Abstract
There is significant interest in the development of agents that can ameliorate radiation damage after exposure to radiation has occurred. Here we report that chronic supplementation of the antioxidant Tempol in the diet of mice can reduce body weight without toxicity, decrease cancer, and extend survival when administered after non-lethal total body radiation (TBI). These effects were apparent in two different strains of mice (C3H, CBA) exposed to TBI (3 Gy). Notably, delaying administration of the Tempol diet 1 month after TBI could also enhance survival. Tempol reduced the incidence of hematopoietic neoplasms (lymphomas) in both strains; whereas, both the onset and incidence of non-hematopoietic neoplasms were reduced in CBA mice. These results encourage further study of Tempol as a chemopreventive, to reduce the incidence of radiation-induced second malignancies after a course of definitive radiation therapy. Tempol may also find applications to reduce the risk of cancers in populations exposed to non-lethal radiation due to nuclear accidents or terrorist attacks.
Keywords: Tempol, radiation, carcinogenesis, antioxidant, chemoprevention
Introduction
Intentional or accidental exposure of humans to ionizing radiation (IR) can lead to a variety of acute effects including lethality or long-term late effects encompassing organ injury (1) and carcinogenesis (2). To protect against these toxicities there has been an ongoing development of chemical and biological radiation protectors or mitigators (3) primarily directed to protection against the acute radiation syndrome following total body irradiation (TBI) or for normal tissue protection in cancer patients treated with radiotherapy (4). A terrorist-mediated nuclear attack or accidental damage to nuclear reactors could place large populations of people exposed to non-lethal IR doses at risk for cancer induction. Such events can occur without warning, necessitating the application of interventions post-IR exposure. Likewise, IR-induced second malignancies can occur after a course of definitive radiation therapy (5, 6). Both of these situations suggest a need for safe and effective interventions to reduce these risks to humans after IR exposure.
The development of chemical and biological radiation protectors or mitigators dates back to the 1940s (7-9). Applications of such agents have been primarily directed to protection against the acute radiation syndrome following TBI or normal tissue protection in radiotherapy patients. Both of these toxicities occur over hours to several weeks post-IR and can be life threatening. Unfortunately IR exposure can also impose late effects including induction of cancer. Most, if not all classical radiation protectors, provide protection only if administered immediately before and during IR treatment (7, 9). Intentional and in some cases accidental IR exposure precludes the use of such protectors for IR-induced carcinogenesis. Therapies or approaches administered post-IR for the reduction in IR-induced carcinogenesis are scarce; however, the long latency period of cancer induction post-IR exposure should provide opportunities for intervention (2). IR-induced cancer induction was significantly reduced after radiation treatment by calorie restriction (CR) (10-12). These studies utilized both rats and mice and employed either a significant reduction in food intake (up to 40% reduction) or reduction in the caloric content in the food to achieve CR resulting in significant reductions in body weight. A 25-40% reduction in calorie intake is necessary to benefit from CR in mice; a calorie reduction that would be difficult for most individuals to reach and maintain who are accustomed to modern dietary patterns (13). Pre-clinical studies conducted in our laboratory have shown that the stable nitroxide free radical, Tempol (14), supplemented in the drinking water or diet of mice results in: a) significant body weight loss without untoward toxicity (15); and b) reduction or delay in the onset of cancer in ATM, p53, and Fanconi anemia knockout mice (16-18). We hypothesized that chronic Tempol supplementation in the diet immediately after non-lethal total body IR may mimic the effects of CR by reducing IR-induced carcinogenesis and enhancing survival in mice. To test this hypothesis the lifespans of two different mouse strains were compared after exposure to non-lethal 3 Gy TBI and administration of Tempol or control diets after IR.
Materials and Methods
Mice
Female C3H/HenTac-MTV- (Taconic Farms, Germantown, NY) and CBA/CaJ (Jackson Laboratory in Bar Harbor, ME) mice were used. A special breeding program for both animal strains was initiated to provide 750 C3H and 600 CBA mice at 4 weeks of age. Mice were housed in a specific pathogen free (SPF) facility on a 12 hr day/night cycle with standard laboratory chow and water ad libitum. When the mice were 8-9 weeks of age, they were divided into various unirradiated control and TBI groups. The groups included 0 Gy control diet (0 Gy C), 3 Gy control diet (3 Gy C), 0 Gy Tempol diet (0 Gy T), 3 Gy Tempol diet (3 Gy T), and 3 Gy Tempol, diet delayed 1 month post-TBI (3 Gy T Delayed; C3H mice only). Immediately following TBI the chow for all groups was switched to bacon-flavored control chow or a bacon-flavored chow containing Tempol and water ad libitum. Powdered Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl; supplied by Mitos Pharmaceuticals, Newport Beach, CA) was uniformly mixed with bacon-flavored mouse chow by a “cold press” technique (Bio-Serv, Frenchtown, NJ, USA) at concentration equivalent to 58 mM (10 mg/g of food). Control animals received bacon-flavored chow minus the Tempol. Mice were maintained on these respective chows for their lifespan in climate controlled, circadian rhythm-adjusted rooms (5 mice/cage on Sani-Chip bedding). Average weights of the mice were determined several times a week for the first few weeks post-TBI by weighing the cage of animals and dividing their total weight by the number of animals. Periodic weight assessments were subsequently made approximately monthly post-TBI. Average food consumption was conducted periodically by randomly selecting 5 cages of mice from each group and determining the food consumed over a two-week period. All experiments were carried out under the aegis of a protocol approved by the National Cancer Institute Animal Care and Use Committee and were in compliance with the Guide for the Care and Use Of Laboratory Animal Resource, (2008) National Research Council.
