Abstract
Attentional impairments are found in a range of neurodegenerative and neuropsychiatric disorders. However, the development of procognitive enhancers to alleviate these impairments has been hindered by a lack of comprehensive hypotheses regarding the circuitry mediating the targeted attentional functions. Here we discuss the role of the cortical cholinergic system in mediating cue detection and attentional control and propose two target mechanisms for cognition enhancers: stimulation of prefrontal α4β2* nicotinic acetylcholine receptors (nAChR) for the enhancement of cue detection and augmentation of tonic acetylcholine levels for the enhancement of attentional control.
Keywords: actelycholine, cognition enhancers, attentional control, cue detection
1. Introduction
Attentional functions are often parsed into “bottom-up” processes, including processes associated with the detection and processing of relevant cues, and “top-down” processes associated with selecting relevant inputs, ignoring irrelevant inputs, and maintaining the appropriate task set over time in the face of competing internal or external demands (Kastner & Ungerleider, 2000; Sarter et al., 2001; Sarter et al., 2006; Treisman & Gelade, 1980). Converging evidence from numerous animal model studies, including lesion and pharmacological manipulations, and human psychopharmacological and pharmaco-fMRI studies demonstrates the importance and necessity of the cortical cholinergic input system for attention (e.g., Robbins et al., 1989; Muir et al., 1994; McGaughy et al., 1996; Sarter et al., 2005; Giocomo & Hasselmo, 2007; Hahn et al., 2007; Deco & Thiele, 2009; Hasselmo & Sarter, 2011). The cortical cholinergic system exerts its effects on attention via sensory, prefrontal, and parietal regions and interactions among those regions (e.g., Sato et al., 1987; Golmayo et al., 2003; Nelson et al., 2005). Prefrontal cortex, particularly right prefrontal cortex, is a key site for cortical cholinergic mediation of both cue detection and top-down attentional control processes, including the enhancement of control in response to challenging or distracting conditions (e.g., Parikh et al., 2007; Gill et al., 2000; Sarter et al., 2006; see also Cabeza & Nyberg, 2000 for a review of neuroimaging studies of attention supporting the involvement of a right-lateralized frontoparietal network, particularly for selective and sustained attention).
Given the cortical cholinergic system’s importance in mediating attentional functions, this system is a frequent target of drug development programs aimed at improving cognition. Here, we propose two target mechanisms for cognition enhancers, cue detection and attentional control. To this end, we describe these attentional functions in healthy individuals and impairments in these functions in patient populations, review the literature on the use of acetylcholinesterase inhibitors and agonists at muscarinic acetylcholine receptors (mAChRs) and nicotinic acetylcholine receptors (nAChRs) to enhance cognition and attention, and detail recent data indicating tonic cholinergic activity mediates top-down attentional control functions while phasic cholinergic activity mediates cue detection. Collectively, the available evidence and literature supports the use of cholinergic drugs, particularly agonists targeting α4β2* nAChRs, for enhancing attentional and cognitive functions and capacities.
2. Cue detection and attentional control in healthy and patient populations
A critical aspect of attention is the ability to detect relevant environmental cues and discriminate these cues from noise. Detection is defined as a cognitive process which consists of “…the entry of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal by an arbitrary response indicated by the experimenter” (Posner et al., 1980). Behaviorally relevant stimuli, particularly unexpected or salient stimuli, are detected by the ventral frontoparietal network, including regions in right inferior frontal and temporoparietal cortex (see review by Corbetta & Shulman, 2002). This ventral, stimulus-driven network directs attention to salient environmental events and interacts with the dorsal frontoparietal attention network, which consists of regions in superior frontal cortex and intraparietal cortex.
In addition to being influenced by the detection of cues, the dorsal attention network is involved in the top-down control of attention. Top-down attentional control processes modulate the detection and processing of cues on the basis of goals and prior knowledge of or experience with relevant stimuli, and allowing the enhancement of behaviorally-relevant cues and the suppression of irrelevant stimuli (e.g., Serences et al., 2005; O’Connor et al., 2002; for review, see Corbetta & Shulman, 2002; Pessoa et al., 2003). Attentional control processes are also engaged when attention is challenged, say by the presentation of distractors, fatigue, changing target stimulus characteristics or presentation parameters, or other detrimental events, in order to increase motivated participants’ attentional effort and stabilize or maintain attentional performance in the face of the challenging conditions (Sarter et al., 2006; Sarter & Paolone, 2011).
Disruption of cholinergic transmission and associated impairments in attention are seen in a variety of neurodegenerative and neuropsychiatric disorders, including age-related cognitive declines, mild cognitive impairment, Alzheimer’s, attention deficit hyperactivity disorder (ADHD) and schizophrenia. In schizophrenia, for example, research has consistently demonstrated impairments in attention and attentional control (Heinrichs & Zakzanis, 1998; Nuechterlein et al., 2004) that persist across both periods of psychosis and remission (Asarnow & Maccrimmon, 1978; Nuechterlein et al., 1992; Wohlberg & Kornetsky, 1973). Patients’ difficulties with controlled, effortful attentional processing become even more apparent when the attentional systems are highly taxed, such as in tasks with high loads, tasks with rapid processing of information requirements, and in tasks with distraction (e.g., Braff & Saccuzzo, 1985; Dawson & Nuechterlein, 1984; Dawson, 1990; Kietzman et al., 1985). Attentional deficits have a significant relationship to functional outcome, including the ability to acquire basic life skills, social problem solving and social competence (Green et al., 2000), suggesting that improving attentional capabilities may benefit several aspects of patients’ lives.
Yet, while the need for pro-cognitive enhancers is there and there has been renewed interest in addressing the cognitive symptoms in disorders such as schizophrenia (i.e., the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative: Carter & Barch, 2007; Carter et al., 2008; see also review by Sarter et al., 2012), the success of preclinical and clinical psychopharmacological research to develop procognitive treatments has been limited. Our previous reviews on the reasons behind this failure have largely pointed to the use of atheoretical research strategies in preclinical drug development, including the widespread use of high-throughput behavioral screening tests with limited or unknown validity in terms of modeling clinically-relevant cognitive outcomes and a focus on accumulating evidence that drugs in development show beneficial effects on a wide range of behavioral measures instead of focusing on defining specific mechanisms underlying pharmacologically-induced cognitive enhancement (see Sarter et al., 1992a,b; Sarter, 2006). Below, we review the evidence supporting the use of acetylcholinesterase inhibitors, mAChR agonists and nAChR agonists for enhancing attention and propose two target mechanisms in order to support hypothesis-guided development of procognitive compounds.
3. Enhancement of attention via acetylcholinesterase inhibitors
The interest in acetylcholinesterase inhibitors like donepezil or galantamine for the enhancement of attention stems largely from their use clinically to treat the cognitive impairments associated with dementia (e.g., Rogers et al., 1996; 1998; Raskind et al., 2000). In animal model studies, selective lesions to the basal forebrain cortical cholinergic system in attention task-performing animals result in a severe and lasting impairment in signal detection, while the ability to correctly reject nonsignals remains intact (McGaughy et al., 1996; McGaughy & Sarter, 1998). While these studies demonstrate the necessity of the cortical cholinergic system for attentional performance, follow-up experiments attempting to rescue attentional performance with cholinesterase inhibitors following limited basal forebrain lesions have seen mixed results, with some experiments finding attenuation of attentional impairments (Muir et al., 1995) and others finding no benefit of cholinesterase inhibitors for attentional performance in lesioned animals (McGaughy & Sarter, 1998). This suggests the usefulness of cholinesterase inhibitors to rescue attention in cases where the cholinergic neurons have degenerated is limited.
