Abstract
Background
Celiac disease (CD) is common but under-diagnosed in the United States. Serological screening studies indicate that, although CD occurs at the same frequency in both genders, women are diagnosed more frequently than men (2:1). CD is less frequently diagnosed among black patients, though the seroprevalence in this group is not known.
Objective
to measure the rates of duodenal biopsy during esophagogastroeduodenoscopy (EGD) for symptoms consistent with CD.
Design
Retrospective cohort study.
Setting
Clinical Outcomes Research Initiative National Endoscopy Database, spanning the years 2004–2009.
Patients
Adults undergoing EGD for the indication of diarrhea, anemia, iron deficiency, or weight loss, in which the endoscopic appearance of the upper gastrointestinal tract was normal.
Main outcome measurement
performance of duodenal biopsy.
Results
Of 13,091 individuals (58% females, 9% blacks) who met the inclusion criteria, duodenal biopsy was performed in 43%, 45% of females and 39% of males (p<0.0001). Blacks underwent duodenal biopsy in 28% of EGD’s, compared to 44% for whites (p<0.0001). On multivariate analysis, male gender (OR 0.81 95%CI 0.75–0.88), older age (OR for ≥70 compared to 20–49 0.51 95%CI 0.46–0.57), and black race (OR 0.55 95%CI 0.48–0.64) were associated with decreased odds of duodenal biopsy.
Limitations
Lack of histopathologic correlation with CD prevalence.
Conclusions
In this multi-region endoscopy database spanning 2004–2009, rates of duodenal biopsy increased modestly over time, but overall remain low in patients with possible clinical indications for biopsy. Non-performance of duodenal biopsy during endoscopy may be contributing to the under-diagnosis of CD in the United States.
INTRODUCTION
Celiac disease (CD) is common, with a seroprevalence of approximately 1% in the United States and Western Europe. 1–3 This autoimmune disease is associated with an increased risk of malignancy4 and death,5 risks that diminish towards that of the general population in the years after diagnosis and institution of the only recognized treatment of CD, a gluten-free diet. Despite increasing rates of diagnosis, CD remains under-diagnosed in the United States, with fewer than 10% of patients with CD having received the diagnosis.6 The proportion of undiagnosed CD patients in the United States far exceeds those of areas in Western and Northern Europe.7–8 As undiagnosed CD is associated with increased mortality,2 efforts to understand the reasons for these low rates are warranted.
Factors related to the performance of gastrointestinal endoscopy contribute to the under-diagnosis of CD. A recent analysis of a national pathology database found that among patients undergoing esophagogastroduodenoscopy (EGD) with duodenal biopsy, only 35% had the recommended four specimens submitted, despite the finding that adherence to this standard led to a doubling of the CD diagnosis rates.9 Similarly, an analysis of the Clinical Outcomes Research Initiative (CORI) National Endoscopy Database found that, among individuals undergoing EGD for indications including symptoms of CD, the vast majority (89%) did not have a duodenal biopsy during the procedure.10 However, the time span of the latter study (spanning the years 2000–2003) was before the major seroprevalence study finding that CD is common,1 and it is unknown whether practice patterns have changed in response to this knowledge.
We aimed to measure whether the performance of duodenal biopsy is increasing over time, by analyzing the CORI database spanning the years 2004–2009. We also aimed to identify sociodemographic and medical factors associated with the performance of duodenal biopsy during EGD.
METHODS
We performed a cross-sectional study of the CORI National Endoscopic Database. This database was established in 1995 with the goal of establishing a network of gastroenterologists to prospectively collect data related to endoscopy for clinical and research purposes.11 Participating sites agree to use a structured computerized report generator to produce all endoscopic reports and comply with quality control requirements. The site’s data files are transmitted electronically to a central data repository. The data that are transmitted from the local site to the National Endoscopic Database do not contain most patient or provider identifiers. After completion of quality control checks, data from all sites are merged in the data repository for analysis. Procedure counts are monitored on a weekly basis for atypical activity. The repository is checked for anomalies on a daily basis.
