Abstract
Background
Parenteral nutrition (PN) increases infectious risk in critically ill patients compared with enteral feeding. Previously, we demonstrated that PN feeding suppresses the concentration of the Paneth cell antimicrobial protein secretory phospholipase A2 (sPLA2) in the gut lumen. sPLA2 and other Paneth cell proteins are released in response to bacterial components, such as lipopolysaccharide (LPS), and they modulate the intestinal microbiome. Since the Paneth cell protein sPLA2 was suppressed with PN feeding, we hypothesized PN would diminish the responsiveness of the small bowel to LPS through reduced secretions and as a result exhibit less bactericidal activity.
Methods
The distal ileum was harvested from ICR mice, washed, and randomized for incubation with LPS (0, 1, or 10 μg/mL). Culture supernatant was collected and sPLA2 Activity was measured. Bactericidal activity of the ileum segment secretions was assessed against P. aeruginosa with and without a sPLA2 inhibitor at two concentrations, 100nM and 1μM. ICR mice were randomized to Chow or PN for 5 days. Tissue was collected for immunohistochemistry (IHC) and ileal segments were incubated with LPS (0 or 10 μg/mL). sPLA2 activity and bactericidal activity were measured in secretions from ileal segments.
Results
The ileal segments responded to 10 ug/mL LPS with significantly greater sPLA2 activity and bactericidal activity. The bactericidal activity of secretions from LPS stimulated tissue was suppressed 50% and 70%, respectively, with the addition of the sPLA2-inhibitor. Chow displayed greater sPLA2 in the Paneth cell granules and secreted higher levels of sPLA2 than PN before and after LPS. Accordingly, media collected from Chow was more bactericidal than PN. IHC confirmed a reduction in Paneth cell granules after PN.
Conclusions
This work demonstrates that ileal segments secrete bactericidal secretions after LPS exposure and the inhibition of the Paneth cell antimicrobial protein sPLA2 significantly diminishes this. PN feeding resulted in suppressed secretion of the sPLA2 and resulted in increased bacterial survival. This demonstrates that PN significantly impairs the innate immune response by suppressing Paneth cell function.
Keywords: Parenteral Nutrition, secretory phospholipase A2, small intestine, innate immunity
Introduction
Parenteral nutrition (PN) provides essential nutritional support to patients with contraindications to enteral feeding. Unfortunately, PN is associated with an increased risk of septic complications from intra-abdominal abscesses and pneumonia in critically ill trauma patients compared to patients fed enterally 1,2. Increased susceptibility to infection following PN is linked to multiple factors including altered intestinal permeability, increased virulence of resident microbes, and alterations in acquired immune defenses manifested through gut associated lymphoid tissue (GALT) changes. GALT changes include fewer Peyer’s patch and lamina propria lymphocytes, reductions in IgA-stimulating cytokines, and lower levels of respiratory and intestinal IgA levels3,4. The lower IgA levels provide less anti-bacterial and anti-inflammatory protection for the host mucosa since IgA normally aggregates pathogens and prevents their attachment to the mucosa. But innate immune mechanisms also protect the mucosa though physical barriers such as a mucin layer produced by Goblet cell, maintenance of enterocyte tight-junctions, and the secretion of innate antimicrobial molecules, the majority of which are peptides and proteins produced by Paneth cells.5,6 These molecules play a vital role in preventing microbial colonization of the mucosa7,8 and their loss is associated with spontaneous inflammation of the small bowel9.
We recently demonstrated that PN feeding with or without injury significantly decreases the activity of one innate antimicrobial molecule, secretory phospholipase A2 (sPLA2), in the gut lumen10. This suggested that route of dietary administration affects Paneth cell production or secretion, since this cell is the source of luminal sPLA2. Paneth cells constitutively express sPLA2 and other antimicrobial proteins, such as lysozyme, RegIIIγ, and defensins or cryptidins which are stored in intracellular granules before release into the gut lumen. sPLA2-IIA is highly expressed in Paneth cell granules and exhibits potent antimicrobial activity11. Its catalytic site cleaves free fatty acids from the sn-2 position of phospholipid glycerol backbones, releasing free fatty acids and lyso-phospholipids. Due to the protein’s cationic charge, sPLA2 preferentially attacks negatively charged phospholipids, such as phosphotidylethanolamine and phosphotidylserine, which are found in high concentrations in bacterial cell membranes 12–13. Accordingly, sPLA2 has been shown to disrupt cell membranes in both gram-positive and gram-negative bacterial strains, inducing bacterial cell death 6,11,14,15.
Although the effects of PN on adaptive gut and respiratory immunity through remodeling of GALT have been studied, the innate gut responses following PN have not been explored mechanistically. This work investigates the effect of route of nutrition on Paneth cell function by measuring LPS-induced sPLA2 secretion and the resulting bactericidal function against Pseudomonas aeruginosa in an ex vivo small intestinal model. We also determined the bactericidal activity attributable to sPLA2 using an sPLA2 inhibitor, since several constitutively expressed antibacterial molecules are secreted in response to bacterial components 16. We hypothesized that PN with the lack of enteral feeding suppresses the intestinal capacity to respond to bacterial stimuli through reduced Paneth cell secretions resulting in suppressed bactericidal activity.
