceschini V. Recovery of the olfactory receptor neurons in the African Tilapia mariae following exposure to low copper level. Aquat Toxicol. 2006;76:321–8. doi: 10.1016/j.aquatox.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- 11.Margolis FL, Roberts N, Ferriero D, Feldman J. Denervation in the primary olfactory pathway of mice: biochemical and morphological effects. Brain Res. 1974;81:469–83. doi: 10.1016/0006-8993(74)90844-0. [DOI] [PubMed] [Google Scholar]
- 12.Matulionis DH. Ultrastructural study of mouse olfactory epithelium following destruction by ZnSO4 and its subsequent regeneration. Am J Anat. 1975;142(1):67–89. doi: 10.1002/aja.1001420106. [DOI] [PubMed] [Google Scholar]
- 13.Cancalon P. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell. 1982;14(4):717–33. doi: 10.1016/0040-8166(82)90061-1. [DOI] [PubMed] [Google Scholar]
- 14.Burd GD. Morphological study of the effects of intranasal zinc sulfate irrigation on the mouse olfactory epithelium and olfactory bulb. Microsc Res Tech. 1993;24:195–213. doi: 10.1002/jemt.1070240302. [DOI] [PubMed] [Google Scholar]
- 15.Williams SK, Gilbey T, Barnett SC. Immunohistochemical studies of the cellular changes in the peripheral olfactory system after zinc sulfate nasal irrigation. Neurochem Res. 2004;29(5):891–901. doi: 10.1023/b:nere.0000021234.46315.34. [DOI] [PubMed] [Google Scholar]
- 16.Kawano T, Margolis FL. Transsynaptic regulation of olfactory bulb catecholamines in mice and rats. J Neurochem. 1982;39(2):342–8. doi: 10.1111/j.1471-4159.1982.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 17.Cancalon P. Influence of a detergent on the catfish olfactory mucosa. Tissue Cell. 1983;15(2):245–58. doi: 10.1016/0040-8166(83)90020-4. [DOI] [PubMed] [Google Scholar]
- 18.Cummings DM, Emge DK, Small SL, Margolis FL. Pattern of olfactory bulb innervation returns after recovery from reversible peripheral deafferentation. J Comp Neurol. 2000;421:362–73. [PubMed] [Google Scholar]
- 19.Iqbal T, Byrd-Jacobs CA. Rapid degeneration and regeneration of the zebrafish olfactory epithelium after Triton X-100 application. Chem Senses. 2010;35:351–61. doi: 10.1093/chemse/bjq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paskin TR, Iqbal TR, Byrd-Jacobs CA. Olfactory bulb recovery following reversible deafferentation with repeated detergent application in the adult zebrafish. Neuroscience. 2011;196:276–84. doi: 10.1016/j.neuroscience.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Hansen A, Zielinski BS. Diversity in the olfactory epithelium of bony fishes: development, lamellar arrangement, sensory neuron cell types and transduction components. J Neurocytol. 2005;34:183–208. doi: 10.1007/s11068-005-8353-1. [DOI] [PubMed] [Google Scholar]
- 22.Lipschitz DL, Michel WC. Amino acid odorants stimulate microvillar sensory neurons. Chem Senses. 2002;27:277–86. doi: 10.1093/chemse/27.3.277. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25(20):4889–97. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koide T, Miyasaka N, Morimoto K, Asakawa K, Urasaki A, Kawakami K, Yoshihara Y. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Natl Acad Sci USA. 2009;106(24):9884–9. doi: 10.1073/pnas.0900470106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamdani EH, Lastein S, Gregersen F, Doving KB. Seasonal variations in olfactory sensory neurons-fish sensitivity to sex pheromones explained? Chem Senses. 2008;33:119–23. doi: 10.1093/chemse/bjm072. [DOI] [PubMed] [Google Scholar]
- 26.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–86. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Miyasaka N, Yoshihara Y. Hierarchical regulation of odorant receptor gene choice and subsequent axonal projection of olfactory sensory neurons in zebrafish. J Neurosci. 2007;27(7):1606–15. doi: 10.1523/JNEUROSCI.4218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baier H, Korsching S. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci. 1994;14(1):219–30. doi: 10.1523/JNEUROSCI.14-01-00219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Døving KB, Selset R, Thommesen G. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiol Scand. 1980;108:123–31. doi: 10.1111/j.1748-1716.1980.tb06509.x. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen PW, Hara TJ, Stacey NE. Sex pheromones selectively stimulate the medial olfactory tracts of male goldfish. Brain Res. 1991;558:343–7. doi: 10.1016/0006-8993(91)90790-3. [DOI] [PubMed] [Google Scholar]
- 31.Hamdani EH, Alexander G, Døving KB. Projection of sensory neurons with microvilli to the lateral olfactory tract indicates their participation in feeding behaviour in crucian carp. Chem Senses. 2001;26:1139–44. doi: 10.1093/chemse/26.9.1139. [DOI] [PubMed] [Google Scholar]
- 32.Nikonov AA, Caprio J. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J Neurophysiol. 2001;86:1869–76. doi: 10.1152/jn.2001.86.4.1869. [DOI] [PubMed] [Google Scholar]