Studies in healthy humans show some support for the ability of cholinesterase inhibitors to enhance attention. Behavioral studies of donepezil in healthy humans demonstrate that donepezil can improve voluntary attention, but does not affect involuntary attention (Rokem et al., 2010). In human pharmaco-fMRI studies, cholinergic enhancement through administration of acetylcholinesterase inhibitors increases activation in sensory regions in response to attended, task-relevant stimuli, and suppresses activation in response to task-irrelevant stimuli (e.g., Furey et al., 2000; Bentley et al., 2004; Silver et al., 2008). Cholinergic enhancement also modulates frontoparietal regions, with findings indicating reductions in frontoparietal activations when sensory activations are heightened and task-demands are low, presumably reflecting increased efficiency in executive attentional processes (e.g., Furey et al., 2000; Ricciardi et al., 2009).
The evidence on the ability of cholinesterase inhibitors to enhance attention in patient populations suggests that these compounds may be more effective for some patient populations than for others. For example, an fMRI study of patients with Alzheimer’s disease and age-matched healthy controls with and without the cholinesterase inhibitor physostigmine found that physostigmine improved stimulus- and attention-dependent responses in patients, normalizing activation in areas known to be affected by Alzheimer’s disease like extrastriate and frontoparietal cortex, while perturbing activation in those same regions in the controls (Bentley et al., 2008). In contrast to the beneficial effects seen in dementia patients, the collective evidence for patients with schizophrenia suggests cholinesterase inhibitors are not effective treatments for the attentional deficits seen in this population (e.g., Buchanan et al., 2008; Keefe et al., 2008; Kohler et al., 2007). Some have speculated that cholinergic activity in patients with schizophrenia is elevated compared to healthy controls (Tandon & Greden, 1989). The ability of cholinesterase inhibitors to improve attentional functions may thus depend on the baseline activity of the cholinergic system, and in cases where cholinergic activity is already high, further augmentation of acetylcholine (ACh) levels may fail to provide a benefit. The lack of procognitive results in schizophrenia may also be due to the fact that cholinesterase inhibitors exert broad effects on ACh concentrations in the extracellular space, and do not act to optimize the tonic and phasic components (described below) of cholinergic activity thought to be the most promising targets for procognitive enhancers (for more discussion of these issues see Hasselmo & Sarter 2011).
4. Enhancement of attention via mAChR agonists
4.1 Effects of nonselective mAChR antagonists
In mammals, there are five mAChR subtypes, M1–M5 (Wess, 1996; Caulfield & Birdsall, 1998). Nonselective mAChR antagonists such as scopolamine impair attention and the encoding of new memories in animal models (Aigner et al., 1991; McGaughy et al., 1994) and in healthy humans (e.g., Ghoneim & Mewaldt, 1975, 1977; Wesnes & Warburton, 1984), and exacerbate the cognitive impairments seen in healthy aged individuals and patients with Alzheimer’s disease (eg, Molchan et al., 1992; Sunderland et al., 1987, 1988). Acute administration of nonselective mAChR antagonists to healthy humans has been used as a pharmacological model of dementia (e.g., Beatty et al., 1986; Broks et al., 1988). While this may be a useful model in some respects, the interpretation of the results from nonselective mAChR antagonists must be informed and modified by the fact that these drugs not only block postsynaptic mAChRs, but also antagonize presynaptic M2 receptors (e.g., Herzog et al, 2003). Antagonism of these presynaptic receptors leads to an increase in ACh release, resulting in extremely high extracellular levels of ACh. This in turn stimulates nAChRs, leading to an interaction between the blockade of mAChRs and the stimulation of nAChRs and a complex pattern of behavioral and cognitive effects. Supporting the idea of this interaction between nAChRs and mAChRs, several studies demonstrated that blocking nAChRs alone does not affect cognition, but co-administration of nAChR antagonists and mAChR antagonists significantly impairs cognition (e.g., Little et al, 1998; Ellis et al, 2006; Erskine et al, 2004; see Hasselmo & Sarter, 2011 for further discussion of these issues).
4.2 Development of mAChR subtype-specific agonists
Relatively little is known about the contribution of specific mAChR subtypes to cognitive functions or the usefulness of mAChR agonists for enhancing attention and other cognitive functions. This is largely due to the fact that development of subtype-selective antagonists and agonists has been hindered due to the high sequence homology among the orthosteric binding sites of the five mAChR subtypes. Alternative strategies, such as the use of immunoprecipitation assays with subtype-specific antibodies (e.g., Levey et al., 1991) and the development of muscarinic receptor knockout mice (e.g., Yamada et al., 2001; Jeon et al., 2010) has helped shed light onto the roles of specific mAChR subtypes. For example, M4 mAChRs are expressed widely throughout the forebrain, but are also expressed in D1 dopamine receptor-expressing cells in striatal projecting neurons (Jeon et al., 2010). Deletion of this specific subpopulation of M4 receptors alters several dopamine-dependent behaviors, including increasing behavioral sensitization following psychostimulant treatment, and leads to an increase in dopamine efflux in the nucleus accumbens. This evidence points to a mechanisms for how mAChRs could play a role in the increased central dopaminergic neurotransmission seen in disorders such as schizophrenia (Jeon et al., 2010). The M5 subtype also influences dopamine neurotransmission, as it has been implicated in aiding muscarinic agonist-induced striatal dopamine release. It is also necessary for the dilation of cerebral blood vessels by acetylcholine (Yamada et al., 2001). This latter fact is of special interest from the perspective of developing targets for cognition enhancers, as impairments in cholinergic dilation of cerebral blood vessels may be important for the pathophysiology of Alzheimer’s disease.
While developing subtype-selective mAChR agonists has been challenging, recent advances on this issue have been made by developing compounds that target allosteric binding sites distinct from the ACh binding site (for review, see Conn et al., 2009). These compounds have helped clarify the roles of mAChR subtypes in mediating cognitive functions. For example, the M1 receptor subtype is thought to be important for memory and attention (Levey et al., 1991; Felder et al., 2000), and selective M1 agonists have been proposed as a treatment for Alzheimer’s disease (e.g., Bodick et al., 1997; Caccamo et al., 2006, 2009). Genetic deletion of these receptors failed to produce impairments in hippocampal-dependent learning and failed to influence impairments following mAChR antagonist administration (Rouse et al., 2000; Miyakawa et al., 2001; Anagnostaras et al., 2003), but did reveal specific deficits in learning and memory tasks requiring engagement of the prefrontal cortex (Anagnostaras et al., 2003). This suggests the M1 receptor may help regulate prefrontal function. Recently, compounds that are positive allosteric modulators (PAMs) at the M1 receptor have been discovered and synthesized (Marlo et al., 2009; Shirey et al., 2009). These compounds substantially increase the affinity of the M1 receptor for ACh and potentiate the response to orthosteric agonist. Investigations with these compounds demonstrate that they can increase medial prefrontal neuronal firing in vivo and reverse impairments in prefrontal cortex-dependent learning in mouse models of Alzheimer’s disease, suggesting the M1 receptor is important for regulating excitation of the prefrontal cortex and that selective activation of M1 receptors could help alleviate impairments in prefrontal cortex-dependent cognition (Shirey et al., 2009).