We queried the database for all adults (age ≥20 years) undergoing EGD during the period spanning January 1, 2004 through December 31, 2009 that listed one of the following indications in the primary indication field: anemia, iron deficiency, diarrhea, or weight loss. We included only those EGD’s in which no focal abnormality anywhere in the upper gastrointestinal tract was noted. These inclusion criteria were the same as those of the previous analysis during the earlier time period,10 with the rationale that the above indications can be manifestations of CD, and that a normal-appearing duodenum is a common endoscopic finding in CD.12
The primary outcome was the performance of duodenal biopsy. We assessed the following variables for possible association with the primary outcome: year of the procedure, indication, patient age, gender, race (black vs. white), ethnicity (Hispanic vs. non-Hispanic), and region, as divided into Northeast (Massachusetts, New York, New Jersey, Ohio, Vermont), North Central (Indiana, Minnesota, Nebraska, North Dakota), Northwest (Oregon, Washington), Southeast (Florida, Georgia, Kentucky, North Carolina), South Central (Mississippi, Oklahoma, Tennessee, Texas), and Southwest (Arizona, California, Colorado, New Mexico, Nevada).
We used the chi square test for univariate analysis and the Cochran-Armitage test to assess for a temporal trend in biopsy performance. We performed multiple logistic regression to assess for independent associations with the performance of small bowel biopsy. The following covariates were included a priori in the multivariate model: year of the procedure, age group, gender, race (categorized as “white,” “black,” and “other”), Hispanic ethnicity, practice setting, region, and indication for the procedure.
All statistical tests were performed using SAS version 9.2 (Cary, NC). The Institutional Review Board of Columbia University Medical Center reviewed this protocol and deemed it exempt as the data did not contain any patient identifiers when provided to the investigators.
RESULTS
We identified 13,091 individuals who underwent EGD meeting the inclusion criteria during this six-year period (Table 1). The majority of patients (7,576; 58%) were female, and 11,489 (88%) were white. The majority of examinations (8,490; 66%) were performed in a community or Health Maintenance Organization (HMO) setting. Anemia was the most common indication for endoscopy (9,074; 69%), followed by diarrhea (2,039; 16%), weight loss (1,601; 12%), and iron deficiency (377; 3%).
Table 1.
Characteristics of adult patients undergoing EGD for the indications of weight loss, diarrhea, iron deficiency, or anemia, 2004–2009 (n=13,091)
| Characteristic | Number of patients (%) |
|---|---|
|
| |
| Year of procedure | |
| 2004 | 2,343 (18) |
| 2005 | 2,380 (18) |
| 2006 | 2,490 (19) |
| 2007 | 2,413 (18) |
| 2008 | 1,919 (15) |
| 2009 | 1,546 (12) |
| Gender | |
| Male | 5,515 (42) |
| Female | 7,576 (58) |
| Age Group | |
| 20–49 | 3,539 (27) |
| 50–69 | 5,281 (40) |
