Abstract
We used behavior and event-related potentials (ERPs) to examine auditory stream segregation in people with schizophrenia and control participants. During each trial, a context pattern was presented, consisting of low (A) and high (B) tones in a repeating ABA pattern, with a frequency separation (Δf) of 3, 6, or 12 semitones. Next, a test ABA-pattern was presented that always had a 6-semitone Δf. Larger Δf during the context resulted in more perception of two streams and larger N1 and P2 ERPs, but less perception of two streams during the test pattern. These effects of Δf were smaller in schizophrenia. Individuals with schizophrenia also showed a reduced effect of prior perceptual judgments. Overall, the findings demonstrate that people with schizophrenia have abnormalities in segregating sounds. These abnormalities result from difficulties utilizing frequency cues in addition to reduced temporal context effects.
Keywords: auditory cortex, auditory scene analysis, event-related potentials, tonotopic organization, context effects
Schizophrenia is characterized by a wide range of symptoms and neurobehavioral deficits that result from diverse genetic and environmental factors. Abnormalities in the perception of auditory information are common in schizophrenia and have been demonstrated across a wide range of tasks. One well-documented finding is reduced performance on simple frequency-discrimination tasks (Javitt, Shelley, & Ritter, 2000; Javitt, Strous, Grochowski, Ritter, & Cowan, 1997; March, et al., 1999; Rabinowicz, Silipo, Goldman, & Javitt, 2000; Strous, Cowan, Ritter, & Javitt, 1995). Importantly, frequency-discrimination difficulties may contribute to impairments in more complex real-world skills such as categorical perception of speech (Cienfuegos, March, Shelley, & Javitt, 1999) or encoding speech prosody (Leitman, et al., 2005). Furthermore, impairments in auditory perception may have important relations with the underlying pathophysiology of the disorder, as they are accompanied by brain abnormalities in cortical regions associated with auditory processing. For example, event-related potential (ERP) and magnetoencephalographic (MEG) studies of auditory cortex have shown abnormal tonotopic organization (Rojas et al., 2002); abnormal processing of tone sequences containing alternating frequencies (Rojas, Slason, Teale, & Reite, 2007); and overall reduced amplitude of auditory brain responses (McCarley, Faux, Shenton, Nestor, & Adams, 1991; Shelley et al., 1991). Also, magnetic resonance imaging (MRI) studies have shown reduced gray matter volume in the superior temporal gyrus (STG), which contains primary and secondary auditory cortex (McCarley et al., 1999; Shenton, Dickey, Frumin, & McCarley, 2001).
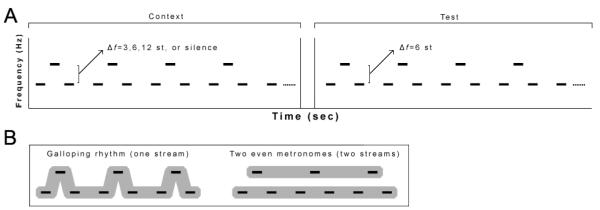
Whether these functional and structural auditory abnormalities affect the ability to segregate sounds coming from different sources is unknown, despite the importance of sound segregation in real-world situations. In particular, it is not clear whether the difficulties in discriminating sound frequency would lead to problems segregating sounds because pure-tone frequency discrimination thresholds are typically smaller than thresholds for perceptually segregating tone patterns (Rose & Moore, 2005). The present study examines these matters in schizophrenia participants and control participants using an auditory stream segregation task. Auditory stream segregation is the phenomenon by which listeners perceptually organize sounds into one or more streams, where a stream can be thought of as a sequence of sounds emanating from a single source. Stream segregation is necessary during noisy social gatherings, such as when trying to segregate a friend’s voice from background noise in order to have a meaningful conversation. In the laboratory, a cue that has been found to greatly increase the likelihood of perceiving multiple streams is the frequency separation (Δf) between sequential sounds. Specifically, in a repeating sequence of low tones (A), a high tone (B), and silence (−) in an ABA-pattern, the likelihood of perceiving the low tones and high tones as separate streams (i.e., A---A---A--- and B---B---B---) increases as Δf increases. In contrast, a sequence with a small Δf is typically perceived as a single stream (i.e., ABA-ABA-ABA-) (see Figure 1).