Assessment, Necropsy, and Pathology
Animals were carefully monitored a minimum of three times per week for their entire lifespan. The endpoint for the study was tumor formation (not to exceed 2 cm diameter) or until the animal reached a humane endpoint (rapid weight loss, debilitating diarrhea, rough hair coat, hunched posture, labored breathing, lethargy, persistent recumbence, jaundice, significantly abnormal neurological signs, bleeding from any orifice, proptosis or abnormal appearance of eyes, impaired mobility, or inability to obtain food or water) at which time the animal was euthanized and evaluated for the presence of tumor and cause of death. Mice were euthanized by CO2 inhalation and blood collected from the thoracic aorta for a complete blood count (CBC). A comprehensive necropsy examination was performed on each mouse with descriptions of gross lesions, collection of all major organs, tissues and lesions and fixation of pathology materials in 10% buffered neutral formalin. Tissues were processed and stained with hematoxylin and eosin (H&E). A board-certified veterinary pathologist performed pathology evaluation. Special stains and immuno-histochemistry were performed on a subset of animals to clarify the major form of hematopoietic neoplasms (HN).
Statistical Analysis
Data were analyzed using R (version 2.14), SAS Software version 8.2, and MATLAB. Overall survival probabilities by age were estimated using the Kapan-Meier method. The logrank test was used to test for significant differences in survival between groups. Cumulative incidence curves by age were calculated for the competing risks of death after hematopoietic neoplasm, death after non-hematopoietic neoplasm, and non-cancer death, using the non-parametric maximum likelihood estimator (19). For mice with both hematopoietic and non-hematopoietic neoplasm, the former was counted as the first event. Relative hazards were estimated using the Cox proportional hazards method. P values < 0.05 were considered to show a statistical trend.
Gene Expression Studies
Brain, liver, and muscle tissues (5-8 mice per time point) were collected from Tempol- and control-fed C3H mice after 14 and 60 days and stored at -80°C. Total RNA was extracted from frozen tissues using TRIzol Reagent (Life Technologies, Inc., Carlsbad, CA) according to the standard protocol. RNA concentration was determined with the NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). Samples were then treated with DNase using the Ambion® DNA-free™ Kit (Life Technologies, Inc., Carlsbad, CA). Amplified antisense RNA was produced from the samples by using the Arcturus® RiboAmp® PLUS Amplification Kit (Life Technologies, Inc., Carlsbad, CA). The mRNA was labeled and hybridized as previously described (20).
Further details on materials and methods can be found in Supplemental Materials and Methods.
Results
Animal Weights and Food Consumption
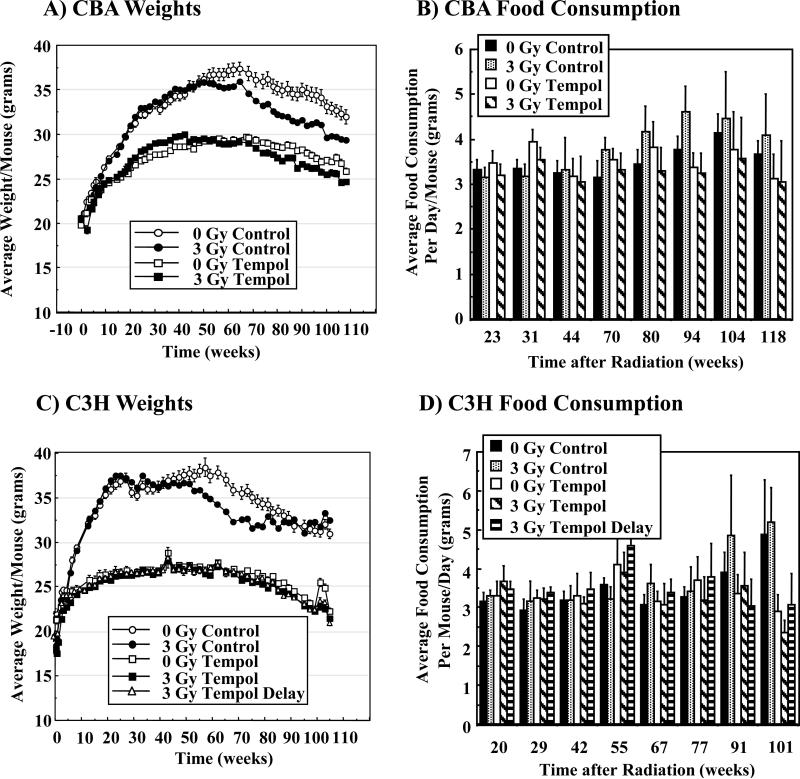
Figures 1A and C show the average weight per mouse for both the CBA and C3H strains over the study. Both strains of mice maintained on the Tempol diet gained significantly less weight compared to controls, yet remained healthy throughout their lifespan as previously reported for C3H mice (15). For CBA mice 0 Gy C and 3 Gy C groups exhibited similar weight profiles to ~ 50 weeks (Figure 1A). 3 Gy C CBA mice exhibited lower weights than 0 Gy C mice beyond 50 weeks post-IR (p < 0.02). The Tempol diet resulted in reduced weight gain starting at ~10 weeks compared to mice on the control diet (p < 0.001). There was no statistical difference in the weights of 0 Gy T or 3 Gy T mice throughout the study. C3H mice exhibited similar weight profiles (Figure 1C) as observed for CBA mice. Weights of 0 Gy C C3H mice increased rapidly to 25 weeks, peaked at 60 weeks (~ 37 g) and then slowly decreased as the animals aged (Figure 1C). The 0 Gy T group of C3H mice gained weight similar to control animals up to ~5 weeks, followed by a plateau in weights to ~50 weeks (~27 g), and then decreased slowly. The 0 Gy C mice had significantly higher weights (p < 0.001) than 0 Gy T mice from ~10-80 weeks with a maximum difference of ~38% at 55 weeks. Weights for 3 Gy C C3H mice were similar to the 0 Gy C to ~ 50 weeks. The weights of the irradiated mice decreased more rapidly from 50-80 weeks than un-irradiated mice (p < 0.005). Irradiated mice on the Tempol diet (both those placed on the Tempol diet immediately after IR or 1 month after IR) exhibited near identical weights to 0 Gy T controls throughout the study. The decreased weight gain of both mouse strains receiving the Tempol diet could not be explained by reduced food consumption as shown in Figure 1B, D. Food consumption monitored from weeks 23-80 (CBA) and weeks 20-77 (C3H) showed no statistical difference among any of the groups.
Figure 1.