5. Enhancement of attention via nAChR agonists
5.1 Effects of nicotine and nonspecific nAChR agonists
Overall, much more is known about the effects of nAChR agonists than mAChR agonists. While only a few animal model studies have assessed the effects of nicotine on attentional performance, nicotine’s effects on attention have been extensively studied in smoker and nonsmoker healthy and patient populations (see Levin, 2002; Kassel, 1997; and Bentley et al., 2011 for reviews). In general, the available evidence indicates nicotine improves basic attentional functions, but these beneficial effects are sometimes difficult to replicate and are usually less robust than the effects seen with specific nAChR agonists. In animal model studies, while some reports have failed to find significant effects of nicotine (e.g., Turchi et al., 1995; Bushnell et al., 1997; Kozak et al., 2007), others have demonstrated that nicotine improves accuracy in attention tasks. Nicotine improves choice-accuracy in a five-choice serial reaction-time task in rats with lesions of the nucleus basalis magnocellularis (Muir et al., 1995). Similarly, Mirza and Stolerman (1998) demonstrated increased accuracy on an attention task with nicotine when the task condition included low rates of stimulus presentation. Blondel et al. (2000) investigated the effects of acute and repeated administration of nicotine on rat performance of the five-choice serial reaction-time task and found nicotine did not improve animals’ accuracy, but did improve response readiness and target scanning as response latencies decreased and performance in the less-well attended stimulus locations improved. Antagonism of specific nicotinic receptor subtypes revealed these beneficial effects were most likely mediated through α4β2*1, α4β4*, or α3β2* but not through α7 nAChRs (Blondel et al., 2000).
Nicotine has been used extensively in human behavioral and pharmaco-fMRI studies to enhance attention. Behavioral studies in non-smokers or deprived smokers consistently find nicotine improves attentional functions (e.g., Le Houezec et al., 1994; Mancuso et al., 1999; Warburton & Mancuso, 1998; Vangkilde et al., 2011), and can also improve memory through the attentional enhancement of items during encoding (Marchant et al., 2008; Rusted et al., 2009). Neuroimaging studies of nicotine generally see changes in activation with nicotine that mirror the patterns seen with cholinesterase inhibitors. Activation in sensory regions increases during attentionally-demanding task conditions compared to less-demanding control conditions, such as when target-detection tasks use low-contrast targets (Hahn et al., 2007) or invalidly cued targets (Thiel et al., 2005), or in visual maze tasks (Ghatan et al., 1998). In response to increased activation in sensory regions and resulting gains in perceptual or encoding efficiency, decreases are often seen in frontal and parietal regions. These effects are interpreted as reflective of reduced demands on top-down attentional control processes (Ghatan et al., 1998; Ettinger et al., 2009). In contrast, when the demands on executive attention functions are high, nicotine increases activation in frontal and parietal regions in order to recruit additional top-down control processes to maintain or improve performance under the attentionally-challenging conditions (Ernst et al., 2001; Lawrence et al., 2002; Thiel et al., 2005; Giessing et al., 2006; Vossel et al., 2008). The modest improvements in attentional functions following nicotine administration seems to generalize from healthy individuals to patient populations, with nicotine or nonspecific nAChR agonists enhancing attentional function in adults with ADHD (Conners et al., 1996; Levin et al., 1996; Wilens et al., 2007), Alzheimer’s disease (Jones et al., 1992; Sahakian et al., 1989; Newhouse et al., 2001) and schizophrenia (Freedman et al., 2008).
5.2 Effects of agonists at α4β2* nAChRs
α4β2 and α7 receptors represent the two most predominate nicotinic receptors in the brain. Unlike the current state of the literature with respect to muscarinic receptor subtypes, much more is known about the potential of selective agonists of nicotinic receptor subtypes as cognition enhancers, particularly for α4β2* agonists. In contrast to the modest and often unreliable effects of nicotine on attention, the available evidence on agonists that specifically target α4β2* nAChR subtypes show much more robust effects. Animal model studies show administration of α4β2* agonists can significantly improve the relative number of hits in a sustained attention task (McGaughy et al. 1999) and improve accuracy on the five-choice serial reaction time task under conditions of enhanced difficulty or in poor-performers (Mohler et al., 2010). Corresponding to the robust attentional enhancement effects seen with α4β2*agonists, nAChR β2 subunit deletions impair attentional performance in mice, an effect that can be reversed by re-expressing functional β2 subunit-containing nAChRs in prefrontal neurons in the prelimbic area (Guillem et al., 2011). While the literature on specific α4β2* agonists in humans is sparse, these agonists appear to have more robust attentional enhancement effects than those seen with nicotine in patient populations such as age associated memory impairment (Dunbar et al., 2007) and schizophrenia (Taylor et al. 2011). Interestingly, clinical trials on the α4β2* agonist ABT-089 demonstrate this agonist can robustly improve attention in adult ADHD patients (Wilens et al., 2006; Apostol et al., 2012), but not in pediatric ADHD patients (Wilens et al., 2011). While still speculative, this evidence seems to support the ideas presented here that nAChR agonists and specifically α4β2* agonists work to enhance attention primarily by enhancing attentional control. As attentional control is still developing well into young adulthood (e.g., Andrews-Hanna et al., 2011) the cognitive mechanisms underlying the attentional deficits seen in pediatric versus adult ADHD may be distinct in certain respects. If deficits in adult, but not pediatric, ADHD are primarily related to deficits in the top-down control of attention, this would account for the findings that α4β2* agonists benefit adult ADHD patients, but do not show the same effects in pediatric ADHD (see Sarter & Paolone, 2011 for further discussion).
5.3 Effects of agonists at the α7 nAChR
In contrast to the evidence that nicotine and especially α4β2* agonists enhance attention, the evidence concerning the effects of selective α7 agonists is limited and conflicted. Several animal model studies of α7 agonists have failed to show effects on attention (Grottick et al., 2000; 2003; Hahn et al. 2003). However, α7 nAChR knockout mice show impairments in acquiring and performing an attention task, and improvements following nicotine administration, suggesting that this receptor plays a role in the effects of nicotine (Young et al., 2004). While α7 agonists may have a place among other procognitive enhancers for treating the cognitive deficits in certain disorders (Olincy et al., 2007), evidence from animal models on the mechanisms underlying the cortical cholinergic system’s role in cue detection and top-down attentional control suggests that selective stimulation of α4β2* receptors represents a critical target mechanism for the enhancement of attention and stimulation of α7 receptors may weaken or blunt these beneficial effects (Howe et al., 2010).
6. Tonic and phasic cholinergic activity mediates attention
As described above, selective lesions of cholinergic neurons in the basal forebrain demonstrate the necessity of the cortical cholinergic system for attentional performance (McGaughy et al., 1996; Turchi & Sarter, 1997). Much of our own work on the cortical cholinergic system’s role in mediating attention utilizes the sustained attention task (SAT), a task developed for use in, and validated for, mice, rats, and humans (St. Peters et al., 2011a; McGaughy & Sarter, 1995; Demeter et al., 2008). In the SAT, a brief, variable duration signal/cue is either presented (signal trial) or not presented (nonsignal trial). Participants must then report the presence or the absence of the cue and are rewarded for correct performance. The attentional control demands of this task can be manipulated through the introduction of a visual distractor (distractor condition sustained attention task, dSAT), allowing for the assessment of multiple aspects of attentional performance. In vivo microdialysis studies of the cholinergic system find that ACh release in right prefrontal cortex increases during performance of the SAT, beyond the levels of release seen in control tasks with matched motor and reward components (Himmelheber et al., 2000; Arnold et al., 2002; Dalley et al., 2001; Kozak et al., 2006, 2007; St. Peters et al., 2011b). While the low temporal resolution of microdialysis (on the scale of minutes) places limitations on the ability to map changes in ACh release onto specific task events, more recent evidence suggested that it samples a separate and indeed more tonically active component of cholinergic neurotransmission that is separate from phasic increases in cholinergic activity (“transients”; Parikh & Sarter, 2008; Paolone et al., 2010; see below).