| ≥70 | 4,270 (33) |
| Race | |
| White | 11,489 (88) |
| Black | 1,141 (9) |
| Other | 456 (3) |
| Ethnicity | |
| Hispanic | 1,006 (8) |
| Non-Hispanic | 12,080 (92) |
| Practice type | |
| Community/HMO | 8,490 (66) |
| University | 2,580 (20) |
| VA | 1,713 (13) |
| Region | |
| North Central | 1,611 (12) |
| Northeast | 2,464 (19) |
| Northwest | 1,494 (11) |
| South Central | 2,016 (15) |
| Southeast | 1,622 (12) |
| Southwest | 3,884 (30) |
| Indication | |
| Anemia | 9074 (69) |
| Iron deficiency | 377 (3) |
| Diarrhea | 2,039 (16) |
| Weight loss | 1,601 (12) |
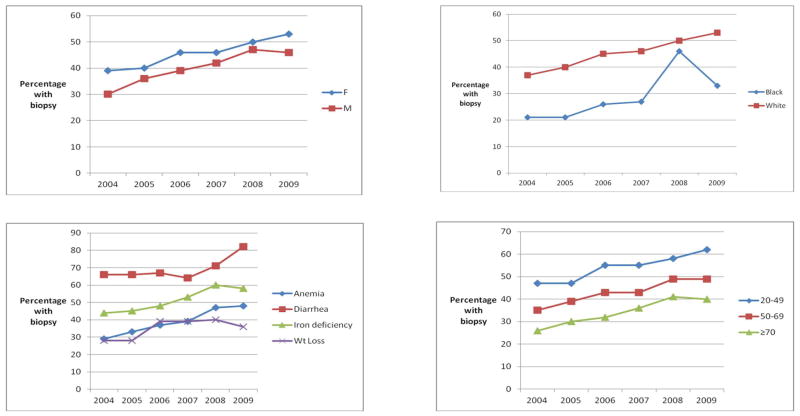
Duodenal biopsy was performed in 43% of all patients (Table 2). The rate of biopsy increased each year of the observation period, from 35% in 2004 to 51% in 2009 (p for trend <0.0001). Female patients were more likely than male patients to undergo duodenal biopsy (45% versus 39%, p<0.0001). Biopsies were performed more frequently in younger patients (age: 20–49: 54%; age 50–69: 43%; age ≥70: 33%, p<0.0001). Only 28% of black patients underwent duodenal biopsy during EGD, as compared to 44% of white patients (p<0.0001). There was marked regional variability in biopsy rates, with the highest rates in the Northwest (59%) and the lowest in the North Central region (19%, p<0.0001). Biopsy rates were lower in academic settings (38%) than in community/HMO settings (43%) or Veterans Affairs centers (44%, p<0.0001). The differences between the genders, age groups, whites and blacks, and among the various indications remained stable over the six year period (Figure 1); during this time, all groups had a modest rise in biopsy rates, but the disparities between these groups persisted.
Table 2.
Variables associated with the performance of small intestinal biopsy during EGD.
| Characteristic | Biopsy performed (%) | P value |
|---|---|---|
|
| ||
| Year of procedure | <0.0001 | |
| 2004 | 822/2,343 (35) | |
| 2005 | 904/2,380 (38) | |
| 2006 | 1,063/2,490 (43) | |
| 2007 | 1,062/2,413 (44) | |
| 2008 | 942/1,919 (49) | |
| 2009 | 783/1,546 (51) | |
| Gender | <0.0001 | |
| Male | 2,162/5,515 (39) | |
| Female | 3,414/7,576 (45) | |
| Age Group | <0.0001 | |
| 20–49 | 1,904/3,539 (54) | |
| 50–69 | 2,247/5,281 (43) | |
| ≥70 | 1,424/4,270 (33) | |
| Race | <0.0001 | |
| White | 5,087/11,489 (44) | |
| Black | 318/1,141 (28) | |
| Other | 168/456 (37) | |
| Ethnicity | 0.7688 | |
| Hispanic | 424/1,006 (42) | |
| Non-Hispanic | 5,149/12,080 (43) | |
| Practice type | <0.0001 | |
| Community/HMO | 3,665/8,490 (43) | |
| University | 988/2,580 (38) | |
| VA | 751/1,713 (44) | |
| Region | <0.0001 | |
| North Central | 311/1,611 (19) | |
| Northeast | 852/2,464 (35) | |
| Northwest | 874/1,494 (59) | |
| South Central | 919/2,016 (46) | |
| Southeast | 496/1,622 (31) | |
| Southwest | 2,124/3,884 (55) | |
| Indication | <0.0001 | |
| Anemia | 3,449/9074 (38) | |
| Iron deficiency | 188/377 (50) | |
| Diarrhea | 1,382/2,039 (68) | |
| Weight loss | 557/1,601 (35) | |
FIGURE 1.