Average weight/mouse (grams) of various groups of CBA (A) and C3H (C) mice as a function of time. Average food consumption (grams) per mouse/day for the various groups of CBA (B) and C3H (D) mice.
Survival and Mortality Assessment
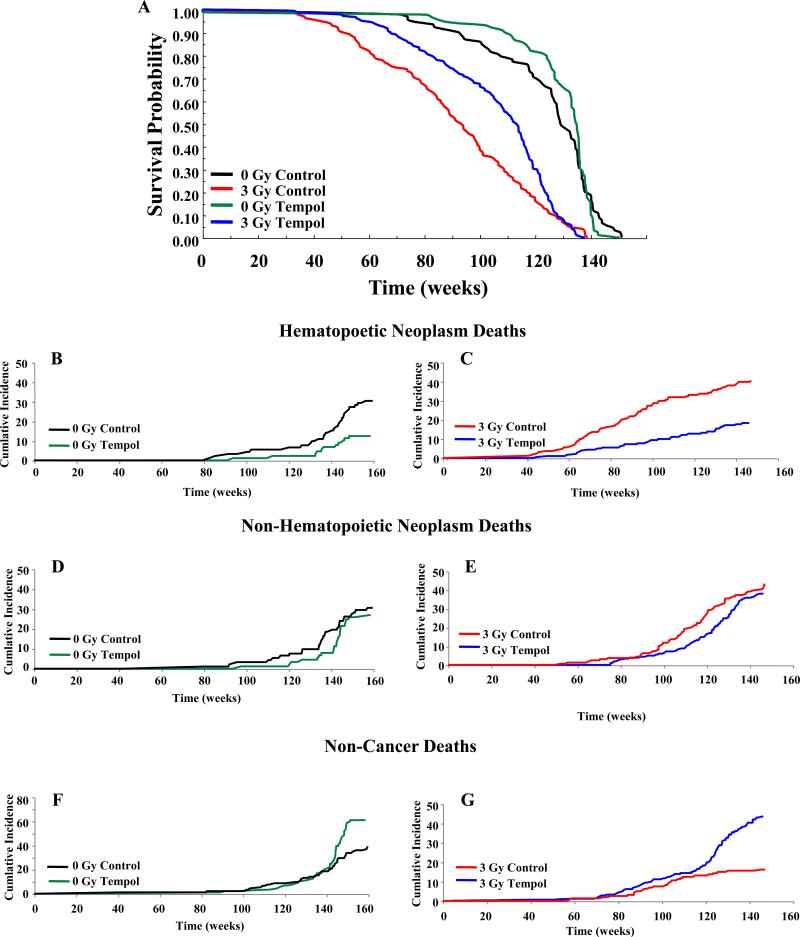
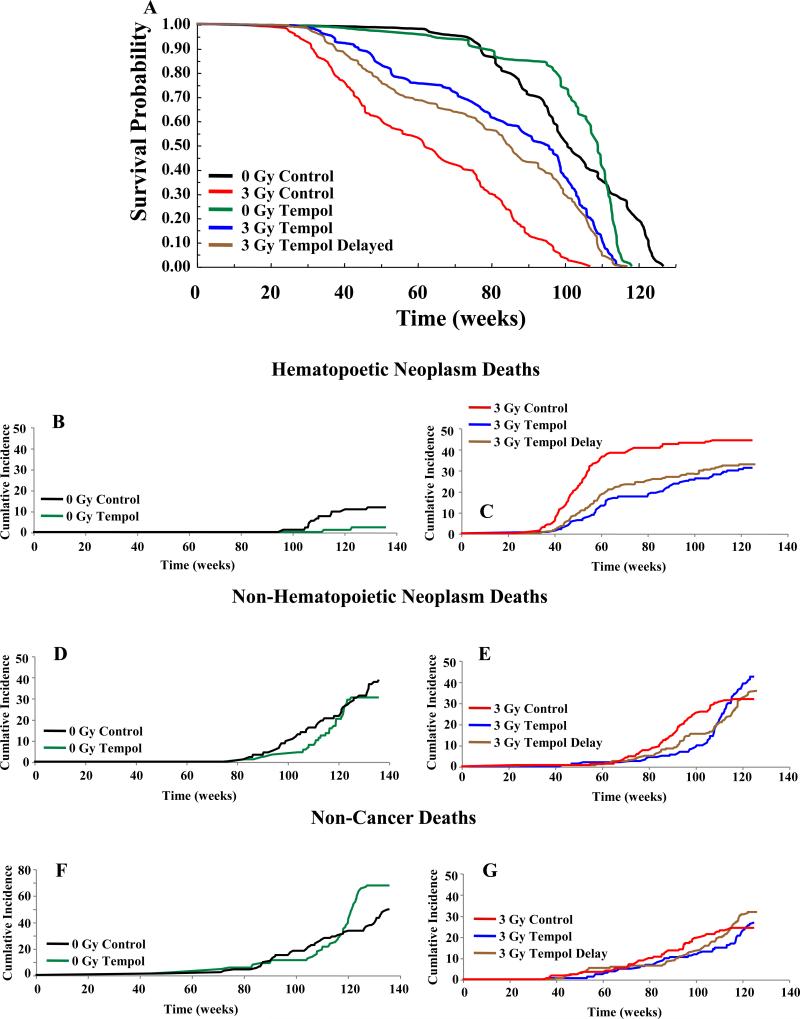
Figures 2A, 3A show Kaplan-Meier survival plots while Tables 1, 2 list the median survival times for CBA and C3H mice, respectively. Log rank analysis of the survival curves showed no significant difference in the lifespan of 0 Gy C versus 0 Gy T (CBA, p = 0.91; C3H, p < 0.06). Overall, chronic Tempol food supplementation was well tolerated by both mouse strains with little untoward toxicity. In the Tempol fed C3H mice toward the end of their lifespan (>98 weeks), transmural proximal duodenal ulcers and peritonitis were noted. This was not observed in the CBA mice. There was a substantial reduction in the lifespan after 3 Gy TBI compared to unirradiated controls for both mice strains; 34 weeks less for CBA mice (p <0.001) and 38 weeks less for C3H (p < 0.001) mice. Tempol diet supplementation initiated immediately after TBI resulted in a significant survival advantage compared to the control diet for both CBA and C3H mice (CBA, p < 0.003; C3H, p < 0.001), representing an extension in survival of ~18 weeks and ~34 weeks for CBA and C3H mice, respectively. Interestingly, delaying the Tempol diet one month post-TBI in C3H mice also provided a significant survival advantage (23 weeks) compared to the 3 Gy C (p < 0.001). However, there was no statistical difference between the 3 Gy T and 3 Gy T Delayed groups (see Figure legend 3).