These limitations have been overcome by recent technological advances that led to the ability to measure the synaptic release of ACh in task-performing animals at a sub-second resolution using choline-sensitive microelectrodes (Parikh et al., 2004). Studies using this microelectrode technology demonstrate the necessity of transient increases in ACh in right prefrontal cortex for cue detection and begin to unravel the neurochemical circuitry responsible for mediating cue detection (e.g., Parikh et al., 2007). Further, we now understand that ACh acts through two separable components: a sub-second phasic component responsible for mediating cue detection, and a tonic, minutes-based component responsible for mediating the top-down attentional control. The circuitry supporting cue detection and attentional control are described in detail below. Collectively, this evidence suggests the cortical cholinergic system can play a dynamic role in optimizing both sensory and frontoparietal control regions in response to behaviorally-relevant stimuli and task demands and provides the rationale for target mechanisms for procognitive enhancers.
7. Target mechanism for enhancing cue detection and attentional switching
The evidence concerning the phasic component of the cortical cholinergic system and its role in cue detection has evolved rapidly in the past few years. Below we describe how transient increases in prefrontal ACh release mediates the detection of cues and how these transient ACh increases are dependent, but not sufficiently, upon prefrontal glutamateric activity. We also explain a complex pattern of data on when increases in transient ACh are seen and reconcile these findings by describing a model for the role of phasic cholinergic activity in facilitating attentional switches between internal and external processing modes.
7.1 Phasic increases in cholinergic activity mediates cue detection
Initial investigations using choline-sensitive microelectrodes in rats to study the phasic release of ACh made use of a simple cued-appetitive task in order to minimize the electrical interference arising from computerized operant tasks (Parikh et al., 2007). Recordings from the medial prefrontal cortex revealed that cues that evoked transient increases in cholinergic activity were correctly detected, while cues that did not evoke transient increases in cholinergic activity were missed. Transient increases in cholinergic activity were not evoked by the motor and reward components of the task. Further, the increase in cholinergic activity for detected cues was specific to the prefrontal cortex, as recordings from control regions in motor cortex did not show these transients. Deafferentiation of the prefrontal cholinergic neurons using the selective immunotoxin 192 IgG-saporin attenuated the cue-evoke cholinergic activity and cue detection, confirming the neuronal origin of the activity observed by the microelectrodes and the necessity of prefrontal cholinergic transients for cue detection (Parikh et al., 2007).
7.2 Glutamatergic-cholinergic interactions
Experiments in anesthetized rats and in rats performing the SAT further elucidate the mechanism underlying the transient increases in cholinergic activity seen when cues were detected (Parikh et al., 2008; Howe et al., 2010). These experiments show the key role that glutamatergic-cholinergic interactions play in mediating cue detection and help explain why selective α4β2* nAChR agonists produce more robust pro-attentional effects than nonspecific nAChR agonists. Using microelectrodes sensitive to glutamate and glutamate antagonists, Parikh and colleagues (2008) demonstrate that prefrontal glutamate release and stimulation of presynaptic ionotropic glutamate receptors (presumably on cholinergic terminals) is necessary for the generation of cue-evoked cholinergic transients. Glutatmatergic inputs to prefrontal cortex arise from the medial dorsal nucleus of the thalamus (Sarter & Markowitsch, 1984). Our current model for the circuitry supporting cue detection proposes that input from sensory regions is projected to the thalamic reticular nucleus, which in turn makes contacts with the medial dorsal nucleus. Together, this circuitry is believed to import a pre-attentionally-processed cue into the prefrontal cortex. Thus, via glutamatergic-cholinergic interactions, the neuronal representation of the pre-attentionally processed cue evokes a cholinergic transient responsible for the conscious detection of a cue.
Related studies also help connect the proposed circuitry model to the pattern of results seen when nicotine or α4β2*agonists are used to enhance attentional performance. Pressure injection of nicotine into the prefrontal cortex of anesthetized animals elicits cholinergic transients; however, injection of the α4β2* specific agonist ABT-089 more potently elicits cholinergic transients, and these transients have higher amplitudes than the transients seen with nicotine (Parikh et al., 2008). Interestingly, the rise and fall of the cholinergic transients elicited with ABT-089 is also substantially sharper than the pattern seen with nicotine (Parikh et al., 2008), and ABT-089 is more potent than nicotine at eliciting the glutamateric signals necessary for cue-evoked cholinergic transients (Parikh et al., 2008). In awake animals performing the SAT, systemic injection of a α4β2* agonist also results in cholinergic transients with higher amplitudes and sharper, faster decay profiles than injections of nicotine, confirming the patterns seen in anesthetized animals (Howe et al., 2010). The necessity of α4β2* nAChR stimulation to evoke the glutamatergic release necessary for the generation of cue-evoked cholinergic transients has been confirmed in studies with mice lacking β2 subunits (Parikh et al., 2010). ABT-089-evoked cholinergic transients are completely abolished in mice missing the β2 subunits, and lesions of the thalamic glutamatergic inputs to prefrontal cortex also abolish the cholinergic transients. Together, these results support the model that cholinergic inputs from the basal forebrain to prefrontal cortex stimulate α4β2* nAChRs on prefrontal glutamatergic projections arising from the thalamus. α4β2* nAChR stimulation evokes transient glutamatergic signals that are necessary for the generation of the prefrontal cholinergic transients mediating cue detection. Positive modulation of glutamate release by nAChR agonists augments the amplitudes of the cholinergic transients necessary for cue detection, supporting enhanced detection capabilities (Howe & Sarter, 2010).
We hypothesize that because specific α4β2* agonists evoke cholinergic transients with larger amplitudes and sharper decay rates than the transients evoked by nicotine, this leads to greater stimulation of the glutamatergic projections. This in turn increases the likelihood of cue-evoked cholinergic transients, as the glutamatergic transients are necessary for the generation of the cue-evoked cholinergic transients, and hence leads to more robust attentional enhancements. The amplitudes and decay rate profiles of the prefrontal cholinergic transients evoked by selective nicotinic agonists are expected to be informative for deciding whether experimental compounds are likely to show procognitive effects. These data also help explain the limited procognitive effects seen with cholinesterase inhibitors, as these compounds would not be capable of enhancing or restoring the phasic glutamateric-cholinergic interactions described here.
7.3 Phasic increases in cholinergic activity facilitates attentional switching
Adding to the complexity of the data described above on the role of cholinergic transients in mediating cue detection, while cholinergic transients are never seen when an animal fails to detect a cue, these transients are only seen on roughly 60% of the trials where cues are accurately detected (“hit” trials; Howe et al., 2007). Interestingly, further analysis reveals that transient increases in cholinergic activity are only seen on hit trials when the preceding trial is a correctly rejected uncued (nonsignal) trial, or when the preceding trial is a miss (undetected cue; Howe et al., 2007; see also Sarter et al., 2009 and Hasselmo & Sarter, 2011 for discussion of these data). When the preceding trial is also a hit trial, no increases in cholinergic activity are seen on the subsequent hit trial.