Temporal trends in small intestinal biopsy stratified by gender (A), race (B), indication (C), and age (D)
The results of the multivariate analysis are shown in Table 3. Later year (OR for 2009 vs. 2004 1.97 95% CI 1.71–2.28) was associated with increased odds of duodenal biopsy, whereas male gender (OR 0.81 95% CI 0.75–0.88), older age (OR for ≥70 compared to 20–49 0.51 95% CI 0.46–0.57), black race (OR 0.55 95% CI 0.48–0.64), and Hispanic ethnicity (OR 0.69 95% CI 0.59–0.80) were associated with decreased odds of duodenal biopsy. Differences between regions, practice types, and clinical indication remained significant on multivariate analysis (Table 3).
Table 3.
Multiple logistic regression identifying variables associated with the performance of small intestinal biopsy during EGD.
| Characteristic | Odds Ratio | 95% CI | p value |
|---|---|---|---|
| Year of procedure | |||
| 2004 | 1.0 | [ref] | [ref] |
| 2005 | 1.15 | 1.01–1.31 | 0.0336 |
| 2006 | 1.36 | 1.20–1.55 | <0.0001 |
| 2007 | 1.41 | 1.24–1.61 | <0.0001 |
| 2008 | 1.89 | 1.65–2.16 | <0.0001 |
| 2009 | 1.97 | 1.71–2.28 | <0.0001 |
| Gender | |||
| Male | 0.81 | 0.75–0.88 | <0.0001 |
| Female | 1.0 | [ref] | [ref] |
| Age Group | |||
| 20–49 | 1.0 | [ref] | [ref] |
| 50–69 | 0.72 | 0.66–0.80 | <0.0001 |
| ≥70 | 0.51 | 0.46–0.57 | <0.0001 |
| Race | |||
| White | 1.0 | [ref] | [ref] |
| Black | 0.55 | 0.48–0.64 | <0.0001 |
| Other | 0.56 | 0.46–0.69 | <0.0001 |
| Ethnicity | |||
| Hispanic | 0.69 | 0.59–0.80 | <0.0001 |
| Non-Hispanic | 1.0 | [ref] | [ref] |
| Practice type | |||
| Community/HMO | 1.0 | [ref] | [ref] |
| University | 0.54 | 0.48–0.60 | <0.0001 |
| VA | 0.78 | 0.68–0.89 | 0.0002 |
| Region | |||
| North Central | 0.42 | 0.36–0.49 | <0.0001 |
| Northeast | 1.0 | [ref] | [ref] |
| Northwest | 2.69 | 2.33–3.11 | <0.0001 |
| South Central | 1.36 | 1.18–1.56 | <0.0001 |
| Southeast | 0.82 | 0.71–0.94 | 0.0061 |
| Southwest | 2.23 | 1.99–2.49 | <0.0001 |
| Indication | |||
| Anemia | 1.0 | [ref] | [ref] |
| Iron deficiency | 1.42 | 1.14–1.78 | 0.0018 |
| Diarrhea | 3.25 | 2.89–3.67 | <0.0001 |
| Weight loss | 0.85 | 0.75–0.96 | 0.0070 |
DISCUSSION
In this analysis of a national endoscopy database encompassing a broad spectrum of endoscopy settings during the years 2004–2009, duodenal biopsy was performed in 43% of patients undergoing EGD for anemia, iron deficiency, diarrhea, or weight loss. Although the rate of biopsy increased over time, even in the last year of the analysis (2009) only 51% underwent duodenal biopsy. Older individuals, males, blacks, and Hispanics were less likely to be biopsied than younger individuals, females, and whites.
This is the first study to measure duodenal biopsy rates nationally since the report in 2003 that the prevalence of CD is nearly 1% in the US,1 significantly greater than previously thought.13 Diagnosis rates appear to be increasing, based on data from Olmsted County6 and from a large insurance claims database.14 Despite these increasing diagnosis rates, there is evidence that CD remains under-diagnosed in this country. The prevalence of diagnosed CD in Olmstead County in 2001 was measured to be 0.04%, one twentieth of the true prevalence as measured by serological screening.1–2, 6 There are multiple potential steps along the path of a patient’s symptomatic presentation during which a CD diagnosis may be missed, and there is evidence that appropriate testing and referral by the patient’s primary care provider is crucial.15 The recent study of biopsy practices, in which only 35% of EGD’s with duodenal biopsy included the recommended number of specimens (≥4) suggests that factors related to the performance of endoscopy are, in part, responsible for low diagnosis rates.9
Our current study found that men undergoing EGD are less likely to have a duodenal biopsy than women. Most seroprevalence studies of CD found a similar prevalence among men and women,1–2, 16 but multiple epidemiological studies in the US and elsewhere have found that women are more likely to be diagnosed with CD,5–6 and multiple studies of patients with CD have a female:male ratio of approximately 2:1.17–18 This may be due to increased health-care seeking by women, but alternatively this may be due to unproven beliefs among patients and physicians that CD predominantly affects women. Low rates of duodenal biopsy among men will lead to fewer diagnoses of CD among men, further reinforcing the notion that men are less likely to develop CD.