Figure 2.
A) Kaplan-Meier survival plots for CBA mice. Mice were exposed to either 0 or 3 Gy TBI with Tempol food supplementation initiated immediately after radiation and maintained throughout the lifespan. Statistics using log rank analysis of the survival curves: 0 Gy C vs 0 Gy T (p = 0.91); 3 Gy C vs 3 Gy T (p < 0.003). B) Cumulative incidence of deaths for various groups of CBA mice shown in A: hematopoietic neoplasms (B, C), non-HN (D, E), and non-cancer deaths (F, G).
Figure 3.
A) Kaplan-Meier survival plots for C3H mice. Mice were exposed to either 0 or 3 Gy TBI with Tempol food supplementation initiated immediately after radiation and maintained throughout the lifespan. Statistics using log rank analysis of the survival curves: 0 Gy C vs 0 Gy T (p = 0.06); 0 Gy C vs 3 Gy C (p < 0.001); 0 Gy T vs 3 Gy T (p < 0.001); 3 Gy C vs 3 Gy T (p < 0.001); 3 Gy C vs 3 Gy T Delayed (p < 0.001); 3 Gy T vs 3 Gy T Delayed (p = 0.07). B) Cumulative incidence of deaths for various groups of C3H mice shown in A: hematopoietic neoplasms (B, C), non-HN (D, E), and non-cancer deaths (F, G).
Table 1.
Median Survival and Percentage Distribution of Neoplasms in CBA Mice‡
| Group | Number of Mice | Median Survival in weeks (CI)# | Number of Mice | |||
|---|---|---|---|---|---|---|
| Malignant Neoplasm† (%) | Non-Hematopoietic Neoplasm* (%) | Hematopoietic Neoplasm* (%) | Benign Neoplasm (%) | |||
| 0 Gy Control | 92 | 128.7 (126.3-134.3) | 56 (61%) | 42 (46%) | 28 (30%) | 72 (78%) |
| 3 Gy Control | 161 | 94.3 (89.3-99.3) | 134 (83%) C:IR p = 0.001 | 96 (60%) C:IR p = 0.036 | 65 (40%) C:IR p = 0.14 | 110 (68%) C:IR p = 0.11 |
| 0 Gy Tempol | 89 | 134.1 (132.3-135.6) | 35 (39%) T:C p = 0.005 | 26 (29%) T:C p = 0.031 | 11 (12%) T:C p = 0.004 | 60 (67%) T:C p = 0.13 |
| 3 Gy Tempol | 181 | 111.7 (108.6-115.1) | 102 (56%) T:IR-T p = 0.005 | 80 (44%) T:IR-T p = 0.012 | 33 (18%) T:IR-T p = 0.22 | 121 (67%) T:IR-T p = 0.78 |
Statistical comparisons: T:C = 0 Gy T to 0 Gy C; C:IR = 0 Gy C to 3 Gy C; T:IR-T = 0 Gy T to 3 Gy T
CI; 95% confidence interval.
Malignant neoplasms include both HN + non-HN.
Percentages in these two categories do not add up to the total percentage of malignant neoplasms because some mice had both hematopoietic and non-HN.
Table 2.
Median Survival and Percentage Distribution of Neoplasms in C3H Mice‡
| Group | Number of Mice | Median Survival in weeks (CI)# | Number of Mice | |||
|---|---|---|---|---|---|---|
| Malignant Neoplasm† (%) | Non-Hematopoietic Neoplasm* (%) | Hematopoietic Neoplasm* (%) | Benign Neoplasm (%) | |||
| 0 Gy Control | 93 | 100.9 (97.1-109) | 47 (51%) | 39 (42%) | 11 (12%) | 46 (49%) |
| 3 Gy Control | 170 | 62 (54.7-70.6) | 129 (76%) C:IR p < 0.01 | 60 (35%) C:IR p = 0.29 | 75 (44%) C:IR p < 0.01 | 81 (47%) C:IR p = 0.79 |
| 0 Gy Tempol | 89 | 109.1 (107-111) | 29 (33%) T:C p = 0.02 | 27 (30%) T:C p = 0.12 | 2 (2%) T:C p = 0.02 | 72 (81%) T:C p < 0.01 |
| 3 Gy Tempol | 161 | 95.7 (87.7-98.9) | 118 (74%) T:IR-T p < 0.01 | 75 (47%) T:IR-T p = 0.015 | 50 (31%) T:IR-T p < 0.01 | 122 (76%) T:IR-T p =0.43 |
| 3 Gy Tempol Delayed | 202 | 84.9 (78.9-92.7) | 138 (68%) T:IR T-D p < 0.01 | 83 (41%) T:IR T-D p = 0.09 | 66 (33%) T:IR T-D p < 0.01 | 132 (65%) T:IR T-D p = 0.01 |
Statistical comparisons: T:C = 0 Gy T to 0 Gy C; C:IR = 0 Gy C to 3 Gy C; T:IR-T = 0 Gy T to 3 Gy T; T:IR T-D = 0 Gy T to 3 Gy T-D
CI; 95% confidence interval.
Malignant neoplasms include both HN + non-HN.
Percentages in these two categories do not add up to the total percentage of malignant neoplasms because some mice had hematopoietic and non-HN.