Based on this data, we propose that the phasic component of the cortical cholinergic system facilitates a switch from an internal mode of processing to an external mode of processing, and suggest that this switch readies the prefrontal cortex for cue detection. Trials where no cue/signal is presented or when the cue is missed encourage an internally-directed processing mode. In contrast, on cued trials the brain needs to switch to an external processing mode in order to correctly detect the cue. Sequences of successive hits therefore do not generate additional cholinergic transients because in those cases shifts from ongoing behavior are not required as the cue-directed behavior and associated response rules have already been activated by the first hit trial. Similarly, trials where the cue is missed also fail to generate cholinergic transients because though the cue may have been perceived on some level, it has failed to initiate the shift from ongoing behavior to the appropriate response. Thus, cholinergic transients are hypothesized to facilitate shifts from ongoing cue-independent attentional processing to cue-dependent processing, specifically the activation of cue-associated response rules and reward expectation.
A role for the cortical cholinergic system in switching between internal and external processing modes also helps explain results from pharmaco-fMRI experiments concerning the effects of cholinergics, particularly nicotine, on brain regions that are typically considered part of the “default” or “resting state” network (Raichle & Synder, 2007). fMRI studies using nicotine frequently observe that nicotine causes further deactivations in medially-located regions such as cingulate, precuneus, and parahippocampal gyri, and in regions in the superior-middle temporal and angular gyri during attentionally-demanding tasks, while not affecting activation in those regions during rest (e.g., Ghatan et al., 1998; Bentley et al., 2004; Hahn et al., 2007, 2009; Ettinger et al., 2009). In addition to these exaggerated deactivations during attentionally-demanding tasks, these studies also observe a concurrent increase in task-related activation in dorsolateral frontoparietal and posterior regions. These data support a role for nAChR agonists in facilitating the switch from the internally-directed processing modes (reflected in the activation of the default network during rest) to externally-directed processing modes capable of reacting to externally-driven tasks and external, task-relevant cues (for further discussion, see also Hahn et al., 2007).
8. Target mechanism for enhancing top-down control
In addition to the second-based phasic component of the cortical cholinergic system that is theorized to mediate attentional switching and hence the detection of external, task-relevant cues, the cortical cholinergic system also operates via another, minutes-based component (“tonic” ACh release). Importantly, the tonic component of the cholinergic system cannot be fully explained by simply summing the cholinergic transients described above over time (Parikh et al., 2007). Tonic cholinergic activity is theorized to mediate top-down attentional control, with increases in tonic cholinergic activity reflecting the engagement of attentional effort processes designed to help stabilize or maintain attentional performance in the face of attentionally-challenging conditions.
As described above, in-vivo microdialysis studies in SAT-performing rats demonstrate that ACh release increases in right prefrontal cortex during attentional performance, above and beyond the release levels seen in tasks with matched motor or reward components, but low demands on attention (Arnold et al., 2002; Dalley et al., 2001; Himmelheber et al., 2000; Passetti et al., 2000). While initial theories proposed that the levels of ACh release would simply mirror the success of attentional performance (low levels of release when performance was poor and high levels of release when performance was strong), the evidence has instead demonstrated that tonic ACh levels vary as a function of the demands on attentional control. For example, pharmaceutical challenges that impair attentional performance increase levels of prefrontal ACh release above and beyond the levels seen during normal attention-task performance (Kozak et al., 2006). This data lead to the development of the theory that tonic ACh levels reflect the amount of attentional effort an individual is exerting (Sarter et al., 2006).
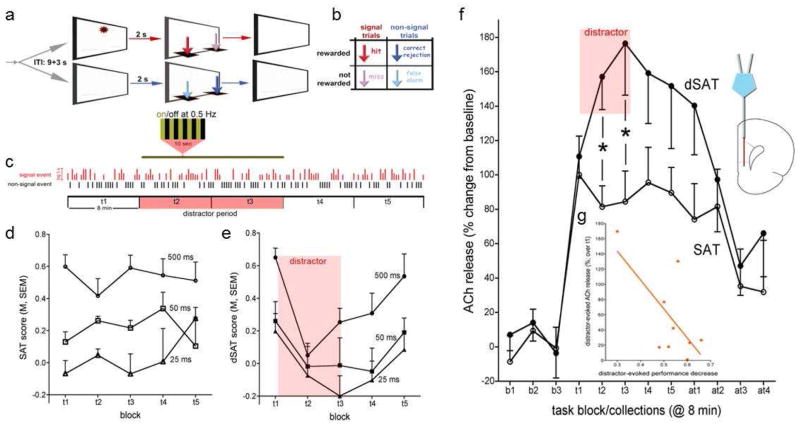
According to the theory of attentional effort, following a challenge to attention, motivated individuals will engage top-down attentional control processes in order to stabilize or recover their performance. This idea was recently tested in an in-vivo microdialysis study using a visual distractor to impair performance of a sustained attention task (the dSAT; St. Peters et al., 2011b; Figure 1). In this study, the distractor robustly impaired attentional performance, but increased the levels of prefrontal ACh release significantly over the levels seen from pre-distractor task performance. Furthermore, the levels of ACh during distraction correlated with how well animals were able to maintain their performance during distraction, with higher levels of ACh seen for animals with less severe distractor effects.
Figure 1.
Distractor-induced impairment in attentional performance and prefrontal acetylcholine (ACh) release in the presence and absence of a distractor. (a) The sustained attention task (SAT) consists of randomly ordered signal (light signals 500, 50, or 25 ms long) and non-signal events, spaced by 9±3 s. Two seconds after an event, levers are made available and animals need to respond within 4 s. Following a lever press or after 4 s, levers are withdrawn. (b) Hits and correct rejections, but not misses and false alarms, are rewarded (note that arrows indicating the 4 response types in a are color-coded and match arrows in b). Sessions lasted 40 min and were blocked post hoc into five 8-min blocks of trials (t1–t5). (c) For testing on the distractor condition sustained attention task (dSAT), the distractor (chamber lights flashing on/off at 0.5 Hz) occurred during blocks 2 and 3. The vertical red and black bars illustrate a random sequence of signal and non-signal trials (signal duration indicated by the length of the red bars. (d) In the absence of a distractor (SAT; n=6), performance varied with signal duration and remained stable over the 5 task blocks (t1–t5). This plot and the plot in e show SAT and dSAT scores, respectively, which are an overall measure of performance calculated as follows: SAT/dSAT = (hit − false alarm) / [2(hit + false alarm) (hit + false alarm)2]. (e) Presentation of the distractor (dSAT; n=9) during task blocks 2 and 3 transiently impaired performance. During the post-distractor blocks, animals’ performance recovered. (f) SAT performance evoked a steep initial increase in ACh release in the medial prefrontal cortex as measured via in-vivo microdialysis. Release levels remained stable throughout the remainder of the performance session. Presentation of the distractor further increased ACh release (b1–b3 depict baseline collections prior to task onset, t1–t5 depict the 5 task blocks and at1–at4 indicate data from four collections following completion of the task (8 min/collection). (g) The severity of the distractor-induced impairment of performance was significantly correlated with distractor-induced increases in cholinergic activity. The abscissa of this graph depicts the differences between t1 and t2/t3 dSAT scores, with larger numbers indicating more severe impairments. Thus, higher increases in ACh release were correlated with less severe distractor effects on performance. (Post hoc multiple comparisons, using t-test or the Least Significance Difference (LSD), are indicated by symbols: *p<0.05). Adapted and modified from Figure 1 in St. Peters, M., Demeter, E., Lustig, C., Bruno, J.P., Sarter, M., 2011. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J. Neurosci. 31, 9760–9771, with permission from the Society for Neuroscience.
