Abstract
In visceral pain, anxiety and pain occur simultaneously, but the etiogenesis of this effect is not yet well-described. The anterior cingulate cortex (ACC) is known to be associated with the affective response to noxious stimuli. The aim of the current study is to define the role of ACC extracellular signal-regulated (ERK)-1 and-2 (ERK1/2) activity in the development of pain-related anxiety/depression and the nocifensive response in acetic acid (AA)-elicited visceral pain. The model of visceral pain was created by intraperitoneal (ip) injection of AA to female Kunming mice. We found that AA injection resulted in a dynamic, bilateral ERK1/2 activation pattern in the ACC. Inhibition of ERK1/2 activation 2 hr after AA injection by subcutaneous (sc) injection of the mitogen-activating extracellular kinase (MEK) inhibitor, SL327, had no effect on the nocifensive responses, but did attenuate anxiety-like behavior, as determined by elevated plus-maze and open-field testing results. These data suggest that AA-induced visceral pain activates expression of ACC ERK1/2, which regulates visceral pain-related anxiety, but not the nocifensive response.
Keywords: anterior cingulate cortex (ACC), extracellular signal-regulated kinase (ERK), visceral pain, anxiety-like behavior
I. Introduction
Pain is related to sensory and affective parameters, accompanied by feelings of unpleasantness. In a state of chronic pain, negative factors, including anxiety and depression, are well-known to be associated with the perception of pain. It is widely accepted that pain-related negative factors influence the perception pain and the anxiety-decreased pain threshold [26]. In a recent study, we demonstrated that pain-related anxiety is dissociated from pain perception following surgery [6]. The clinical features of visceral pain, the most common form of pathological pain, differ from those of somatic pain—a difference likely due to differences in the underlying neurobiology [4, 11]. However, the underlying mechanism(s) responsible for the influence of the negative factors associated with visceral pain is still poorly understood.
Results from numerous human and animal studies indicate that the anterior cingulate cortex (ACC), which forms one of the largest parts of the limbic system, plays an important role in the affective component of pain [8, 20]. It has been reported that surgical lesions to the ACC region produce attenuation of the pain-related depression and unpleasantness experienced by patients suffering from chronic pain [13]. Another study indicated that ACC modulates anxiety-like behavior in adult mice [14]. Anatomically, the interconnections between the ACC and other limbic regions [1, 21] involved in pain modulation [22, 28] provide pathways through which the ACC influences in the emotional component of pain as well as the information processing and modulation of the transmission of noxious pain.
Members of the family of mitogen-activated protein kinases (MAPKs), particularly extracellular signal-regulated kinase (ERK), have received a great deal of attention in the past few years. The ERK pathway is involved in the regulation of a variety of cellular functions [3]. Our previous studies showed that pain-related anxiety and mechanical hypersensitivity are tightly linked and regulated by ACC ERK1/2 activation during the early phase of postoperative pain, while pain-related anxiety—but not mechanical hypersensitivity—requires ACC ERK1/2 activation in the late phase [6]. In the present study, therefore, we addressed the role of ACC ERK1/2 in hypersensitivity and pain-related anxiety behavior in visceral pain. We found that ERK1/2 was rapidly activated in non-gamma-aminobutyric acid (GABA)ergic neurons after acetic acid (AA) injection in mice. Inhibition of ERK1/2 activation by subcutaneous (sc) injection of the inhibitor, SL327, had no effect on abdominal contractions, but did reduce the anxiety-like behavior of the mice. These findings reveal a novel role for ACC ERK1/2 in regulating pain-related anxiety in visceral pain, and suggest that ERK1/2 inhibitors represent potential therapeutic strategies for the treatment of pain-related anxiety disorders.