Specific mortality analyses (cancer) for both mouse strains are described in Tables 1 and 2 and Figures 2B-G, 3B-G. The spectrum of neoplasms were not changed in either mouse strain by Tempol food supplementation (see Supplemental Tables 1, 2), consistent with previous reports (15-18). Over their lifespan 61% of 0 Gy C CBA mice had malignant neoplasms (hematopoietic and non-HN), compared to 39% (p < 0.005) for 0 Gy T mice. As anticipated, TBI increased malignant neoplasms by 22% and 17% for 3 Gy C and 3 Gy T CBA mice, respectively. Hematopoietic neoplasms (HN) were primarily lymphomas of B cell origin (data not shown). Table 1 also lists the percentages of neoplasms, for both malignant and benign tumors. For un-irradiated mice, the Tempol diet lowered the incidence of all forms of neoplasms (non-HN: 37%↓, HN: 60%↓, and benign: 14%↓). Likewise, 3 Gy T mice reduced the incidences of malignant neoplasms compared to 3 Gy C mice (non-HN: 27%↓, HN: 55%↓). Temporal analyses of the cumulative incidence of malignant HN and non-HN, and non-cancer related deaths for CBA mice are shown in Figures 2B-G. Tempol treatment delayed the onset of both HN and non-HN in IR and un-irradiated mice. The onset for HN death for 0 Gy C versus T mice changed from 87 to 106 weeks (p = 0.008), and for 3 Gy C versus T mice from 39 to 55 weeks (p = 0.03). Similarly, Tempol treatment delayed the onset of non-HN death for 0 Gy C versus T from 84 weeks to 111 weeks (p < 0.001) and for 3 Gy C versus T from 55 weeks to 77 weeks (p < 0.001). Thus, TBI shortened the lifespan of mice and Tempol treatment delayed the onset of radiation-induced neoplasms for CBA mice. Tempol treatment increased the non-cancer deaths for CBA mice as they approached the end of their lifespan.
As was seen for CBA mice, Tempol exhibited significant reductions in HN and non-HN for un-irradiated C3H mice (Table 2, Figure 3B-G). In this strain, lymphomas were also the most common HN. Over their lifespan 51% of 0 Gy C C3H mice had malignant neoplasms, compared to 33% (p < 0.001) for 0 Gy T mice (Table 2). TBI significantly increased malignant neoplasms in C3H mice by 25% (p < 0.01) from the 0 Gy C; whereas, the increase was 41% for 3 Gy T mice and 35% for 3 Gy T delayed. In contrast to the CBA mice however, the number of C3H mice with benign neoplasms on Tempol diet increased (49%, 0 Gy C to 81%, 0 Gy T, p < 0.01, Table 2). The increase in benign neoplasms was dominated by increases in hepatocellular adenomas (30% 0 Gy C versus 74% in 0 Gy T mice (p < 0.001); see Supplemental Table 4). It is well known that C3H mice are susceptible to hepatocellular tumors (21), which was not as prominent in CBA mice (Supplemental Table 3). However, it is important to note that the number of hepatocellular carcinomas in unirradiated C3H mice was not increased with the Tempol diet (9% to 6%) suggesting that Tempol was likely not facilitating the conversion of adenomas to carcinomas.
There were several differences between the two mouse strains with respect to IR-induced neoplasms. Supplemental Table 3 shows that the normalized ratio of HN to non-HN after 3 Gy IR was approximately 4.5 fold higher in C3H mice as opposed to CBA mice, where the ratio remained unchanged compared to 0 Gy C mice. Tempol reduced both HN and non-HN incidence in the 0 Gy C groups of both mouse strains; however, its effects were greatest on HN (compare 0 Gy C to 0 Gy T, Supplemental Table 5). A second major difference in the 3 Gy C groups between CBA and C3H mice was the non-HN incidence, which increased in CBA mice by 14% and decreased in C3H mice by 7%. Non-HN in C3H mice did increase after 3 Gy in the Tempol fed mice from 30% to 47% (Tables 1, 2). No significant differences with respect to either HN or non-HN were observed in the 3 Gy T delay group compared to the 3 Gy T group (Table 2). Lastly, the rate of HN incidence was accelerated in C3H mice compared to CBA mice (compare HN incidence in Figure 3C versus Figure 2C).
The data presented thus far was analyzed for survival and cumulative neoplasm incidences over the entire life span of the mice. To obtain a more refined analysis of the different modes of death for the study, relative hazard (RH) ratios were calculated for both animal strains for two intervals; 0 - 104 weeks and beyond 104 weeks (Supplemental Tables 6, 7). For the first two years of life Tempol provided a significant overall survival benefit for both 0 Gy and 3 Gy groups in CBA mice (RH CBA = 0.33 and 0.47, respectively, Supplemental Table 6). Beyond 104 weeks there was no overall survival advantage for Tempol treatment for 0 Gy or 3 Gy (RH = 1.14 and 1.05, respectively). For 0 Gy, Tempol treatment reduced both HN and non-HN death over the entire time span (Supplemental Table 6). There was a significant increase in non-cancer deaths beyond 104 weeks (RH = 1.92, Supplemental Table 6). The non-cancer deaths did not become apparent until after week 139 (Figure 2F; maximum lifespan = 160 weeks). Similarly, for the 3 Gy T CBA group, there was a highly significant reduction in both HN and non-HN (RH = 0.29 and 0.36, respectively) and an increase in non-cancer deaths beginning at week 120 (Figure 2B; RH = 3.31, Supplemental Table 6). Likewise, Tempol treatment afforded an overall survival benefit in C3H mice for both 0 Gy, 3 Gy, and 3 Gy delayed up to 104 weeks (RH = 0.45, 0.34, and 0.46, respectively; Supplemental Table 7). Unlike the CBA mice, Tempol treatment beyond 104 weeks resulted in a decrease in overall survival for 0 Gy mice (RH = 2.26). The decrease was evident at approximately week 110 (Figure 3A; maximum lifespan = 136 weeks), contributed predominantly by non-cancer deaths (RH = 4.5). Unlike the 0 Gy group, 3 Gy T treatment groups beyond 104 weeks had RH values < 1.0, indicating that Tempol had beneficial effects in all categories (Supplemental Table 7).