Less is known about the prevalence of CD among black and Hispanic patients in the United States. Black patients in the United States have been included in two prevalence studies. Not, et al19 screened 2000 healthy blood donors for CD and this cohort included 230 black patients. One patient out of 230 (0.4%) had a positive endomysial antibody. In the multicenter study of celiac disease prevalence by Fasano, et al,1 blacks comprised 3% of 13,145 screened individuals (n=395). The prevalence of celiac disease among asymptomatic blacks was not reported, but among symptomatic blacks it was reported as 1:48, similar to that of whites. The overall prevalence of celiac disease among all asymptomatic minorities (blacks, Hispanics, and Asians) was reported as 1:236. Apart from these two studies, there are no investigations of the prevalence of CD among blacks or Hispanics in the United States. Black individuals are underrepresented among patients with diagnosed CD, as they comprise only 1.3% of patients in the Celiac Disease Center at Columbia University (9 of 700 patients with biopsy-proven celiac disease).20 Although the prevalence of CD among blacks and Hispanics in the United States is unknown, there are several studies from South America and the Caribbean reporting on CD, either prevalence or case series.21–28 In a prevalence study in Argentina, 12/2000 (0.6%) healthy adults in Buenos Aires screened positive; given the large proportion of patients with Italian ancestry, that population may not be generalizable to Hispanics in the United States.29 A study of healthy blood donors in Mexico found a seroprevalence of approximately 2%.22 This study demonstrates that physicians are less likely to search for a diagnosis of CD in black and Hispanic patients, which may perpetuate the unproven notion that CD is rare in these groups.
Younger age was predictive of duodenal biopsy, with patients the oldest category (≥70 years) nearly half as likely to have a biopsy compared to patients ages 20–49 (multivariate OR 0.51 95% CI 0.46–0.57). Previously thought to primarily present in childhood, CD diagnosis can occur at any age, and is most commonly diagnosed during the fourth through sixth decade.30 However, CD can present in the elderly, either as longstanding mild/subclinical disease31 or as a de novo development.32 Diagnosis and treatment of CD in the elderly may be especially important, as this age group is most at risk for the subsequent development of refractory celiac disease, and enteropathy-associated T cell lymphoma.33–34 Although our knowledge regarding celiac disease in the elderly has increased in the previous decade, the low rates of duodenal biopsy in the highest age group relative to the youngest age group have not changed over time (Figure 1).