II. Materials and Methods
Animals
Adult female Kunming mice, weighing 18–22 g, obtained from Central South University Animal Services (Changsha, China) were used as visceral pain models. Mice were adapted to their new environment for 3–4 days after arrival. The experimental protocol was approved by the Animal Care and Use Committee of Central South University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Visceral pain and ERK1/2 inhibitor SL327 intervention models
Experimental mice (n=25) were gently grasped and injected ip with 0.6% AA in saline (10 ml/kg). The mitogen-activating extracellular kinase (MEK) inhibitor—alpha - [amino [( 4-aminophenyl ) thio ] methylene] - 2 - (trifluoromethyl)benzeneacetonitrie (SL327) (Sigma, St. Louis, MO, USA)—was dissolved in 99.9% dimethyl sulfoxide (DMSO), final concentration 7.5 mg/ml. Thirty min before AA injection, one group of mice (n=8) were injected sc with SL327 (30 mg/kg); the same amount of DMSO was administered to another cohort of mice (n=8), as the vehicle control group.
Assessment of visceral pain behavior
Visceral pain behavior was assessed blindly by counting abdominal contractions for 60 min after AA injection, as described in one of our previous studies [17].
Immunohistochemistry
Mice were treated with either saline or AA. Mice were sacrificed 10 min, 30 min, 1 hr, and, 2 hr after injection by administration of an overdose of 10% chloral hydrate (80 mg/kg) and were then perfused transcardially with warm saline, followed by 100 ml of ice-cold 4% paraformaldehyde solution (pH 7.4). Brains were removed and fixed for 24 hr in 4% paraformaldehyde, and cryoprotected with 30% phosphate-buffered sucrose (pH 7.4) overnight. Coronal sections (30 µm) of brains were made, and free-floating sections were treated with 3% H2O2 to remove endogenous peroxidase, which was then blocked by placing sections in 5% normal bovine serum in 0.01 M phosphate-buffered saline (PBS) containing 0.1% Triton with X-100 for 2 hr. Sections were then incubated with rabbit anti-p-ERK1/2 antibody (1:1,000) (Cell Signaling Technology, Danvers, MA, USA) at room temperature for 2 hr, followed by incubation at 4°C overnight. Secondary reagents comprised biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA). Diaminobenzidine tetrahydrochloride (DAB) (Sigma) was used as a peroxidase substrate. Washing for all procedures was accomplished in 0.01 M PBS, except for the blocking step. Sections treated as above, but with no primary antibody were used as negative controls, no immunostaining was seen in these sections.
Immuno-positive phospho (p)-ERK1/2 cells in both sides of the ACC were counted in six to eight sections from each animal. Counts were averaged into a single value per section per animal, and the average of measurements from each mouse was used to calculate the group means [18]. In order to avoid any bias, the researcher quantifying the number was blinded to the treatment used.
Double labeling immunofluorescence of p-ERK1/2 and GAD67
Localization of p-ERK1/2 and glutamic acid decarboxylase (GAD)-67 in the ACC was identified by using double-labeling, with several modifications to the method used in our previous study [18]. Briefly, free-floating sections were blocked for 2 hr with 5% normal bovine serum in 0.01 M PBS containing 0.1% Triton X-100. They were then incubated in a mixture of rabbit anti-p-ERK1/2 antibody (1:1,000) (Cell Signaling Technology) and mouse anti GAD67 (1:1,000) (Abcam, HongKong) at room temperature for 2 hr, followed by incubation overnight at 4°C. After being thoroughly washed with 0.01 M PBS containing 0.05% Triton X-100 solution, sections were incubated in a mixture of Alexa 488- or Cy3-labeled donkey anti-rabbit or -mouse secondary antibodies (1:250) (Invitrogen, Grand Island, NY, USA) in the dark at room temperature for 2 hr. Following three washes, sections were mounted onto slides using vectashield mounting medium (Vector Laboratories). Negative controls comprised slides treated as above, but without primary or secondary antibodies. Sections were viewed on a Nikon immunofluorescent microscope (Nikon, Japan).