Early Gene Expression Patterns in Tempol Supplemented C3H Mice
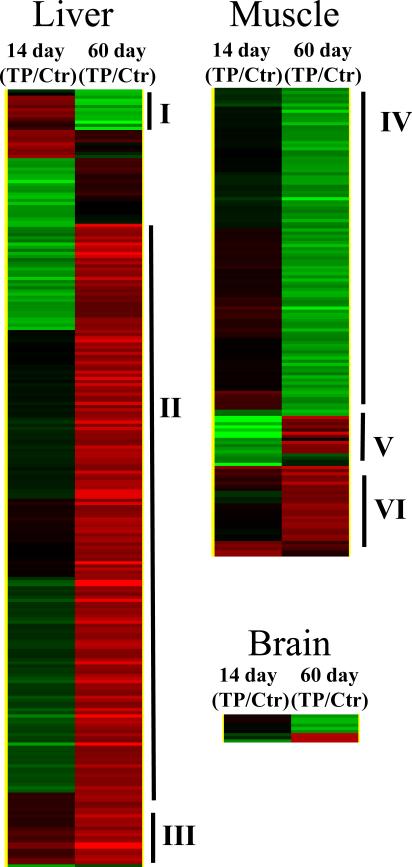
Gene expression profile studies of selected tissues were conducted to obtain early gene expression changes by Tempol in mice prior to changes in weight (14 days) and later when animal weights were reduced by Tempol (60 days). Figure 4 shows hierarchical cluster maps monitoring 2-fold gene changes in muscle, liver, and brain (at both time points) comparing Tempol to control mice. The majority of gene changes (both up- and down-regulated) resulting from the Tempol diet occurred in the liver (248 genes), followed by the muscle (150 genes), and brain (9 genes). Numbers to the right of each map indicates clustered gene profiles and gene ontology categories are listed in Supplemental Table 8. The prominent genes in cluster I were IGF-1 and Ghr, which were down-regulated after 60 days of Tempol diet (I). The Tempol diet up-regulated genes related to mitochondrial function and fatty acid and/or lipid synthesis in cluster II. Drug metabolizing genes (cluster III) were elevated at both 14 and 60 days of Tempol treatment; the only cluster up-regulated at both time points. For muscle, the majority of genes were down regulated with exception to cluster VI where a number of mitochondrial genes were up regulated with the Tempol diet.
Figure 4.
Gene expression patterns in muscle, liver, and brain tissues taken from C3H mice on control or Tempol supplemented diets for 2 weeks or 2 months. Expression patterns were analyzed by hierarchical clustering monitoring 2-fold gene changes in the tissues. The cluster maps were normalized to the 2 week control diet mice. (gene expression: green, up-regulated; red, down-regulated)
Discussion
The hypothesis was tested that Tempol food supplementation post-TBI would mimic the effects of CR in mice by providing a reduction in IR-induced cancers and a survival advantage. Two mouse strains were evaluated having different life spans, HN incidence post-IR, and the rate and time of HN onset. The data presented in this study clearly show that Tempol reduced the incidence of cancer post-TBI in C3H and CBA mice, thereby providing a significant survival advantage. Tempol treatment (for both unirradiated and irradiated groups) exerted a major effect on HN as shown in Supplemental Table 5. For CBA mice IR exposure resulted in a near equal proportion of HN to non-HN neoplasms compared to 0 Gy C; whereas, IR-induced HN was approximately 5 fold higher in C3H as compared to CBA mice. Of note, HN incidence was elevated approximately 11-fold over non-HN for individuals receiving > 2 Gy in the Japanese atomic bomb survivors (22). Therefore, the C3H strain used in the present study may be a more relevant model for predicting tumor incidence after IR exposure. Additionally, Tempol treatment reduced HN incidence in both strains for unirradiated and irradiated mice; however, the overall decrease was greater in C3H mice. These differences could explain the greater survival afforded by Tempol in C3H mice compared with CBA mice. Tempol administered one-month post-IR exposure was as effective as administration immediately after IR. This latter finding is important in that it suggests that there may be a modifiable time threshold for IR-induced life shortening. Should this finding translate to humans, it would suggest that treatment for non-lethal IR exposure with respect to carcinogenesis might be delayed for some time allowing for IR dose assessment under less chaotic conditions that often accompanies an accident or terrorist event.
Mice exposed to 3 Gy TBI exhibited a reduced life span compared to un-irradiated control mice as evidenced by shorter median survival times (Tables 1, 2). Tempol food supplementation provided significant median survival advantages post-TBI in both mouse strains. These findings are in agreement with previous studies using cancer-prone mice where Tempol food supplementation afforded significant extensions in median survival (16-18).
There was no observable effect of Tempol on non-cancer deaths following IR in C3H mice. Overall, RH analysis of the C3H data clearly show an advantage for Tempol treatment in mice up to 104 weeks for both control and IR treated groups. Beyond 104 weeks, RH analysis shows an advantage for irradiated mice on Tempol; however, in unirradiated mice Tempol exerted a modest adverse effect relative to control with regard to overall survival, non-HN, and non-cancer deaths (Table 4). The reason for this latter finding is unclear; however, it may reside in compromised or reduced drug metabolism in elderly mice (23). Mice greater >104 weeks can be considered geriatric with expected perturbations in homeostasis. Alternatively, weights in the Tempol group may have dropped below a minimal acceptable level, which in elderly mice could have contributed to late adverse effects observed as discussed below.
With respect to animal weights, Tempol administration resulted in similarities and differences to CR. First, Tempol did not significantly extend the lifespan of un-irradiated CBA or C3H mice. CR has been shown to substantially extend lifespan of a number of mouse strains (24). However, not all mouse strains exhibit this phenomenon (25). It appeared from the Kaplan-Meier plots (Figure 2A, 3A) that Tempol was indeed extending the lifespan of both strains prior to the median survival; however, as the animals neared the end of their lifespan there was an abrupt drop in the survival curve. While the median life spans for both strains on Tempol were not statistically different from controls, the RHs clearly indicate that Tempol exhibited adverse effects on survival near the end of the lifespan. The reason for this observation is unclear; however, it could relate to the reduced weights in elderly mice. Maintenance of a normal body weight is an important survival parameter in aging. Reduced weight in the elderly is a poor prognostic indicator (26, 27). Further an increase in mortality was shown when 17-24 month old mice were switched from an ad libitum diet to a CR diet (25), suggesting that substantial weight loss or dropping below a minimum weight in old age is detrimental. Conversely, when mice were maintained on a CR diet for 24 months and then switched to an ad libitum diet, the lifespan was extended comparable to the continuous CR diet (25). Thus, terminating Tempol administration and preventing reduced weight in the last 25% of the lifespan might have extended the lifespan.