MATERIALS AND METHODS
Animals
All protocols were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison, and the William S. Middleton Memorial Veterans Hospital, Madison. Male Institute of Cancer Research (ICR) mice were purchased from Harlan (Indianapolis, IN) and housed 5 per covered/filtered box under controlled temperature and humidity conditions with a 12:12 hour light:dark cycle in an American Association for Accreditation of Laboratory Animal Care accredited conventional facility. Animals were fed standard mouse chow (Rodent Diet 5001; LabDiet, PMI Nutrition International, St. Louis, MO) water ad libitum for 1 week prior to initiation of study protocol.
Experimental Designs
Kinetic of sPLA2-IIA secretion by the small bowel following LPS stimulation
To quantify the dose responsiveness and kinetics of sPLA2 release from small bowel segments after LPS stimulation for subsequent experiments, chow (Rodent Diet 5001; LabDiet) fed mice (7 to 8 weeks old, n = 6) were anesthetized by intramuscular injection (ketamine 100 mg/kg and acepromazine maleate 10 mg/kg mixture) and euthanized by exsanguination prior to harvesting the small intestine (SI). The SI lumen was flushed with 20 mL of ice cold calcium-magnesium-free Hank’s balanced salt solution (CMF-HBSS) (Bio Whittaker, Walkersville, MD) to collect the SI wash fluid. An additional 120 mL of ice cold CMF-HBSS was used to thoroughly rinse the tissue. The distal ileum was sectioned into six 2 cm segments, opened longitudinally, and placed in 24-well multidishes (Nunc, Roskilde, Denmark) containing 1 mL CMF-HBSS on ice. Segments were then randomized to receive Control LPS0 (PBS), LPS1 (1 μg/mL LPS from Salmonella enterica,(Cat L6511, Sigma, St. Louis, MO)) or LPS10 (10 μg/mL LPS,) in duplicate. Tissues were incubated at 37°C and culture media was collected at 0, 5, 10, 20, 40, 60 and 120 min. Collected culture media was centrifuged at 3000 rpm for 5 min at 4°C to remove cellular debris, passed through a 0.22 μm filter, and stored at −80°C until sPLA2 analysis. The kinetics of sPLA2 activity in culture media were examined by Continuous Fluorescence Assay at the time points specified. sPLA2-IIA concentration was confirmed with Western blot. Analysis of bactericidal activity was performed on culture media from the 120 minute time point.
Bacteria Preparation and Bactericidal Assay
To determine bacterial activity, Pseudomonas aeruginosa (P. aeruginosa) was cultured overnight in a LB broth (Fisher Scientific, Fair Lawn, NJ) for 24 hr at 37°C. The culture was centrifuged at 2060 x g for 10 min at 4°C, washed, and resuspended in sterile CMF-HBSS. A bacterial suspension was adjusted to 2 × 103 CFU /mL with CMF-HBSS. To evaluate bactericidal activity, 10 μL (~100 CFU) of bacterial stock was combined with 200 μL culture media or CMF-HBSS (Control) and incubated for 1 hr at 37°C. Surviving CFUs were determined in duplicate by plating on LB agar plates (Fisher Scientific, Pittsburg, PA) under aerobic condition at 37°C overnight in triplicate. CFUs were normalized to control plates in respective experiments and results were expressed as bactericidal activity as measured by the reduction in % CFUs.
Bactericidal Activity following sPLA2 inhibitor
To determine the contribution of sPLA2 to the bactericidal activity fluid released by the isolated small bowel segments after LPS stimulation, wash fluid from the intestine of chow (Rodent Diet 5001; LabDiet) fed mice (8 weeks old, n = 4) were collected and prepared as described above using CMF-HBSS (control) or LPS10 (10 μg/mL) for 60 min at 37°C. The bactericidal assay was performed on collected secretions with CMF-HBSS (control), LPS10, or LPS10 with the addition an sPLA2-IIA inhibitor at two concentrations, 100 nM and 1 μM, (Cat. 525145, cyclic (2-NaphthylAla-Leu-Ser-2-Naphthyl Ala-Arg)TFA, EMD Chemicals, Gibbstown, US). Each treatment was inoculated with 10 uL (~100 CFU) bacteria for 60 min at 37°C. Surviving CFUs were plated in duplicate on LB plates and incubated overnight at 37°C.
The effect of PN on small intestine sPLA2 secretion and bactericidal function following LPS stimulation
Male ICR mice (7 to 8 weeks old, n = 10) were randomized to the Chow (n = 5) or the PN (n = 5) group. Animals were anesthetized by intramuscular injection, weighed, and underwent placement of silicon rubber catheter (0.012-inch I.D./0.025-inch O.D.; Helix Medical, Inc., Carpinteria, CA) in the vena cava through the right external jugular vein. The catheter was tunneled subcutaneously over spine and existed at the midpoint of the tail. The animals were housed individually in metabolic cages with wire floors to prevent coprophagia and bedding ingestion and partially immobilized by tail restraint to protect the catheter during infusion. This technique has proven to be an acceptable method of nutritional support and does not produce physical or biochemical evidence of stress 17.