Western blotting
Mice were anesthetized with an overdose of 10% chloral hydrate (80 mg/kg), and the brains removed rapidly following decapitation. The ACC was dissected on ice, according to the method of Franklin and Paxinos [9], then quickly frozen with liquid nitrogen. Frozen samples were homogenized in a lysis buffer containing phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktails (CWbio tech, Beijing, China). Samples were then centrifuged at 12,000 rpm for 15 min at 4°C. The supernatants were collected and used for Western blotting. The BCA protein assay kit (CWbio tech, Beijing, China) was used to measure protein concentration in the ACC. ACC extracts were subjected to SDS-polyacrylamide gel electrophoresis in 10% polyacrylamide gels and transferred to a nitrocellulose membrane (BOSTER, Wuhan, China). The membrane was blocked with 10% nonfat milk for 3 hr at room temperature and then incubated overnight at 4°C with rabbit anti-p-ERK1/2 (1:1,000) (Cell Signaling Technology) and mouse anti-GAP dehydrogenase (GAPDH) (1:1,000) (BOSTER) antisera. After washing with 0.02 M tris-buffered saline/Tween 20 (TBST) buffer, blots were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin (1:1,000) (Pierce, Rockford, IL, USA) at room temperature for 2 hr. Membranes were washed three times with TBST and signals detected by enhanced chemiluminescence (ECL) (Thermo Scientific, Shanghai, China), followed by exposure to X-ray films for 1–10 min. Films were scanned with a laser scanner, and densitometry analysis was performed using the BIORAD Image Analysis System (Bio-Rad Laboratories, Hercules, CA, USA).
Elevated plus-maze test
Anxiety-like behavior was examined by the elevated plus-maze (EPM) test, as described previously [6, 16]. Testing was carried out 2 hr after injection of AA. Briefly, after adaptation for ~1 day in the experimental room, each animal was placed in the center square facing the closed arms, and allowed to move freely for 5 min. The test was recorded for 5 min with a video camera. The percentage of time spent in the open arms was recorded and used for statistical analysis.
Open field test
The open field test was used to detect anxiety-like behavior, as described in our previous studies [6, 16]. Movements of the animal within the area were recorded during the 5-min testing session. An observer, blinded to the experimental conditions, coded the videotapes.
Statistical analyses
Statistical analyses were performed using Graphpad Prism 5.01 software (Graphpad Software, San Diego, CA, USA). Data are presented as mean±SEM. Differences between groups were compared using one-way analysis of variance (ANOVA), followed by post hoc Dunnett testing. Unpaired two-tailed Student’s t-test was used when only two groups were applied. A value of p<0.05 was considered as statistically significant.
III. Results
Biphasic ERK1/2 activation in the ACC Following AA injection
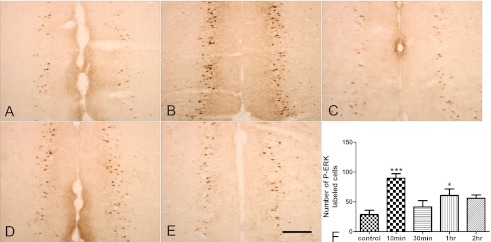
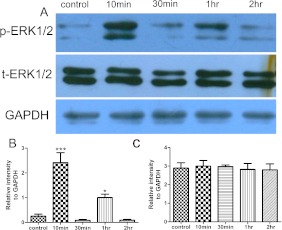
We determined that 0.6% AA injection induces abdominal contractions in female Kunming mice (data not shown). We also found that ERK1/2 was rapidly activated (phosphorylated) after AA injection. Expression of p-ERK1/2 was localized mainly in pyramidal-shaped neurons (Fig. 1). One-way ANOVA revealed that there was a significant increase in the number of p-ERK1/2-labeled cells compared with those of the control group (89.72±7.316 versus 28.6±7.201 (Fig. 1B, F), F4,20=7.415, p<0.001) 10 min after AA injection. However, this increase was transient and returned to baseline levels 30 min after AA injection (41.46±10.543) (Fig. 1C). Interestingly, p-ERK1/2 expression increased again 1 hr after AA injection (60.8±10.673) (Fig. 1D, F), but returned to baseline levels 2 hr after AA injection (56.12±5.266) (Fig. 1E). Western blotting further confirmed this fluctuating expression pattern of ACC p-ERK1/2 (Fig. 2A). Both p-ERK-1 (44 kDa) and p-ERK-2 (42 kDa) significantly increased in the ACC at 10 min (2.415±0.404 versus 0.248±0.088, respectively) in the control group (Fig. 2A, B), decreased at 30 min, and increased again at 1 hr (1.005±0.134 versus 0.248±0.088, respectively) (Fig. 2A, B) after AA injection. No significant changes in total ERK1/2 levels were found between groups at any of the time points examined (F4,25=0.2602, p=0.9006) (Fig. 2A, C).