Both CR and Tempol diet supplementation have been shown to result in decreased protein levels of leptin (15, 24) and circulating IGF-1 levels (24, 28, 29). Both of these metabolic hormones have been implicated in the pathogenesis and/or progression of cancer (30, 31). It is interesting to note that gene expression profiles (Figure 4, cluster I, Supplemental Table 8) showed IGF-1 expression levels down-regulated after 60 days on the Tempol diet, consistent with the decreased systemic levels reported (29). Leptin, a cytokine primarily produced by adipocytes, while important in energy balance, weight homeostasis, and food intake, can influence hematopoietic progenitor cell growth and drive myelocytic cell growth (32, 33). Thus, reduced levels of leptin may inhibit or impede carcinogenesis. However, evidence in the literature is not clear as to whether altered leptin levels can inhibit carcinogenesis in experimental models (24). IGF-1 regulates tissue growth and metabolism and elevated levels have been shown to be associated with increased risk of several types of human cancer (34, 35). Interestingly, restoration of IGF-1 levels in CR mice removes the anti-proliferative effects on leukemia cell growth in CR mice (36, 37), suggesting that reduced circulating IGF-1 levels are involved in the reduction of carcinogenesis in CR mice. Collectively, the reduced levels of leptin and IGF-1 in mice on the Tempol diet could explain in part the reduced incidence of neoplasms and delay of onset of neoplasms in the present study.
The Tempol diet significantly exerted dramatic effects on gene expression profiles in the liver shortly after administration (Figure 4). Since Tempol is not an endogenous molecule it was of little surprise that drug metabolism/detoxification genes (Figure 4, Supplemental Table 8) relating to acute phase I and II genes were up regulated in the liver. Gene expression profiles for times longer than 60 days have not been done. It would interesting to determine if elevated steady state levels of detoxification gene expression occur over the lifespan of the mice, particularly toward the end of the lifespan when the Tempol diet exerted adverse effects. As mentioned above, detoxification enzyme activities in mice have been shown to diminish with aging (38), hence the detoxification of Tempol could have been compromised in the older mice. Tempol has been shown to be as effective as CR in mice with regard to inhibition of transcriptional markers related to aging (39). Conversely, there was a significant difference between CR and Tempol with regard to fatty acid/lipid synthetic genes. CR has been shown to down-regulate SREBP-1c and fatty acid synthetase, while 60 days on Tempol significantly up-regulated these genes. The reason for this discrepancy is not known, but clearly Tempol, like CR, reduces adipose tissue.
Tempol has been shown to exert potent antioxidant activity in a variety of in vitro and in vivo models (14, 40). Because Tempol delayed the onset and incidence of cancer, one interpretation of the findings might be that the promotion/progression steps of IR-induced carcinogenesis may involve free radicals or reactive oxygen species (ROS) processes that can be modified by Tempol. Further, this process occurs over time since Tempol provided a survival advantage and decreased the HN incidence in C3H mice when administered 1 month post-IR. In support of this notion are studies where irradiated bone marrow cells from CBA mice exhibited significant ROS generation 7 days post-IR (41). ROS can inflict DNA damage resulting in chromosomal instability and aberrations leading to carcinogenesis (42). Delayed induction of chromosome damage in BALB/c epithelial cells, reflecting genomic instability over as many as 28 population doublings times post-IR (43) have been reported suggesting that the progression to IR-induced cancer requires considerable time from the initiating event. Additionally, IR can induce chronic inflammation in tissues (44, 45) resulting in cytokine signaling lasting for months post-IR (46). ROS generation often accompanies chronic inflammation and cytokine signaling (47). The role of antioxidants as chemoprevention agents is controversial in that several clinical trials have shown no benefit of antioxidants in reducing or preventing carcinogenesis (48); however, pre-clinical studies, including the present study, have shown that selected antioxidants, protease inhibitors, and dietary supplements can suppress certain IR-induced cancers (49).
With the exception of CR, many rodent carcinogenesis studies evaluating potential chemopreventive agents or strategies do not include exposure to the agent for the entire lifespan. Chemical carcinogens often used in many of these studies result in tumor formation well before the end of the animal's lifespan. Thus, potential toxicities of the agents are usually not evaluated for the entire lifespan. In the present study Tempol was evaluated for the lifespan and exhibited no toxicities until near the end of the lifespan. All RH ratios for both mouse strains up to 104 weeks indicated positive effects on overall survival, neoplasms, and non-cancer deaths by Tempol (0 Gy and 3 Gy). Maintaining a CR diet for extended periods would be difficult for humans making a simple food additive or time-release daily capsule a more viable option to reduce IR-induced carcinogenesis. Such approaches should be considered for cancer patients successfully treated with IR, but potentially susceptible for secondary cancers or for individuals accidently or intentionally exposed to non-lethal IR. Thus, agents such as Tempol might be a reasonable initial therapy option. Further research will have to done to better define the maximum delay after IR for initiating Tempol treatment and the optimal treatment duration to positively impact survival and reduce cancer incidence.
Supplementary Material
Acknowledgements
The authors thank Drs. RJ Michael Fry, Michael Potter, and C. Norman Coleman for helpful discussions regarding the experimental design of the study, Darlene Green and the necropsy staff of the Pathology/Histotechnology Laboratory for technical assistance, and Drs. Deborah Citrin, Kevin Camphausen, and Blanche Alter for critical review of the manuscript. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This research was supported by the NIAID Medical Countermeasures against Radiological and Nuclear Threats Program and the Intramural Research Program of the Center of Cancer Research, National Cancer Institute, National Institutes of Health and in part with federal funds from the NCI, NIH, under Contract No. HHSN261200800001E. The authors declare that there are no potential conflicts of interest.