- 33.Hamdani EH, Doving KB. The alarm reaction in crucian carp is mediated by olfactory neurons with long dendrites. Chem Senses. 2002;27:395–8. doi: 10.1093/chemse/27.4.395. [DOI] [PubMed] [Google Scholar]
- 34.Riddle DR, Oakley B. Immunocytochemical identification of primary olfactory afferents in rainbow trout. J Comp Neurol. 1992;324:575–89. doi: 10.1002/cne.903240410. [DOI] [PubMed] [Google Scholar]
- 35.Fuller CL, Yettaw HK, Byrd CA. Mitral cells in the olfactory bulb of adult zebrafish (Danio rerio): morphology and distribution. J Comp Neurol. 2006;499:218–30. doi: 10.1002/cne.21091. [DOI] [PubMed] [Google Scholar]
- 36.Gayoso JA, Castro A, Anadón R, Manso MJ. Differential bulbar and extrabulbar projections of diverse olfactory receptor neuron populations in the adult zebrafish (Danio rerio) J Comp Neurol. 2011;519:247–76. doi: 10.1002/cne.22518. [DOI] [PubMed] [Google Scholar]
- 37.Michel WC, Lubomudrov LM. Specificity and sensitivity of the olfactory organ of the zebrafish, Danio rerio. J Comp Physiol A. 1995;177:191–9. doi: 10.1007/BF00225098. [DOI] [PubMed] [Google Scholar]
- 38.Vitebsky A, Reyes R, Sanderson MJ, Michel WC, Whitlock KE. Isolation and characterization of the laure olfactory behavioral mutant in the zebrafish, Danio rerio. Dev Dynam. 2005;234:229–42. doi: 10.1002/dvdy.20530. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Hara TJ. Lake char (Salvelinus namaycush) olfactory neurons are highly sensitive and specific to bile acids. J Comp Physiol A. 2009;195:203–15. doi: 10.1007/s00359-008-0399-y. [DOI] [PubMed] [Google Scholar]
- 40.Baier H, Rotter S, Korsching S. Connectional topography in the zebrafish olfactory system: Random positions but regular spacing of sensory neurons projecting to an individual glomerulus. Proc Natl Acad Sci USA. 1994;91:11646–50. doi: 10.1073/pnas.91.24.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolmakov NN, Hubbard PC, Lopes O, Canario AVM. Effect of acute copper sulfate exposure on olfactory responses to amino acids and pheromones in goldfish (Carassius auratus) Environ Sci Technol. 2009;43:8393–9. doi: 10.1021/es901166m. [DOI] [PubMed] [Google Scholar]
- 42.Zippel HP, Lago-Schaaf T, Caprio J. Ciliated olfactory receptor neurons in goldfish (Carassius auratus) partially survive nerve axotomy, rapidly regenerate and respond to amino acids. J Comp Physiol A. 1993;173:537–47. [Google Scholar]
- 43.Zippel HP, Sorensen PW, Hansen A. High correlation between microvillous olfactory receptor cell abundance and sensitivity to pheromones in olfactory nerve-sectioned goldfish. J Comp Physiol A. 1997;180(1):39–52. [Google Scholar]
- 44.Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiol Behav. 1997;62(6):1241–52. doi: 10.1016/s0031-9384(97)00301-6. [DOI] [PubMed] [Google Scholar]
- 45.Slotnick B, Glover P, Bodyak N. Does intranasal application of zinc sulfate produce anosmia in the rat? Behav Neurosci. 2000;114(4):814–29. [PubMed] [Google Scholar]
- 46.Harding JW, Getchell TV, Margolis FL. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978;140:271–85. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- 47.McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem Senses. 2003;28:659–70. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- 48.Zielinski BS, Hara TJ. Ciliated and microvillar receptor cells degenerate and then differentiate in the olfactory epithelium of rainbow trout following olfactory nerve section. Microsc Res Tech. 1992;23:22–7. doi: 10.1002/jemt.1070230103. [DOI] [PubMed] [Google Scholar]
- 49.Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: timing and extent of reinnervation. J Comp Neurol. 1999;412:439–57. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 50.Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in the mouse olfactory bulb. Brain Res. 1993;614:109–16. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- 51.Starcevic SL, Muruganandam A, Mutus B, Zielinski BS. Glutathione in the olfactory mucosa of rainbow trout (Oncorhyncus mykiss) Chem Senses. 1993;18:57–65. [Google Scholar]
- 52.Mannervik B, Danielson UH, Ketterer B. Glutathione transferases-structure and catalytic activity. Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- 53.Ogura T, Szebenyi SA, Krosnowski K, Sathyanesan A, Jackson J, Lin W. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol. 2011;106:1274–87. doi: 10.1152/jn.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekerkova G, Zheng L, Loomis PA, Changyaleket B, Whitlon DS, Mugnaini E, Bartles JR. Espins are multifunctional actin cytoskeletal regulatory proteins in the microvilli of chemosensory and mechanosensory cells. J Neurosci. 2004;24(23):5445–56. doi: 10.1523/JNEUROSCI.1279-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinnikov YA. Glycocalyx of receptor cell membranes. Chem Senses. 1986;11:243–57. [Google Scholar]
- 56.Foster JD, Getchell ML, Getchell TV. Ultrastructural localization of sialylated glycoconjugates in cells of the salamander olfactory mucosa using lectin cytochemistry. Cell Tissue Res. 1992;267:113–24. doi: 10.1007/BF00318697. [DOI] [PubMed] [Google Scholar]