All mice were given chow and water ad libitum with normal saline (0.9%) infusion at 4 mL/day for 48 hr to allow surgical recovery as was previously measured by serum cytokines and normalized oral intake. Chow mice continued to receive intravenous saline (0.9%) at 4 mL/day with free access to chow (Rodent Diet 5001; LabDiet) and water throughout the study period. PN animals received onlyPN solution at rates 4 mL/d (day 1) , 7 mL/d (day 2) and 10 mL/d (day 3 to 5) because a graded infusion period was demonstrated to be necessary for the mice to adapt to the glucose and fluid loads. The PN solution contained 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, containing 1440 kcal/L and a non-protein calories/nitrogen ratio of 128:1. These values were calculated to meet the nutrient requirements of mice weighting 25 to 30 g 18.
After 5 days of feeding, mice were euthanized as described above. The small bowel was harvested, cleaned of mesenteric fat and vascular tissue, and the lumen was flushed with 20 mL of ice cold CMF-HBSS. This wash fluid was then centrifuged at 2000 xg for 10 min at 4°C and a 1 mL supernatant sample was stored at −80°C for sPLA2 activity. Next, the intestinal lumen was rinsed thoroughly with an additional 120 mL of ice cold CMF-HBSS. Ileum segments were randomized to Control (PBS) or LPS10 (LPS 10 μg/mL) in 1 mL CMF-HBSS for 1hr at 37°C. Culture media was centrifuged at 3000 rpm for 5 min at 4°C, passed through a 0.22 μm filter, and stored at −80°C. Western blot was used to identify sPLA2-IIA in culture media. sPLA2 activity was determined in both the intestinal wash fluid and culture media and a bactericidal assay was performed on the culture media from LPS stimulated tissues. Additional Ileum segments were fixed for Immunohistochemistry of sPLA2-IIA.
Continuous fluorescent assay for sPLA2 Activity
Fluorescent assay for sPLA2 activity was performed as described previously by Tsao et al 19, with some modification to substrate preparation10. This assay uses a specific probe, Bis-BODipy FL, which is designed to fluoresce when the Sn2 position of the phospholipid glyceraldehyde backbone is cleaved. This method was established as a high throughput method to rapidly analyze sPLA2 activity. Briefly, substrate was prepared by mixing 10 uL Bis-BODipy FL C11-PN (Molecular Probes, Eugene, OR) in a 1 mL aliquot of phosphatidylglycerol (Sigma, St. Louis, MO) dissolved in chloroform (2 mg/mL) and evaporated under nitrogen. The chloroform-free phospholipids were re-dissolved in 100% ethanol and used as substrate. The ethanol substrate solution was stable for month stored at −20°C. The assay reaction mixture was prepared in a glass tube on ice that contained 10 μL of substrate ethanol solution (20 μg of phospholipids) and 12.3 μL of sample in a final volume of 1 mL that was made up with buffer of 0.01 M Tris-HCl (pH 7.4) containing 10 mM Ca2. An aliquot of 0.3 mL of the reaction mixture was promptly transferred in triplicate to the wells of a white polystyrene microplate (Porvair PS White, PerkinElmer Instruments, Norwalk, CT). The microplate was placed in a temperature-controlled (30°C) microplate reader attached to a PerkinElmer Luminescence Spectrometer LS50B. The fluorescence intensity (FI) in each well was recorded every 10 sec for 60 cycles at 488 nm excitation (excitation slit: 2.5 nm) and 530 nm emission (emission slit: 5.0 nm). ). To confirm calcium dependent sPLA2 activity, samples were also run with EGTA-Buffer (0.01 mol/l Tris-HCL (pH 7.4) containing 10 mmol/l Ca+2 and 20 mmol/l EGTA) which contains ample EGTA for complete calcium sequestration. After the reactions reached equilibrium temperature, the reaction curve was fit to a second-order polynomial equation and the first-degree coefficient was taken as the initial rate of reaction (expressed as change in FI/min/uL sample). Blank wells containing only substrate and buffer were used to find coefficient rates determined as background activity.
Western blot for sPLA2 in the tissue culture media
10 uL of tissue culture media was denatured at 95°C for 10 min with sodium dodecylsulfate and β-mercaptoethanol and separated in a 4–15% polyacrylamide gel (Ready Gel, Bio-Rad Laboratories, Hercules, CA) by electrophoresis at 150 V for 42 min. with molecular weight markers (Dual Color, Precision plus protein standards, Bio-Rad Laboratories). Proteins were then transferred to polyvinylidene fluoride membranes using a Tris-glycine buffer plus 20% methanol at 80 V for 50 min. Membranes were blocked with 5% nonfat dry milk prepared in Tris-buffered saline with 0.5% Tween-20 (TBS-Tween) for 1 hr with constant agitation. Membranes was incubated with the anti-group II sPLA2 antibody (sc-14468, Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted 1:100 overnight at 4°C with agitation. Then membranes were washed and incubated with donkey anti-goat goat IgG-HRP conjugate (sc-2020, Santa Cruz Biotechnology) diluted 1:5000 for 1 hr at room temperature with agitation. Following washing, the membranes was incubated with the ECL reagent (Super Signal West Femto Maximun Sensitivity Substrate, Pierce Biotechnology, Rockford, IL) for 5 min at room temperature and bands were exposed to photographic film within 30 sec.