References
- 1.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 2.Kohn HI, Fry RJ. Radiation carcinogenesis. N Engl J Med. 1984;310:504–11. doi: 10.1056/NEJM198402233100807. [DOI] [PubMed] [Google Scholar]
- 3.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 4.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–71. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010;20:67–78. doi: 10.1016/j.semradonc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newhauser WD, Durante M. Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer. 2011;11:438–48. doi: 10.1038/nrc3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patt HM, Tyree EB, Staube RL, Smith DE. Cysteine protection against X-irradiation. Science. 1949;110:213–4. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- 8.Yuhas JM, Storer VB. Differential chemoprotection of normal and malignant tissues. J Natl Cancer Inst. 1969;42:331–5. [PubMed] [Google Scholar]
- 9.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85:539–73. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 10.Gross L, Dreyfuss Y. Reduction in the incidence of radiation-induced tumors in rats after restriction of food intake. Proc Natl Acad Sci U S A. 1984;81:7596–8. doi: 10.1073/pnas.81.23.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross L, Dreyfuss Y. Inhibition of the development of radiation-induced leukemia in mice by reduction of food intake. Proc Natl Acad Sci U S A. 1986;83:7928–31. doi: 10.1073/pnas.83.20.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida K, Inoue T, Nojima K, Hirabayashi Y, T S. Calorie restriction reduces the incidence of myeloid leukemia induced by a single whole-body radiation in C3H/He mice. Proc Natl Acad Sci. 1997;94:2615–9. doi: 10.1073/pnas.94.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–50. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soule BP, Hyodo F, Matsumoto K, et al. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–50. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JB, Xavier S, DeLuca AM, et al. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 16.Schubert R, Erker L, Barlow C, et al. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13:1793–802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 17.Erker L, Schubert R, Yakushiji H, et al. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum Mol Genet. 2005;14:1699–708. doi: 10.1093/hmg/ddi181. [DOI] [PubMed] [Google Scholar]
- 18.Zhang QS, Eaton L, Snyder ER, et al. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Res. 2008;68:1601–8. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]
- 19.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–9. [Google Scholar]
- 20.Cook JA, Chuang EY, Tsai MH, et al. Radiation-induced changes in gene-expression profiles for the SCC VII tumor cells grown in vitro and in vivo. Antioxid Redox Signal. 2006;8:1263–72. doi: 10.1089/ars.2006.8.1263. [DOI] [PubMed] [Google Scholar]
- 21.Drinkwater NR, Ginsler JJ. Genetic control of hepatocarcinogenesis in C57BL/6J and C3H/HeJ inbred mice. Carcinogenesis. 1986;7:1701–7. doi: 10.1093/carcin/7.10.1701. [DOI] [PubMed] [Google Scholar]
- 22.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- 23.Zhang YK, Saupe KW, Klaassen CD. Energy restriction does not compensate for the reduced expression of hepatic drug-processing genes in mice with aging. Drug Metab Dispos. 2010;38:1122–31. doi: 10.1124/dmd.110.032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–2. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losonczy KG, Harris TB, Cornoni-Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol. 1995;141:312–21. doi: 10.1093/aje/141.4.312. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–18. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 28.Dunn SE, Kari FW, French J, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–72. [PubMed] [Google Scholar]
- 29.Samuni Y, Cook JA, Choudhuri R, et al. Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic Biol Med. 2010;49:667–73. doi: 10.1016/j.freeradbiomed.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu MH, Chou YC, Chou WY, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–82. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 32.Umemoto Y, Tsuji K, Yang FC, et al. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood. 1997;90:3438–43. [PubMed] [Google Scholar]
- 33.Yun JP, Behan JW, Heisterkamp N, et al. Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev Res (Phila) 2010;3:1259–64. doi: 10.1158/1940-6207.CAPR-10-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 35.Petridou E, Dessypris N, Spanos E, et al. Insulin-like growth factor-I and binding protein-3 in relation to childhood leukaemia. Int J Cancer. 1999;80:494–6. doi: 10.1002/(sici)1097-0215(19990209)80:4<494::aid-ijc2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.Hursting SD, Switzer BR, French JE, Kari FW. The growth hormone: insulin-like growth factor 1 axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats. Cancer Res. 1993;53:2750–7. [PubMed] [Google Scholar]
- 37.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 38.Schmucker DL. Aging and drug disposition: an update. Pharmacol Rev. 1985;37:133–48. [PubMed] [Google Scholar]
- 39.Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8:484–95. doi: 10.1111/j.1474-9726.2009.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell JB, Samuni A, Krishna MC, et al. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–7. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- 41.Clutton SM, Townsend KM, Walker C, Ansell JD, Wright EG. Radiation-induced genomic instability and persisting oxidative stress in primary bone marrow cultures. Carcinogenesis. 1996;17:1633–9. doi: 10.1093/carcin/17.8.1633. [DOI] [PubMed] [Google Scholar]
- 42.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 43.Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: the difference is as clear as black and white. Radiat Res. 1997;147:121–5. [PubMed] [Google Scholar]
- 44.Randall K, Coggle JE. Long-term expression of transforming growth factor TGF beta 1 in mouse skin after localized beta-irradiation. Int J Radiat Biol. 1996;70:351–60. doi: 10.1080/095530096145085. [DOI] [PubMed] [Google Scholar]
- 45.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–90. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 46.Lorimore SA, Mukherjee D, Robinson JI, Chrystal JA, Wright EG. Long-lived inflammatory signaling in irradiated bone marrow is genome dependent. Cancer Res. 2011;71:6485–91. doi: 10.1158/0008-5472.CAN-11-1926. [DOI] [PubMed] [Google Scholar]
- 47.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hail NJ, Cortes M, Drake EN, Spallholz JE. Cancer chemoprevention: a radical perspective. Free Radic Biol Med. 2008;45:97–110. doi: 10.1016/j.freeradbiomed.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy AR. Factors that modify radiation-induced carcinogenesis. Health Phys. 2009;97:433–45. doi: 10.1097/HP.0b013e3181ac9262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.