The reasons for the modest increase in biopsy rates over time are not obvious, but this is likely due in part to greater awareness of celiac disease; this analysis begins in 2004, shortly after publication of the first national prevalence study in the United States, establishing the seroprevalence rate of 0.8%. 1 It could also reflect knowledge of low biopsy rates as established by a previous study. 10 This change could also be patient-driven, given increased patient awareness of celiac disease. Regardless of this cause, it is congruent with the modest annual increase in the number of specimens submitted during duodenal biopsy in a separate database study.9
This study has a number of limitations. The CORI database is not linked to pathology results, and although rates of duodenal biopsy could be measured, the results of said biopsies were not available. As such, the rate of CD diagnosis was not measured in this study, and so the impact of non-performance of duodenal biopsy on CD diagnosis rates could not be quantifed. Moreover, important clinical information that would impact on the pre-test likelihood of CD, such as the presence of positive serologies or a family history, was lacking in this database. As the aim of this study was to quantify endoscopist behavior in scenarios in which duodenal biopsy was likely indicated, the inclusion criteria were chosen so as to be most applicable to a patient who may have celiac disease. Most patients undergoing EGD for the indication of anemia, iron deficiency, diarrhea, or weight loss would potentially benefit from duodenal biopsy to diagnose or exclude CD, especially if no obvious explanatory lesion is identified in the rest of the upper gastrointestinal tract. Even a patient with negative serological studies should undergo duodenal biopsy if EGD is being performed, given the imperfect sensitivities of serologies, which in some studies have been less than 80%.35 Race/ethnicity may be subject to misclassification, as it was entered by the endoscopist and not by the patient. Strengths of this analysis include its multi-center national setting, representing a broad spectrum of practice types throughout the US, the six-year time span so as to evaluate for temporal trends, and the presence of racial and ethnic minorities that have been underrepresented in the study of CD.
We conclude that physicians performing EGD in the US for a variety of indications that are compatible with CD presentation (anemia, iron deficiency, diarrhea, and weight loss) perform duodenal biopsy at variable rates, and are less likely to perform duodenal biopsy on patients who are male, black or Hispanic, or elderly. Although biopsy rates have increased over time, the overall rate of duodenal biopsy during EGD’s done for the above indications was only 51% in 2009, lending further support to the notion that endoscopic practice is in part responsible for the under-diagnosis of CD in the United States. Future efforts should focus on increasing duodenal biopsy rates in the appropriate context, and increasing the rate of CD diagnosis in symptomatic individuals.
Acknowledgments
Grant Support:
BL: the National Center for Research Resources, a component of the National Institutes of Health (KL2 RR024157).
DAL: NIH (NIDDK U01DK57132). The practice network (Clinical Outcomes Research Initiative) has received support from the following entities to support the infrastructure of the practice-based network: AstraZeneca, Novartis, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
Abbreviations
- CD
Celiac Disease
- EGD
Esophagogastroduodenoscopy
- CORI
Clinical Outcomes Research Initiative
Footnotes
Author Contributions:
Study concept and design: BL, PG, AIN
Acquisition of data: BL, DAL, JH
Analysis and interpretation of data: BL, CAT, PG, AIN, DAL, JH
Drafting of the manuscript: BL, PG, AIN
Critical revision of the manuscript for important intellectual content: BL, CAT, PG, AIN, DAL, JH
Statistical analysis: BL
Study supervision: PG, AIN
Potential Competing Interests:
DAL: Dr. Lieberman is the executive director of the Clinical Outcomes Research Initiative, a non-profit organization that receives funding from federal and industry sources. This potential conflict of interest has been reviewed and managed by the OHSU and Portland VA Conflict of Interest in Research Committees.
BL, CAT, JLH, AIN, PHRG: No conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191–5. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 6.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 7.Virta LJ, Kaukinen K, Collin P. Incidence and prevalence of diagnosed coeliac disease in Finland: results of effective case finding in adults. Scand J Gastroenterol. 2009;44:933–8. doi: 10.1080/00365520903030795. [DOI] [PubMed] [Google Scholar]