Fig. 1.
Time course of ERK1/2 activation in the ACC after AA injection. A, B: Representative photomicrographs showing p-ERK1/2 immunoreactivity in the ACC from coronal brain sections in control group (A) or at 10 min (B), 30 min (C), 1 hr (D) and 2 hr (E) after AA injection. F: Quantitative analysis of number of p-ERK1/2 positive cells in both sides of ACC at indicated times points (n=5) for each group per indicated time. Bar=200 µm. ACC, anterior cingulate cortex; p-ERK, phosphorylated extracellular signal-regulated kinase. *** p<0.001 versus control group. * p<0.05 versus control group.
Fig. 2.
Effect of AA injection on p-ERK1/2 expression in the ACC by Western blot. A: Representative Western blot of p-ERK1/2, total-ERK1/2, and GAPDH in the ACC after AA injection. B, C: Semiquantitative analysis of p-ERK1/2 (B) and total-ERK1/2 (C) relative to GAPDH. AA injection induces an increase in the intensity of the band for p-ERK1/2. Increased p-ERK1/2 expression appears at 10 min after AA injection and returns to baseline level 30 min after injection. At 1 hr after injection, p-ERK1/2 expression is increased again, and returns to baseline level 2 hr after injection (n=6 for each group). *** p<0.001 versus control, * p<0.05 versus control. GAPDH is the loading control. ACC, anterior cingulate cortex; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
ACC localization of p-ERK1/2 and GAD following AA injection
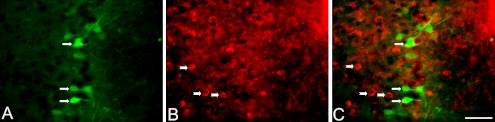
To reveal neurochemical phenotypes of activated ACC neurons following AA injection, p-ERK1/2 and GAD67 (a marker for GABAergic-neurons) double immunofluorescence labeling was performed. As shown in Figure 3, p-ERK1/2 (shown in green in Fig. 3A) was localized mainly in neuronal cytoplasm and dendrites, whereas GAD67 (shown in red in Fig. 3B) was localized in the cell membrane of neurons. No co-localization of p-ERK1/2 and GAD67 was found in ACC neurons following AA injection (Fig. 3C).
Fig. 3.
Localization of p-ERK1/2 and GAD67 in the ACC 10 min after AA injection. A, B: Representative photomicrographs of the double labeling of p-ERK1/2 (green, A) with GAD (red, B). Arrows (A) indicate p-ERK1/2 immunoreactive cells. Arrowheads (B) indicate GAD67 immunoreactive cells. C: Merged pictures of A and B. Bar=50 µm. GAD, glutamic acid decarboxylase.
SL327 reduces AA-induced anxiety-like behavior but not nociceptive pain
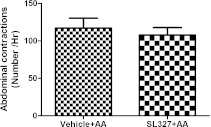
One-way ANOVA showed that there was no significant difference in abdominal contractions between the AA, DMSO, and SL327 groups (107.75±9.915, 92.44±11.699, and 117±13.291, respectively; F2,22=1.146, p=0.3362) (Fig. 4). These results suggest that blocking ERK1/2 activation has no effect on AA-induced abdominal contractions.
Fig. 4.
Effect of SL327 on abdominal contractions of mouse after AA injection. SL327 was administered by sc injection 30 min before AA injection. Abdominal contractions were recorded 1 hr after AA injection. There was no significant difference in the SL327-treated group compared with vehicle group (n=8 for each group). SL327=alpha-[Amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile.