Quantification of sPLA2 in Chow and PN Ileum segments
To quantify the concentration of sPLA2 in Paneth cell granules, we performed IHC on the ileum segments following Chow or PN feeding. Samples were fixed in 4% paraformaldehyde overnight, transfer to 70% ethanol, and stored at 4 Cº until processing. Samples were processed in a Tissue-Tek V.I.P tissue processor which included the following steps: 70% ETOH, 45 min; 80% ETOH, 45 min; 95% ETOH, 2x1h; 100% ETOH, 2x1h; Xylene, 2x1h; Paraffin MP 60ºC (Surgipath, Richmond, IL), 2x45 min; Paraffin, 2x1h. Samples were then embedded in paraffin, sectioned at 5 μm with a microtome, and placed on Adhesive Coated Slides (White Aminosilane, Newcomer Supply, Madison, WI). Paraffin was melted by placing slides in 60 C for 35 mins. Slides were placed in xylene to remove remaining paraffin and rehydrated in graded alcohol baths. Antigen retrieval was performed by boiling slides in 10mM sodium citrate buffer (pH 6.0). Samples were outlined using a pap-pen. 10% BSA-PBS was used to block non-specific binding for 1 hour. Samples were then incubated with primary antibody (group II sPLA2 (G-15) goat polyclonal IgG, sc-14468, Santa Cruz Biotechnology) overnight in 1% BSA-PBS at 4 C in a humidity chamber. Remaining solution was tapped off and samples were incubated in the dark with secondary antibody (Alexa Fluor 594, donkey anti- goat IgG, 2mg/mL, A11058, Invitrogen, Grand Island, NY) for 30 mins in 1% BSA-PBS at room temperature. DAPI (P36935, Invitrogen) was placed on each slide before covering to image nuclei. After imaging, densitometric measurements of sPLA2 intesity were quantified in 12 individual crypts per animal using NIH ImageJ software.
Statistical analysis
The data are expressed as means ± standard error of the mean. Statistical significance was determined using ANOVA with Fisher’s protected least significant difference post hoc test or Student’s t-test. Bactericidal activity was measured by normalizing treatment group Colony Forming Units (CFUs) to the control group using paired analysis in each respective experiment. Treatment group CFUs were compared using ANOVA. Differences were considered to be statistically significant at p<0.05. All statistical calculations were performed with StatView (Abacus Concepts, Berkeley, CA).
RESULTS
Kinetics of sPLA2-IIA secretion following LPS Stimulation
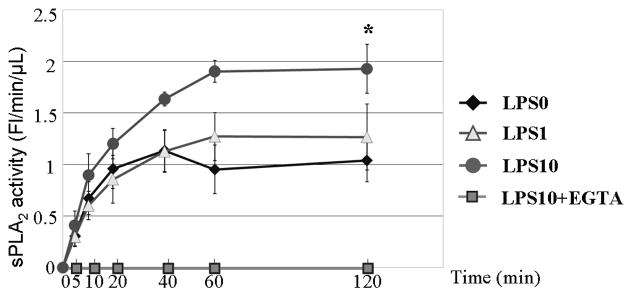
Increased sPLA2 activity occurred in all treatments, reached a peak at 60 minutes, and remained stable through 120 minutes [Figure 1A]. At 120 minutes, LPS10 stimulated significantly more sPLA2 activity (1.93 ± 0.24 Fluorescence/min/μL) than LPS0 (1.04 ± 0.19, p < 0.05) but not LPS1 (1.26 ± 0.32, p = 0.08) with no significant differences between LPS0 and LPS1 (p = 0.57). All sPLA2 activity was absent in LPS10 culture medium with EGTA confirming that the activity measured in the treatments was calcium dependent, excluding calcium-independent cytosolic PLA2 as the source. Western blot for sPLA2-IIA in secretions also showed no change between LPS0 and LPS1, while LPS10 demonstrated an increased sPLA2 concentration consistent with the quantified activity [Figure 1B].
Figure 1. sPLA2 release following LPS Stimulation of Intestinal Segments.

Figure 1A Kinetics of sPLA2 activity (FL/min/μL) in culture media following LPS stimulation at LPS doses of 0 (LPS0), 1 (LPS1), and 10 μg/mL (LPS10). sPLA2 activity reached a plateau at 60 minutes and remained constant through 120 minutes. At 120 minutes LPS10 was significantly greater than LPS0 but failed to reach significance vs LPS1. LPS1 did not significantly differ from LPS0. * p<0.05 vs. LPS0
Figure 1B Representative sPLA2-IIA western blot of tissue culture media after LPS stimulation. LPS0 and LPS1 did no differ, while LPS10 showed an increase in sPLA2-IIA concentration consistent with the observed activity. Bands are 14 kDa molecular weight.
Bactericidal activity of tissue culture medium
Both LPS0 (p < 0.08) and LPS10 (p < 0.003) samples demonstrated bactericidal activity compared to control HBSS [Figure 2]. There was no significant difference between LPS0 and LPS10.The bactericidal activity in LPS0 was consistent with baseline levels of sPLA2 secretion observed in un-stimulated tissue.
Figure 2. Bactericidal Activity of tissue secretions ± LPS Stimulation.