- 8.Catassi C, Fabiani E, Ratsch IM, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl B, Kapel RC, Neugut AI, Green PH, Genta RM. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–9. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harewood GC, Holub JL, Lieberman DA. Variation in small bowel biopsy performance among diverse endoscopy settings: results from a national endoscopic database. Am J Gastroenterol. 2004;99:1790–4. doi: 10.1111/j.1572-0241.2004.40176.x. [DOI] [PubMed] [Google Scholar]
- 11.Accessed at http://www.cori.org
- 12.Dickey W, Hughes D. Disappointing sensitivity of endoscopic markers for villous atrophy in a high-risk population: implications for celiac disease diagnosis during routine endoscopy. Am J Gastroenterol. 2001;96:2126–8. doi: 10.1111/j.1572-0241.2001.03947.x. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Valdovinos M, Petterson TM, Carpenter HA, Melton LJ., 3rd Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994;89:843–6. [PubMed] [Google Scholar]
- 14.Green PH, Neugut AI, Naiyer AJ, Edwards ZC, Gabinelle S, Chinburapa V. Economic benefits of increased diagnosis of celiac disease in a national managed care population in the United States. J Insur Med. 2008;40:218–28. [PubMed] [Google Scholar]
- 15.Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454–60. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 16.Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011;106:1333–9. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebwohl B, Stavsky E, Neugut AI, Green PH. Risk of colorectal adenomas in patients with coeliac disease. Aliment Pharmacol Ther. 2010;32:1037–43. doi: 10.1111/j.1365-2036.2010.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci. 2008;53:1573–81. doi: 10.1007/s10620-007-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Not T, Horvath K, Hill ID, et al. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494–8. doi: 10.1080/00365529850172052. [DOI] [PubMed] [Google Scholar]
- 20.Brar P, Lee AR, Lewis SK, Bhagat G, Green PH. Celiac disease in African-Americans. Dig Dis Sci. 2006;51:1012–5. doi: 10.1007/s10620-005-9000-5. [DOI] [PubMed] [Google Scholar]
- 21.Remes-Troche JM, Rios-Vaca A, Ramirez-Iglesias MT, et al. High prevalence of celiac disease in Mexican Mestizo adults with type 1 diabetes mellitus. J Clin Gastroenterol. 2008;42:460–5. doi: 10.1097/MCG.0b013e318046ea86. [DOI] [PubMed] [Google Scholar]
- 22.Remes-Troche JM, Ramirez-Iglesias MT, Rubio-Tapia A, Alonso-Ramos A, Velazquez A, Uscanga LF. Celiac disease could be a frequent disease in Mexico: prevalence of tissue transglutaminase antibody in healthy blood donors. J Clin Gastroenterol. 2006;40:697–700. doi: 10.1097/00004836-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Parada A, Araya M, Perez-Bravo F, et al. Amerindian mtDNA haplogroups and celiac disease risk HLA haplotypes in mixed-blood Latin American patients. J Pediatr Gastroenterol Nutr. 2011;53:429–34. doi: 10.1097/MPG.0b013e31821de3fc. [DOI] [PubMed] [Google Scholar]
- 24.Araya M, Mondragon A, Perez-Bravo F, et al. Celiac disease in a Chilean population carrying Amerindian traits. J Pediatr Gastroenterol Nutr. 2000;31:381–6. doi: 10.1097/00005176-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Blanco Rabasa E, Sagaro Gonzalez E, Fragoso Arbelo T, Castaneda Guillot C, Gra Oramas B. Demonstration of celiac disease in Cuba. Bol Med Hosp Infant Mex. 1980;37:587–97. [PubMed] [Google Scholar]
- 26.Sagaro E, Jimenez N. Family studies of coeliac disease in Cuba. Arch Dis Child. 1981;56:132–3. doi: 10.1136/adc.56.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung JC, Phillips AD, Walker-Smith JA. Coeliac disease in children of West Indian origin. Arch Dis Child. 1995;73:166–7. doi: 10.1136/adc.73.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandolfi L, Pratesi R, Cordoba JC, Tauil PL, Gasparin M, Catassi C. Prevalence of celiac disease among blood donors in Brazil. Am J Gastroenterol. 2000;95:689–92. doi: 10.1111/j.1572-0241.2000.01847.x. [DOI] [PubMed] [Google Scholar]
- 29.Gomez JC, Selvaggio GS, Viola M, et al. Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am J Gastroenterol. 2001;96:2700–4. doi: 10.1111/j.1572-0241.2001.04124.x. [DOI] [PubMed] [Google Scholar]
- 30.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–31. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee R, Egbuna I, Brar P, et al. Celiac disease: similar presentations in the elderly and young adults. Dig Dis Sci. 2010;55:3147–53. doi: 10.1007/s10620-010-1142-4. [DOI] [PubMed] [Google Scholar]
- 32.Vilppula A, Kaukinen K, Luostarinen L, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 34.Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]