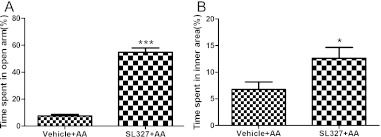
Two hours after AA injection, as shown by unpaired two-tailed Student’s t-test, the percentage of time spent in the open arm (54.73±3.301 versus 7.486±1.138, respectively) and the percentage of time in the inner area (12.63±2.026 versus 6.75±1.424, respectively) were significantly longer in SL327-treated mice compared with control animals. These data imply that inhibition of ERK1/2 activation contributes to decreased anxiety-like behavior, at least at 2 hr after AA injection.
IV. Discussion
The present study investigated the mechanism(s) underlying nocifensive responses and anxiety-like behavior in an animal model of acute visceral pain—a state that mimics clinical peritonitis or irritable bowel syndrome (IBS) pain.
Several important findings emerge from the current study. First, we found that p-ERK1/2 was dynamically activated after AA injection. Significantly, AA-induced increases in expression of p-ERK1/2 reached a peak at 10 min, decreased briefly to baseline levels at 30 min, increased again at 1 hr, and returned to baseline at 2 hr after injection. Second, as shown by results of double-labeling, AA-induced p-ERK1/2 activation occurred only in non-GABAergic ACC neurons. Third, inhibition of p-ERK1/2 activation had no effect on abdominal contractions, but did reduce the anxiety-like behavior resulting from administration of AA.
AA-induced ERK biphasic activation in ACC
Phosphorylation of ERK1/2 in the ACC is implicated in the affective and sensory dimensions involved with chronic [27], incisional [6] and inflammatory pain [2]. More recently, ERK1/2 was reported to be extensively involved in visceral pain hyperalgesia [15]. Some studies have shown that stimulation of noxious visceral pain, e.g., intracolonic application of mustard oil and capsaicin [10], gastric distension [23] and colorectal distension [19], induced ERK1/2 phosphorylation in spinal dorsal horn neurons, indicating that spinal ERK1/2 may be involved in visceral pain processing. In agreement with results of our previous study, we hereby demonstrated that AA-induced ACC ERK1/2 activation displays a biphasic pattern, despite differences in the time points used in the two studies, which may be attributed to the different pain models (visceral or incisional pain) used as well as to differences in species (mice versus rats) [6]. Furthermore, the p-ERK1/2 expression pattern in the present study is consistent with recently reported findings, i.e., that in formalin-induced pain rapid (<30 min) ERK1/2 activation is induced in the deep layers (laminae V–VI) of the bilateral rostral ACC [2]. GABA is the main inhibitory neurotransmitter in the nervous system, and GABAergic inhibition is abnormal in pain states [24]. A number of studies have suggested the involvement of spinal GABAergic neurotransmission in modulating pain behavior evoked by chronic inflammatory pain states [29]. GABAergic neurons are critical components of the ACC, and are rich in GAD67, the GABA-synthesizing enzyme that is widely used as the marker for activated GABA neurons [7, 25]. Surprisingly, as revealed by double-labeling, no co-localization of GAD67 and p-ERK1/2 was found in the ACC. One possible explanation for this is that p-ERK1/2 is expressed in projection neurons, which transmit messages to target neurons in other affective, associated brain regions, e.g., the amygdalla and hippocampus.
AA-induced activation ACC ERK1/2 regulates anxiety-like behavior but not the nocifensive response
Although pain-related anxiety is tightly coupled to pain itself, growing evidence indicates that pain-related anxiety is dissociated from pain perception in chronic back pain patients [12]. Pain-related negative sequelae are more disabling than pain itself, and have been shown to have severe effects on daily activities of chronic pain patients [5]. Thus, it is crucial to explore the molecular signaling mechanism(s) underlying pain-related anxiety behavior. In the current study, we found that SL327 injection did not reverse abdominal contractions. This finding is in accordance with our recent data that pain-related anxiety lasts longer than does mechanical hypersensitivity in incisional pain [16]. The fact that ERK1/2 inhibitors attenuated induction of pain-related anxiety, but not mechanical hypersensitivity, indicates that the late-phase of ACC ERK1/2 activation is responsible for the affective and perceptive aspects of visceral pain. Moreover, our finding that ACC ERK1/2 activation modulates pain perception after AA injection is in agreement with those of recent studies which demonstrated that the ERK1/2 activation in the rostral ACC is required for both induction and expression of affective pain, but not nociceptive pain, in formalin-induced pain [2]. Additionally, previous studies demonstrated that the late phase of ACC ERK1/2 activation regulates only the expression of pain-related anxiety, not mechanical hypersensitivity [6]. Taken together, these findings further demonstrate that pain effects and mechanical hypersensitivity are tightly coupled, whereas their expression may be dissociated.