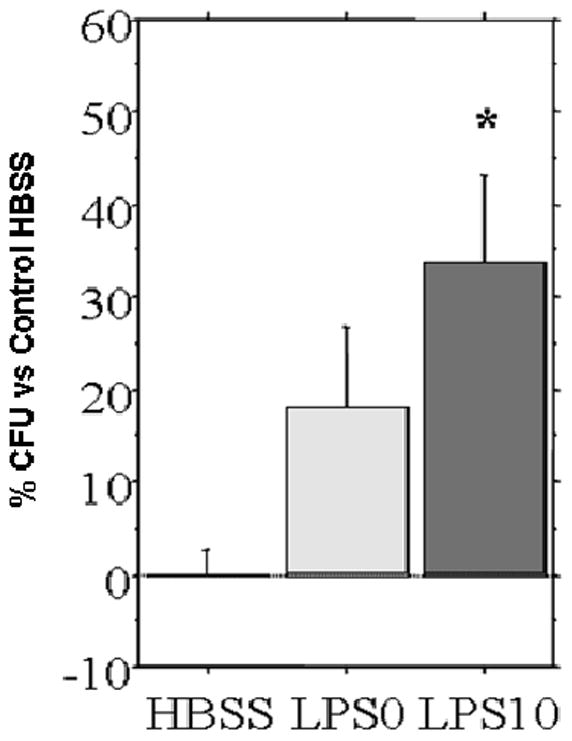
Bactericidal activity against Ps. Aeruginosa from culture media unstimulated, LPS0 (0 μg/mL LPS), and LPS10 (10 μg/mL LPS) compared with HBSS control. LPS10 had significantly greater bactericidal activity than HBSS control, while LPS0 failed to reach significance (p < 0.08). * p<0.05 vs HBSS
Bactericidal Activity following sPLA2 inhibitor
Consistent with the kinetics, LPS10 induced significantly greater bactericidal activity than control HBSS samples [Figure 3]. Compared with LPS10 alone, addition of sPLA2-IIA inhibitor reduced bactericidal activity at both inhibitor concentrations but only the 1μM concentration reached statistical significance ( p = 0.03). There was no statistical difference in bactericidal activity between the control HBSS and either inhibitor doses (100nM : p = 0.18 and 1μM : p=0.35) or between the two inhibitor concentrations (p = 0.6).
Figure 3. Bactericidal Activity of LPS stimulated tissue secretions with sPLA2 Inhibitor.
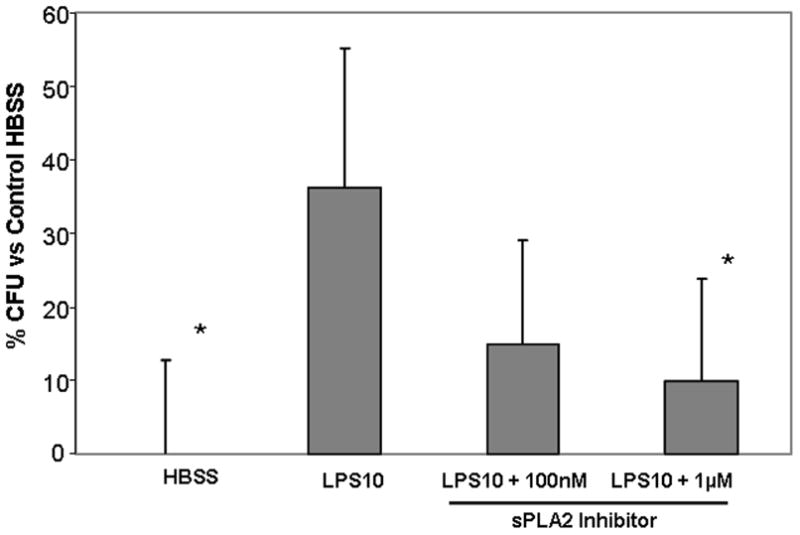
Bactericidal Activity of culture media against Ps. Aeruginosa with an sPLA2 Inhibitor. LPS10 (10 μg/mL LPS) demonstrated significant bacterial killing compared with the HBSS control. The sPLA2 inhibitor 100 nM suppressed killing non-significantly (p = 0.07), while the higher concentration 1 μM significantly suppressed bacterical activity. There was no change between inhibitors and HBSS control. * p<0.05 vs LPS10
The effect of Chow and PN on secretion and bactericidal function of sPLA2-IIA
Body Weight
There were no significant differences in initial body (Chow vs PN: 34.3 ± 2.3 vs. 33.1 ± 1.8 grams, p = 0.69) or weight change between Chow and PN groups (−3.4 ±1 vs. −5.5 ±1.1 grams, p = 0.20).
sPLA2 activity of SIWF
Consistent with our previous findings10, SI wash fluid sPLA2 activity was significantly lower in PN than Chow (2.6 ± 0.5 vs. 11.9 ± 1.1 FL/min/μL, p < 0.001).
sPLA2 activity of culture medium
At baseline (0 μg/mL LPS), chow fed mice demonstrated greater sPLA2 activity (0.9 ± 0.1 FL/min/μL) than PN (0.45 ± 0.1, p = 0.01) [Figure 4]. Following LPS stimulation (10 μg/mL LPS), Chow sPLA2 activity significantly increased compare to baseline (1.3 ± 0.15 vs. 0.9 ± 0.1, p = 0.02), while PN was non-significantly increased due to variation in tissue responsiveness (0.8 ± 0.3 vs. 0.45 ± 0.1, p = 0.3). However, despite LPS stimulation, PN sPLA2 activity (0.8 ± 0.3) only reached baseline Chow levels (0.9 ± 0.1, p = 0.5). Western blot results demonstrated sPLA2-IIA concentrations consistent with quantified sPLA2 activity in respective treatments [Figure 4B].