Mirroring the increases seen in ACh release in rats during distraction, an arterial spin labeling (ASL) fMRI study using the human version of the dSAT found that activation in right frontal regions increases during distraction (Demeter et al., 2011). Interestingly, however, this study found that higher levels of activation during distractor were correlated with worse attentional performance – the opposite relationship to what was seen in ACh release in rats. We hypothesize that increases in right prefrontal ACh in response to attentionally challenging conditions acts to optimize the prefrontal circuitry necessary for the top-down control of attention, leading to reduced metabolic demands and hence less blood flow to this region.
The neuronal circuitry supporting top-down attention control is believed to include cholinergic projections from the basal forebrain to the prefrontal cortex and glutamatergic projections from prefrontal cortex back to the basal forebrain, both directly and indirectly via projections to mesolimbic regions including the nucleus accumbens, the ventral tegmentum, and the amygdala (Mogenson et al., 1980; Gruber et al., 2009; Zaborszky et al., 1986; Ingham et al., 1988; Zaborszky & Cullinan, 1992, 1996; Zaborszky, 2002; Zaborszky et al., 2005; Zaborszky et al., 1993; Zaborszky et al., 1997; Zaborszky et al., 1984; Zaborszky et al, 1999). In response to increases in attentional control demands, tonic release of ACh in right prefrontal cortex is believed to be upregulated, helping to enhance the preattentional representation of cues. Augmenting the tonic levels of ACh is therefore hypothesized to represent a central mechanism for enhancing attentional control and optimizing cue detection processes. In addition to this somewhat simplified story, the top-down effects of cholinergic activity may also act through cortex-wide mechanisms, including cortico-cortico mechanisms (Nelson, Sarter & Bruno, 2005), and feedback projections from prefrontal cortex to thalamus (e.g., see Crick, 1984; McAlonan et al., 2006).
9. Conclusions
Here, we propose two target mechanisms for the development of procognitive enhancers: stimulation of α4β2* nAChRs on prefrontal glutamatergic neurons in order to facilitate attentional switching and cue detection, and augmentation of the tonic levels of ACh in order to improve attentional control functions. While cholinesterase inhibitors and nAChR agonists have been intensely studied in regards to their putative pro-attentional effects, the recent mechanistic evidence on the circuitry supporting cue detection and attentional control suggest that specific α4β2* nAChR agonists are likely to be the most successful cognition enhancers. Thus, the two target mechanisms proposed here allow for the hypothesis-driven development and characterization of new cognitive enhancers.
Highlights.
Cue detection and top-down attentional control represent key attentional functions.
Agonism of specific nicotinic receptor subtypes aids cue detection.
Augmentation of tonic acetylcholine levels aids attentional control.
Development of cognition enhancers should focus on these aspects of attention.
Acknowledgments
The authors would like to thank David Cron for assistance with preparation of this manuscript.
Footnotes
Asterisks indicate possible presence of other subunits in the nAChR subtypes. For example in the layer VI pyramidal neurons of prefrontal cortex, the less common α5 subunit is also incorporated into the α4β2 subtype. This additional subunit enhances the conductance and currents of these neurons (Ramirez-Latorre et al., 1996; Bailey et al., 2010). nAChR activation of layer VI pyramidal neurons results in a net increase in neuronal activity (see Poorthuis et al., 2012 for a description of the layer-specific effects of nAChR stimulation). These neurons are important for attention through their feedback projections onto the thalamus (Crick, 1984; Zikopoulos & Barbas, 2006; Briggs & Usrey, 2011), and deletion of the α5 subunit in mice results in deficits on attentional tasks when demands on attention are high (Bailey et al., 2010). Hence, this additional α5 subunit may contribute to the mechanisms supporting top-down control described in this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner TG, Walker DL, Mishkin M. Comparison of the effects of scopolamine administered before and after acquisition in a test of visual recognition memory in monkeys. Behav Neural Biol. 1991;55:61–7. doi: 10.1016/0163-1047(91)80127-Z. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–8. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: Neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/S0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Maccrimmon DJ. Residual performance deficits in clinically remitted schizophrenics – marker of schizophrenia. J Abnorm Psychol. 1978;87:597–608. doi: 10.1037/0021-843X.87.6.597. [DOI] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, Pritchett YL, Feifel D, Collins MA, Saltarelli MD. Efficacy and safety of the novel α4β2* neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology. 2012;219:715–725. doi: 10.1007/s00213-011-2393-2. [DOI] [PubMed] [Google Scholar]
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–52. doi: 10.1523/JNEUROSCI.2258-10.2010. 0.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Butters N, Janowsky DS. Patterns of memory failure after scopolamine treatment: implications for cholinergic hypotheses of dementia. Behav Neural Biol. 1986;45:196–211. doi: 10.1016/S0163-1047(86)90772-7. [DOI] [PubMed] [Google Scholar]
- Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–82. doi: 10.1016/S0896-6273(04)00145-X. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain. 2008;131:409–424. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94:360–388. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, van Lier R, Paul SM. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl 4):S16–22. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology. 2000;149:293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Braff DL, Saccuzzo DP. The time course of information-processing deficits in schizophrenia. Am J Psychiatry. 1985;142:170–174. doi: 10.1176/ajp.142.2.170. [DOI] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Corticogeniculate feedback and visual processing in the primate. J Physiol. 2011;589:33–40. doi: 10.1113/jphysiol.2010.193599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia. 1988;26:685–700. doi: 10.1016/0028-3932(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, McMahon RP. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry. 2008;165:82–89. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Padnos BK. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology (Berl) 1997;134:230–41. doi: 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–82. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Fisher A, LaFerla FM. M1 agonists as a potential disease-modifying therapy for Alzheimer’s disease. Curr Alzheimer Res. 2009;6:112–7. doi: 10.2174/156720509787602915. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: The CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, Geyer M, Green M, Nuechterlein KH, Robbins T, Silverstein S, Smith EE, Strauss M, Wykes T, Heinssen R. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the cognitive neuroscience treatment research to improve cognition in schizophrenia initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–90. [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30:148–55. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci. 1984;81:4586–90. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–14. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME. Psychophysiology at the interface of clinical science, cognitive science, and neuroscience. Psychophysiology. 1990;27:243–255. doi: 10.1111/j.1469-8986.1990.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Nuechterlein KH. Psychophysiological dysfunctions in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:204–232. doi: 10.1093/schbul/10.2.204. [DOI] [PubMed] [Google Scholar]