In conclusion, the present study demonstrates that the activation of p-ERK1/2 in the ACC, the brain area in control of mood disorders and pain processing, is responsible for acute visceral pain-related anxiety behaviors. Attenuation of the overactivity of ACC p-ERK1/2 represents a potential, valuable therapeutic strategy for relief of pain-related anxiety.
V. Acknowledgments
This study was supported by the National Science Foundation of China (Grant No: 31000525) and the Free Exploration Program of Central South University (201012200188) to Fang Li, Master’s Thesis Innovation Program of Central South University (2011ssxt105) to Xiao-Lin Zhong, National Science Foundation of China (Grant No: 81070897) and Excellent Young Investigator Program of Central South University to Dr. Dai, National Science Foundation of China (Grant No: 81100908) and Free Exploration Program of Central South University (2011QNZT143) to Dr. Fang-Fang Bi. We wish to thank Xin-Fu Zhou for critical reading of the manuscript.
VI. Conflict of Interest Statement
The authors affirm that no conflict of interest exists.
Fig. 5.
Effect of SL327 on anxiety-like behavior after AA injection. A: The percentage of time spent in the open arm in EPM test in vehicle and SL327 groups. B: Open-field test shows increases in time spent in the inner square after SL327 injection (n=8 for each group). SL327=alpha-[Amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile. * p<0.05 versus the vehicle group, *** p<0.001 versus vehicle group.
VII. References
- 1.Allen G. V., Hopkins D. A. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J. Comp. Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- 2.Cao H., Gao Y. J., Ren W. H., Li T. T., Duan K. Z., Cui Y. H., Cao X. H., Zhao Z. Q., Ji R. R., Zhang Y. Q. Activation of extracellular signal regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci. 2009;29:3307–3321. doi: 10.1523/JNEUROSCI.4300-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Célia C. D., Cruz F. The ERK 1 and 2 pathway in the nervous system: from basic aspects to possible clinical applications in pain and visceral dysfunction. Curr. Neuropharmacol. 2007;5:244–252. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervero F., Laird J. M. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 5.Crombez G., Vlaeyen J. W., Heuts P. H., Lysens R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339. doi: 10.1016/s0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 6.Dai R. P., Li C. Q., Zhang J. W., Li F., Shi X. D., Zhang J. Y., Zhou X. F. Biphasic activation of extracellular signal-regulated kinase in anterior cingulate cortex distinctly regulates the development of pain-related anxiety and mechanical hypersensitivity in rats after incision. Anesthesiology. 2011;115:604–613. doi: 10.1097/ALN.0b013e3182242045. [DOI] [PubMed] [Google Scholar]
- 7.Erlander M. G., Tillakaratne N. J., Feldblum S., Patel N., Tobin A. J. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 8.Foltz E. L., White L. E. Pain “relief” by frontal cingulumotomy. J. Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 9.Franklin K. B. J., Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 10.Galan A., Cervero F., Laird J. M. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res. Mol. Brain Res. 2003;116:126–134. doi: 10.1016/s0169-328x(03)00284-5. [DOI] [PubMed] [Google Scholar]
- 11.Giamberadino M. A. Recent and forgotten aspects of visceral pain. Eur. J. Pain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- 12.Grachev I. D., Fredickson B. E., Apkarian A. V. Dissociating anxiety from pain: mapping the neuronal marker N-acetyl aspartate to perception distinguishes closely interrelated characteristics of chronic pain. Mol. Psychiatry. 2001;6:256–258. doi: 10.1038/sj.mp.4000834. [DOI] [PubMed] [Google Scholar]