Figure 4. sPLA2 Activity of Chow or PN tissue secretions ± LPS stimulation.
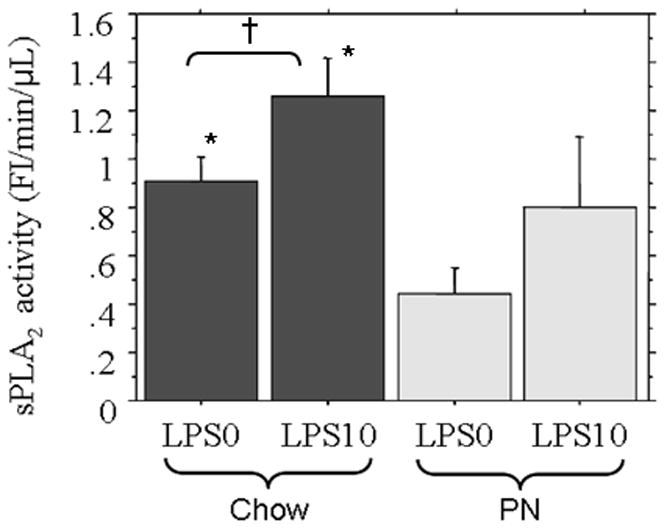
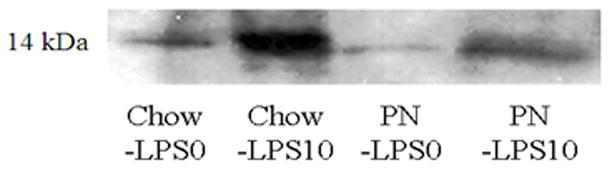
Figure 4A Following LPS stimulation (10 μg/mL LPS) chow significantly increased tissue culture sPLA2 activity compared with baseline (LPS0) and PN was increased but failed to reach significance due to variation in response. Both Chow LPS0 and LPS10 secretions were significantly greater than PN + LPS0. * p<0.05 vs PN+LPS0, † p=0.02.
Figure 4B Representative western blot of sPLA2-IIA in tissue culture media from Chow or PN tissue showed LPS10 increased sPLA-IIA concentrations consistent with the quantified increase in sPLA2 acitivty observed in both groups. Bands are 14 kDa molecular weight.
Bactericidal activity of culture medium
Bactericidal activity in Chow (17 ± 3%) culture media was significantly greater than control HBSS (0 ± 4%, p < 0.01) or PN (5 ± 4%, p < 0.04)[Figure 5]. PN failed to reach significance over control HBSS (p = 0.40).
Figure 5. Bactericidal Activity of LPS stimulated secretions from Chow and PN.
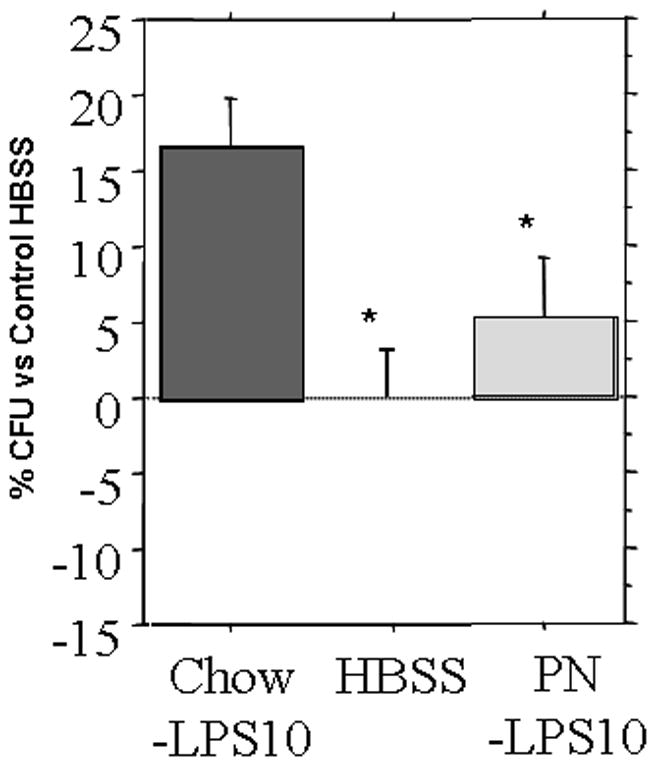
Bactericidal activity of Chow or PN tissue secretions following LPS (10 μg/mL LPS) stimulation. Chow had significantly greater bactericidal activity than HBSS control or PN. PN stimulated a sub-significant level of bactericidal activity. * p<0.05 vs Chow + LPS10
Immunohistochemistry of sPLA2-IIA in Ileum
sPLA2-IIA was clearly visible in the Paneth cell granules, located at the base of the intestinal crypts [Image 1]. Densitometric analysis of sPLA2-IIA intensity was significantly reduced in PN (386.7 ± 180.5 Relative intensity (RI), p = 0.01) compared with Chow (3118.0 ± 1017 RI) [Figure 6], consistent with decreased luminal sPLA2 activity and reduced tissue secretions following LPS challenge in PN tissue.