- Deco G, Thiele A. Attention – oscillations and neuropharmacology. Eur J Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: Cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54:1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI) J Psychopharmacol. 2007;21:171–178. doi: 10.1177/0269881107066855. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–89. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci U S A. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine FF, Ellis JR, Ellis KA, Stuber E, Hogan K, Miller V, Moore E, Bartholomeusz C, Harrison BJ, Lee B, Phan KL, Liley D, Nathan PJ. Evidence for synergistic modulation of early information processing by nicotinic and muscarinic receptors in humans. Hum Psychopharmacol. 2004;19:503–9. doi: 10.1002/hup.613. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Williams SC, Patel D, Michel TM, Nwaigwe A, Caceres A, Mehta MA, Anilkumar AP, Kumari V. Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity. Neuroimage. 2009;45:549–561. doi: 10.1016/j.neuroimage.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Felder CC, Bymaster FP, Ward J, DeLapp N. Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem. 2000;43:4333–53. doi: 10.1021/jm990607u. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl) 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP. Effects of diazepam and scopolamine on storage, retrieval and organizational processes in memory. Psychopharmacologia. 1975;44:257–62. doi: 10.1007/BF00428903. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP. Studies on human memory: the interactions of diazepam, scopolamine, and physostigmine. Psychopharmacology (Berl) 1977;52:1–6. doi: 10.1007/BF00426592. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Rosler F, Fink GR. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137:853–864. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical–prefrontal–basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/S0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/S0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Haman M, Wyler R, Higgins GA. Reversal of a vigilance decrement in the aged rat by subtype-selective nicotinic ligands. Neuropsychopharmacology. 2003;28:880–887. doi: 10.1038/sj.npp.1300102. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O’Donnell P. The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One. 2009;4:e5062. doi: 10.1371/journal.pone.0005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spiker S, Mansvelder HD. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–67. doi: 10.1016/S0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Wolkenberg FA, et al. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: An fMRI study. Cereb Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037/0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Nowak KA, Sarter M, Bruno JP. Microdialysis without acetylcholinesterase inhibition reveals an age-related attenuation in stimulated cortical acetylcholine release. Neurobiol Aging. 2003;24:861–3. doi: 10.1016/S0197-4580(02)00226-9. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9:313–325. doi: 10.1016/S0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Howe WM, Parikh V, Martinez V, Sarter M. Prefrontal cholinergic switching from associational processing to cue detection: evidence from sub-second measures of prefrontal cholinergic neurotransmission, using choline-sensitive microlectrodes, in animals performing an operant sustained attention task. Society for Neuroscience Annual Meeting; San Diego, CA. 2007. [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of a α4β2* nAChRs: Underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Sarter M. Prefrontal; glutamatergic-cholinergic interactions for attention: Glutamatergic coding of signal salience as a function of performance levels. In: Westerink B, Clinckers R, Smolders S, Sarre S, Michotte Y, editors. Monitoring Molecules in Neuroscience. Vrije Universiteit Brussels; Brussels, Belgium: 2010. pp. 57–59. [Google Scholar]
- Ingham CA, Bolam JP, Smith AD. GABA-immunoreactive synaptic boutons in the rat basal forebrain: comparison of neurons that project to the neocortex with pallidosubthalamic neurons. J Comp Neurol. 1988;273:263–282. doi: 10.1002/cne.902730210. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wörtwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schütz G, Sager TN, Mørk A, Li C, Deng CX, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–405. doi: 10.1523/ JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzheimer’s disease. Psychopharmacology (Berl) 1992;108:485–494. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- Kassel JD. Smoking and attention: a review and reformulation of the stimulus-filter hypothesis. Clin Psychol Rev. 1997;17:451–478. doi: 10.1016/S0272-7358(97)00032-9. [DOI] [PubMed] [Google Scholar]
- Keefe R, Malhotra A, Meltzer H, Kane J, Buchanan R, Murthy A, Sovel M, Li C, Goldman R. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology. 2008;33:1217–1228. doi: 10.1038/sj.npp.1301499. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kietzman ML, Spring B, Zubin J. Perception, cognition and attention. In: Kaplan HI, Freedman AM, Sadock BJ, editors. Comprehensive Textbook of Psychiatry. 3. Vol. 1. Williams & Wilkins; Maryland: 1985. pp. 334–370. [Google Scholar]
- Kohler C, Martin E, Kujawski E, Bilker W, Gur R, Gur R. No effect of donepezil on neurocognition and social cognition in young persons with stable schizophrenia. Cogn Neuropsychiatry. 2007;12:412–421. doi: 10.1080/13546800701307263. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Kozak R, Lipinski W, Kantarowksi A, Lankford D, Williams S, Michel J, Meltzer LT, Sarter M. Behavioral screening of cognition enhancers: effects of standard compounds on attentional performance and in interaction with performance challenges. Society for Neuroscience Annual Meeting; San Diego, CA. 2007. [Google Scholar]
- Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. Toward a neuro- cognitive animal model of the cognitive symptoms of schizophrenia: Disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing, but not non-performing, rats. Neuropsychopharmacology. 2007;32:2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/S0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Le Houezec J, Halliday R, Benowitz NL, Callaway E, Naylor H, Herzig K. A low dose of subcutaneous nicotine improves information processing in nonsmokers. Psychopharmacology (Berl) 1994;114:628–634. doi: 10.1007/BF02244994. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633– 640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–26. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JT, Johnson DN, Minichiello M, Weingartner H, Sunderland T. Combined nicotinic and muscarinic blockade in elderly normal volunteers: cognitive, behavioral, and physiologic responses. Neuropsychopharmacology. 1998;19:60–9. doi: 10.1016/S0893-133X(98)00002-5. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Warburton DM, Melen M, Sherwood N, Tirelli E. Selective effects of nicotine on attentional processes. Psychopharmacology (Berl) 1999;146:199–204. doi: 10.1007/s002130051107. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Trawley S, Rusted JM. Prospective memory or prospective attention: physiological and pharmacological support for an attentional model. Int J Neuropsychopharmacol. 2008;11:401–11. doi: 10.1017/S146114570700819X. [DOI] [PubMed] [Google Scholar]
- Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, Austin CA, Williams MB, Kim K, Williams R, Orton D, Brown HA, Lindsley CW, Weaver CD, Conn PJ. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75:577–88. doi: 10.1124/mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Turchi J, Sarter M. Crossmodal divided attention in rats: effects of chlordiazepoxide and scopolamine. Psychopharmacology (Berl) 1994;115:213–20. doi: 10.1007/BF02244774. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: Task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG- saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037/0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: Effects of physostigmine and FG 7142. Behav Neurosci. 1998;112:1519–1525. doi: 10.1037/0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138:266–274. doi: 10.1007/s002130050671. 0.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus- dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–50. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Martinez RA, Hill JL, Weingartner HJ, Thompson K, Vitiello B, Sunderland T. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Rev. 1992;17:215–26. doi: 10.1016/0165-0173(92)90017-G. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mohler EG, Franklin SR, Rueter LE, Fox GB, Decker MW, Browman KE. ABT-594 improves performance in the 5-choice serial reaction time task under conditions of increased difficulty, sub-chronic dosing, and in poorly-performing subjects. Pharmacol Biochem Behav. 2010;95:146–157. doi: 10.1016/j.pbb.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci. 1994;14:2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. Reversal of visual attentional dysfunction following lesions of the cholinergic basal forebrain by physostigmine and nicotine but not by the 5-HT3 receptor antagonist, ondansetron. Psychopharmacology (Berl) 1995;118:82–92. doi: 10.1007/BF02245253. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Kelton M, Corwin J. Nicotinic treatment of Alzheimer’s disease. Biol Psychiatry. 2001;49:268–78. doi: 10.1016/S0006-3223(00)01069-6. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Snyder KS, Mintz J. Paths to relapse – possible transactional processes connecting patient illness onset, expressed emotion, and psychotic relapse. Br J Psychiatry. 1992;161:88–96. [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Olincy A, Stevens KE. Treating schizophrenia symptoms with an α7 nicotinic agonist, from mice to men. Biochem Pharmacol. 