- 13.Hurt R. W., Ballantine H. T. Stereotactic anterior cingulated lesions for persistent pain: A report on 68 cases. Clin. Neurosurg. 1974;21:334–351. doi: 10.1093/neurosurgery/21.cn_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- 14.Kim S. S., Wang H., Li X. Y., Chen T., Mercaldo V., Descalzi G., Wu L. J., Zhuo M. Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol. Brain. 2011;4:6. doi: 10.1186/1756-6606-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai H. H., Qiu C. S., Crock L. W., Morales M. E., Ness T. J., Gereau R. W., 4th Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain. 2011;152:2117–2124. doi: 10.1016/j.pain.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C. Q., Zhang J. W., Dai R. P., Wang J., Luo X. G., Zhou X. F. Surgical incision induces anxiety-like behavior and amygdala sensitization: effects of morphine and gabapentin. Pain Res. Treat. 2010;2010:705874. doi: 10.1155/2010/705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F., Zhang J. W., Wei R., Luo X. G., Zhang J. Y., Zhou X. F., Li C. Q., Dai R. P. Sex-differential modulation of visceral pain by brain derived neurotrophic factor (BDNF) in rats. Neurosci. Lett. 2010;478:184–187. doi: 10.1016/j.neulet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Li F., Wang X. S., Dai R. P., Zhang J. Y., Zhou X. F., Hao W., Li C. Q. The activation of NMDA receptor-ERK pathway in the central amygdala is required for the expression of morphine-conditioned place preference in the rat. Neurotox. Res. 2011;20:362–371. doi: 10.1007/s12640-011-9250-2. [DOI] [PubMed] [Google Scholar]
- 19.Million M., Wang L., Wang Y., Adelson D. W., Yuan P. Q., Maillot C., Coutinho S. V., Mcroberts J. A., Bayati A., Mattsson H., Wu V., Wei J. Y., Rivier J., Vale W., Mayer E. A., Taché Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK 1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainville P., Duncan G. H., Price D. D., Carrier B., Bushnell M. C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 21.Reep R. L., Corwin J. V. Topographic organization of the striatal and thalamic connections of rat medical agranular cortex. Brain Res. 1999;841:43–52. doi: 10.1016/s0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- 22.Royce G. J. Cortical neurons with collateral projections to both the caudate nucleus and the centromedian-parafascicular thalamic complex: a fluorescent retrograde double labeling study in the cat. Exp. Brain Res. 1983;50:157–165. doi: 10.1007/BF00239179. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai J., Obata K., Ozaki N., Tokunaga A., Kobayashi K., Yamanaka H., Dai Y., Kondo T., Miyoshi K., Sugiura Y., Matsumoto T., Miwa H., Noguchi K. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology. 2008;134:1094–1103. doi: 10.1053/j.gastro.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Schoffnegger D., Heinke B., Sommer C., Sandkühler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J. Physiol. 2006;577:869–878. doi: 10.1113/jphysiol.2006.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soghomonian J. J., Martin D. L. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 26.Tang J., Gibson S. J. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J. Pain. 2005;6:612–619. doi: 10.1016/j.jpain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Wei F., Zhuo M. Activation of Erk in the anterior cingulate cortex during the induction and expression of chronic pain. Mol. Pain. 2008;4:28. doi: 10.1186/1744-8069-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyss J. M., Sripanidkulchai K. The topography of the mesencephalic and pontine projections from the cingulate cortex of the rat. Brain Res. 1984;293:1–15. doi: 10.1016/0006-8993(84)91448-3. [DOI] [PubMed] [Google Scholar]
- 29.Zeilhofer H. U., Zeilhofer U. B. Spinal dis-inhibition in inflammatory pain. Neurosci. Lett. 2008;437:170–174. doi: 10.1016/j.neulet.2008.03.056. [DOI] [PubMed] [Google Scholar]