Image 1. Immunohistochemistry of sPLA2 in Chow and PN ileum tissue.
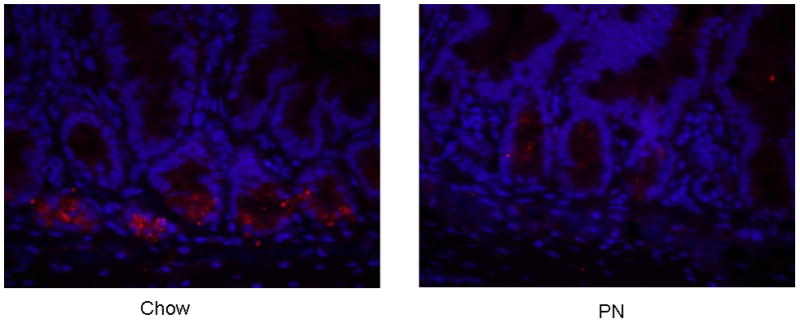
sPLA2-IIA (red) was localized in Paneth cell granules and was assessed in ileum samples of Chow and PN. Cell nuclei are stained with DAPI (blue).
Figure 6. Relative Density of sPLA2 in Chow vs PN Ileum Tissue.
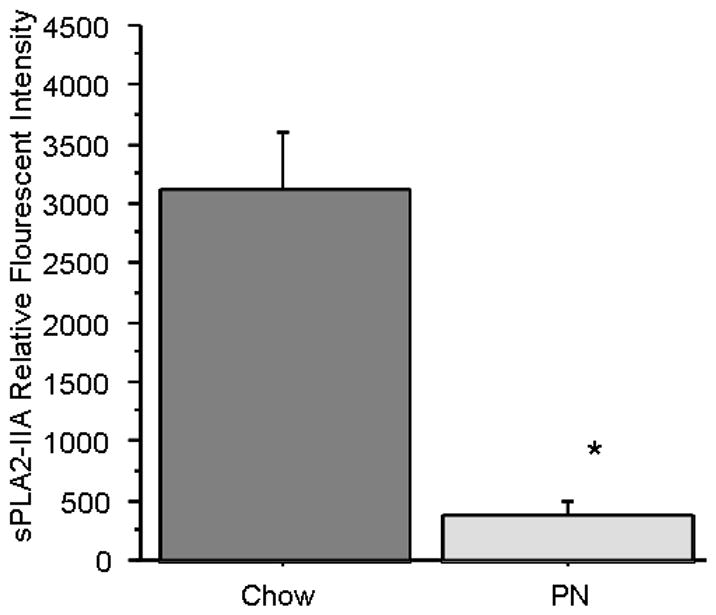
Immunohistochemical analysis of sPLA2-IIA, performed by quantifying relative intensity of Fluorescent signal, showed Chow had significantly higher expression of sPLA2 compared with PN. * p<0.05 vs Chow
DISCUSSION
The intestinal mucosal surface constitutes a dynamic interface between the host and microbiome that plays essential roles in human health by enhancing nutrient metabolism, promoting immune development, and limiting pathogen colonization. Despite the dense and diverse community of microorganisms, both the host and bacteria benefit illustrating the highly effective mechanisms for monitoring and regulating bacterial interactions with the intestinal mucosal interface. During illness or following injury, this balance may be impaired due to interruptions in nutrient intake, the use of parenteral nutition, intestinal ischemia and acidosis, antibiotics and other aspects of clinical care and the clinical environment.
Recently we observed that PN with lack of enteralstimulation reduced the activity of sPLA2 in small intestinal wash fluid both prior to and following surgical stress compared to chow fed mice 10. sPLA2 is an innate immune molecule produced by Paneth cells, which are secretory epithelial cells located atthe base of intestinal crypts throughout the intestine that produce a variety of bactericidal peptides and proteins, including human α-defensins or murine cryptidins, lysozyme, Reglll-γ, in addition to sPLA2. These agents remain stored in intracellular granules before their release into the lumen.6. Production of Paneth cell granular products is not dependent on microbial presence, but secretion is regulated in part through response to binding of pathogen-associated molecular pattern (PAMP) to innate receptors, such as extracellular Toll-Like Receptors (TLRs) and intracellular Nucleotide- Oligermerization Domains (NODs). The innate receptors respond to various bacterial antigens, including Lipopolysaccharide (LPS) - a well conserved gram-negative cell wall component -that binds TLR -4.
This work focused on sPLA2 activity and bactericidal activity of secretions from small intestinal segments following LPS-stimulation.Then, we analyzed these parameters following Chow and PN feeding regimens. We first established the kinetics of sPLA2 secretion from intestinal tissue following LPS-stimulation using two LPS doses, which was consistent with others work showing release of innate bactericidal molecules after LPS stimulation16,20. We demonstrated that sPLA2 secretion from ex vivo tissue segments reaches maximal levels after 60 minutes under our experimental conditions, and that 10 μg/mL LPS significantly stimulates sPLA2 compared to 0 or 1 μg/mL LPS. Interestingly, there was no measurable increase between 0 and 1 μg/mL LPS, perhaps due to the constant bacterial presence in the bowel and a threshold of tolerance. We also observed a baseline level of sPLA2 secretion, even in the absence of experimental LPS-stimulation. We then determined the bactericidal activity against Pseudomonas aeruginosa and found secretions from LPS-stimulated tissue contained the most activity, but un-stimulated tissue still possessed activity consistent with baseline secretions.