2007;74:1192–1201. doi: 10.1016/j.bcp.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Howe MW, Gopalakrishnan M, Decker MW, Sarter M. Regulation and function of the tonic component of cortical acetylcholine release. In: Westerink B, Clinckers R, Smolders I, Sarre S, Michotte Y, editors. Monitoring Molecules in Neuroscience. Vrije Universiteit Brussels; Brussels, Belgium: 2010. pp. 363–365. [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: the contributions of phasic versus tonic components of prefrontal cholinergic activity. In: Pfaff D, Kieffer B, editors. Molecular and Biophysical Mechanisms of Arousal, Alertness and Attention Annals of the New York Academy of Sciences. Vol. 1129. The New York Academy of Sciences; NY: 2008. pp. 225–235. [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal β2 subunit-containing and α7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O’Connell MT, et al. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. Layer- Specific Modulation of the Prefrontal Cortex by Nicotinic Acetylcholine Receptors. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr390. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen. 1980;109:160–174. doi: 10.1037/0096-3445.109.2.160. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–51. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD - A 6-month randomized, placebo-controlled trial with a 6-month extension. Neurology. 2000;54:2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Cholinergic modulation of visual working memory during aging: a parametric PET study. Brain Res Bull. 2009;79:322–332. doi: 10.1016/j.brainresbull.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res. 1989;35:221–240. doi: 10.1016/S0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double- blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: Results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA. Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology. 2010;35:2538–2544. doi: 10.1038/npp.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse ST, Hamilton SE, Potter LT, Nathanson NM, Conn PJ. Muscarinic- induced modulation of potassium conductances is unchanged in mouse hippocampal pyramidal cells that lack functional M1 receptors. Neurosci Lett. 2000;278:61–4. doi: 10.1016/S0304-3940(99)00914-3. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Sawyer R, Jones C, Trawley SL, Marchant NL. Positive effects of nicotine on cognition: the deployment of attention for prospective memory. Psychopharmacology. 2009;202:93–102. doi: 10.1007/s00213-008-1320-7. [DOI] [PubMed] [Google Scholar]
- Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- Sato H, Hata Y, Hagihara K, Tsumoto T. Effects of cholinergic depletion on neuron activities in the cat visual cortex. J Neurophysiol. 1987;58:781–794. doi: 10.1152/jn.1987.58.4.781. [DOI] [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Collateral innervation of the medial and lateral prefrontal cortex by amygdaloid, thalamic, and brain-stem neurons. J Comp Neurol. 1984;224:445–460. doi: 10.1002/cne.902240312. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hagan J, Dudchenko P. Behavioral screening for cognition enhancers: from indiscriminate to valid testing: Part I. Psychopharmacology (Berl) 1992a;107:144–59. doi: 10.1007/BF02245132. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hagan J, Dudchenko P. Behavioral screening for cognition enhancers: from indiscriminate to valid testing: Part II. Psychopharmacology (Berl) 1992b;107:461–73. doi: 10.1007/BF02245257. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/S0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo M, Bruno J, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring W, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M. Preclinical research into cognition enhancers. Trends Pharmacol Sci. 2006;27:602–608. doi: 10.1016/j.tips.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: Integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Paolone G. Deficits in attentional control: Cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci. 2011;125:825–835. doi: 10.1037/a0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2012;62:1544–1553. doi: 10.1016/j.neuropharm.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, Christian EP, Doherty JJ, Quirk MC, Snyder DH, Lah JJ, Levey AI, Nicolle MM, Lindsley CW, Conn PJ. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–86. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Shenhav A, D’Esposito M. Cholinergic enhancement reduces spatial spread of visual responses in human early visual cortex. Neuron. 2008;60:904–914. doi: 10.1016/j.neuron.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StPeters M, Koshy Cherian A, Bradshaw M, Sarter M. Sustained attention in mice: Expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav Brain Res. 2011a;225:574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StPeters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011b;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Arch Gen Psychiatry. 1987;44:418–26. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Tariot PN, Newhouse PA. Differential responsivity of mood, behavior, and cognition to cholinergic agents in elderly neuropsychiatric populations. Brain Res. 1988;472:371–89. doi: 10.1016/0165-0173(88)90013-6. [DOI] [PubMed] [Google Scholar]
- Tandon R, Greden J. Cholinergic hyperactivity and negative schizophrenic symptoms. A model of cholinergic/dopaminergic interactions in schizophrenia. Arch Gen Psychiatry. 1989;46:745–753. doi: 10.1001/archpsyc.1989.01810080075010. [DOI] [PubMed] [Google Scholar]
- Taylor K, Decker MW, Sarter M, Parikh V. Viability of α4β2* nAChRs as a target for treating the cognitive symptoms of schizophrenia in the presence of chronic nicotine and risperidone. Paper presented at the Society for Neuroscience Annual Meeting; Washington, DC. 2011. [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Turchi J, Holley LA, Sarter M. Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology (Berl) 1995;118:195–205. doi: 10.1007/BF02245840. [DOI] [PubMed] [Google Scholar]
- Vangkilde S, Bundesen C, Coull JT. Prompt but inefficient: nicotine differentially modulates discrete components of attention. Psychopharmacology. 2011;218:667–680. doi: 10.1007/s00213-011-2361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, Fink GR. Behavioral and neural effects of nicotine on visuospatial attentional reorienting in non-smoking subjects. Neuropsychopharmacology. 2008;33:731–738. doi: 10.1038/sj.npp.1301469. [DOI] [PubMed] [Google Scholar]
- Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology (Berl) 1998;135:305–310. doi: 10.1007/s002130050514. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology (Berl) 1984;82:147–50. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: Results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74:1212–1223. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Gault LM, Childress A, Kratochvil CJ, Bensman L, Hall CM, et al. Safety and efficacy of ABT-089 in pediatric attention-deficit/hyperactivity disorder: Results from two randomized placebo-controlled clinical trials. J Am Acad Child Adolesc Psychiatry. 2011;50:73–84. doi: 10.1016/j.jaac.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlberg GW, Kornetsky C. Sustained attention in remitted schizophrenics. Arch Gen Psychiatry. 1973;28:533–537. doi: 10.1001/archpsyc.1973.01750340065011. [DOI] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci. 2001;98:14096–101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the α7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Leranth C, Heimer L. Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neurosci Lett. 1984;52:219–225. doi: 10.1016/0304-3940(84)90165-4. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: An ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250:282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: A correlated light and electron microscopic double immunolabeling study in rat. Brain Res. 1992;570:92–101. doi: 10.1016/0006-8993(92)90568-T. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE, Luine VN. Catecholaminergic-cholinergic interaction in the basal forebrain. Prog Brain Res. 1993;98:31–49. doi: 10.1016/S0079-6123(08)62379-1. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Direct catecholaminergic-cholinergic interactions in the basal forebrain. I. Dopamine-betahydroxylase and tyrosine hydroxylase input to cholinergic neurons. J Comp Neurol. 1996;374:535–554. doi: 10.1002/(SICI)1096-9861(19961028)374:4<535::AID-CNE>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/S0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: The last frontier. Prog Brain Res. 2002;136:359–372. doi: 10.1016/S0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: Spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience. 2005;136:697–713. doi: 10.1016/j.neuroscience.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–61. doi: 10.1523/ JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]