To determined the contribution of sPLA2 to the observed bactericidal activity, we added two concentrations, 100 nM and 1μM of a potent sPLA2 inhibitor, cyclic(2-NaphthylAla-Leu-Ser-2-Naphthyl-Ala-Arg)TFA, to the secretions obtained from LPS10 stimulated tissue. The inhibitor reduced bactericidal by approximately half, 54%, at a concentration of 100 nM, and approximately 70% of bactericidal activity was lost with 1 μM. This confirmed that sPLA2 contributed a large percentage of bactericidal activity against Ps. Aeruginosa. sPLA2 works synergistically with a myriad of molecules within the gut lumen including lysozyme, Reg-IIIγ, and angiogenin-4 to modulate bacteria. We did not measure the contribution of these individual molecules but such studies are currently underway.
After establishing LPS dose and time points, we examined the effect of PN with the lack of enteral stimulation on the intestinal sPLA2 responsiveness to LPS and the associated bactericidal function compared with chow feeding. Our previous showed PN feeding reduced sPLA2 activity in the small intestinal wash fluid10. Consistent with those data, we demonstrated that PN blunted the sPLA2 response to LPS by intestinal tissue potentially indicating reduced levels of sPLA2 in the Paneth cell granules, a reduced ability to release granules, or both. We addressed both possibilities using immunohistochemistry (IHC) and the LPS stimulation technique. IHC demonstrated significantly reduced sPLA2-IIA expression within the Paneth cell granules following PN feeding [Figure 6] indicating that sPLA2-IIA production, and perhaps production of other constitutively expressed antimicrobial proteins, is down-regulated following reduced enteral feeding, such as occurs with PN. The significantly suppressed bactericidal activity from PN secretions, even despite LPS stimulation, confirms the relevance of these findings.
These data confirm our hypothesis that reduced enteral stimulation suppresses innate mucosal immunity in the small bowel and provide the first mechanistic experimental work into the effect of dietary route on innate mucosal responsiveness to bacterial components. The impaired innate mucosal defense following PN suggests an impaired ability to influence intestinal microbiota.
Clinical Relevancy.
Prolonged PN administration is associated with increased incidence of infections. This work demonstrates that PN reduces the innate immunity response of the GI tract to LPS as measured by reduced secretions. This impairment results in less bactericidal activity towards Pseudomonas aeruginosa, a pathogen which contributes to infectious complications
Acknowledgments
This research is supported by National Institute of Health (NIH) Grant R01 MG53439. This material is also based upon work supported in part by the Department of Veteran Affairs, Veteran Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Service. The contents of this article do not represent the views of the Veterans Affairs or the United States Government.
We would like to thank Glen Leverson, PhD, for assistance with statistical analysis. Kaz Fikatsu, MD, Wentong Xu, MD., PhD, & Kazuo Hase, MD., PhD, for valuable input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore KA, Feliciano DV, Andrassy RJ, McArdle AH, Booth McL, Morgenstein-Wagner TB, Kellum JM, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduced postoperative septic complications: the results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudsk KA, Crove MA, Fabian TC, Minard G, tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding: effects on septic morbidity adter blunt and penetrating abdominal trauma. Ann Surg. 1991;215:503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Kudsk KA, Gocinsky B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. Faseb J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 6.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee S, Vaishnava S, Hooper LV. Multilayered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019–3027. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, et al. A key role for auophagy and the autophagy gene Atg 16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierre JF, Heneghan AF, Tsao FHC, Sano Y, Jonker MA, Omata J, Lan J, Kudsk KA. Route and Type of Nutrition and Surgical Stress Influence Secretory Phospholipase A2 (sPLA2) Secretion of the Murine Small Intestine. J Parenter Enteral Nutr. 2011;35(6):748–756. doi: 10.1177/0148607111414025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harwig SS, Tan L, Qu XD, Cho Y, Eisenhauer PB, Lehrer RI. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95(2):603–10. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, Wilton DC. The antibacterial properties of secreted phospholipase A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. Jour Biol Chem. 2002;277:1788–1793. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 13.Koduri RS, Gronroos JO, Laine VJO, Le Calvez C, Lambeau G, Nevalainen TJ, Gelb MH. Bacteridical properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. Jour biol chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 14.Weinrauch Y, Elsbach P, Madsen LM, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996 Jan 1;97(1):250–7. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest. 1998;102(3):633–8. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbial alpha-defenins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 17.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 18.National Academy of Science. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy of Science; National Research Publication No. 10, 1978. [Google Scholar]
- 19.Tsao FHC, Shanmuganayagam D, Zachman DK, Khosravi M, Folts JD, Meyer KC. A continuous fluorescence assay for the determination of calcium-dependent secretory phospholipase A2 activity in serum. Clinica Chimica Acta. 2007;379:119–126. doi: 10.1016/j.cca.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Cunliffe RN, Rose FR, Keyta J, et al. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48:176–185. doi: 10.1136/gut.48.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]