Figure 1.
Stimulus design and perceptual organization. (A) Trials consisted of a 6.72 s context sequence, a 1.44 s silent interval, and a 6.72 s test sequence. Context and test sequences both consisted of 14 repeating ABA-patterns where A represents a low tone, B a high tone, and - a silence. The Δf between the low and high tones during the test sequence was always 6 semitones (st) where A=300 Hz and B=424 Hz. During the context sequence, four of the following conditions could occur: (1) A=300 Hz, B=357 Hz (Δf=3 st), (2) A=300 Hz, B=424 Hz (Δf=6 st), (3) A=300 Hz, B=600 Hz (Δf=12 st), or (4) silence. Each context sequence was paired with the test sequence to make a total of 4 trial types. (B) Both context and test patterns could be heard as either a galloping rhythm (one stream) or two even metronomes (two streams).
The importance of Δf as a cue to streaming is likely to be a consequence of the tonotopic organization of the peripheral (Hartmann & Johnson, 1991) and central (Snyder & Alain, 2007b) auditory system. Specifically, alternating tone sequences with small Δf activate overlapping frequency-selective neurons in the auditory cortex and are consequently perceived as one stream; in contrast, sequences with large Δf activate two non-overlapping frequency-selective neuronal populations and are consequently perceived as two streams. Therefore, the non-overlapping neural populations activated by large Δf sequences should produce larger responses, as there will be less mutual suppression of the two neural populations. Support for this was provided by MEG and ERP studies showing changes in auditory cortical N1 and P2 components (Gutschalk et al., 2005; Snyder & Alain, 2007a; Snyder, Alain, & Picton, 2006; Snyder, Holder, Weintraub, Carter, & Alain, 2009), such that increases in Δf were coupled with increases in response amplitudes and these amplitude modulations correlated very strongly with perception of streaming.
To our knowledge, only one study has examined auditory stream segregation in schizophrenia (Bourdet, Brochard, Rouillon, & Drake, 2003). In this study, schizophrenia participants showed no streaming impairments compared to controls, as measured indirectly based on temporal change detection that should have been easier when two segregated streams were perceived. An alternative strategy for assessing streaming is to have participants directly report their perception. This task is particularly well suited for investigating streaming because it is possible to rapidly assess behavior, in contrast to indirect tasks that are often extremely time consuming and cognitively demanding. An additional limitation of the study by Bourdet and colleagues is that they used Δf values that were all larger than one octave. Such large values may be unable to reveal robust group differences.
In addition to low-level processes such as Δf-based segregation, streaming also involves higher-level aspects of auditory processing (for reviews, Moore & Gockel, 2002; Snyder & Alain, 2007b; Snyder, Gregg, Weintraub, & Alain, 2012), as indicated by context effects on perception of streaming. Specifically, the perception of streaming during a current pattern is greatly influenced by the previous Δf and the perception of previous patterns. When a context ABA-pattern is presented with a variable Δf, followed by a test ABA-pattern, the larger the Δf during context, the less likely listeners hear the test pattern as two streams (Snyder, Carter, Hannon, & Alain, 2009; Snyder, Carter, Lee, Hannon, & Alain, 2008; Snyder, Holder, et al., 2009; Snyder & Weintraub, 2011). This effect of prior Δf is not due to a suppressive effect of the prior perceptual interpretation because when the Δf is kept constant during the context and test, perception of two streams at the end of the context actually results in listeners more likely to again report perceiving two streams compared to one stream during the test pattern. Thus, there are two separate context effects that can be observed in streaming, one that is due to the influence of prior Δf and another that is due to the prior percept. Studying these two implicit memory effects together should shed light on higher-level aspects of streaming.
To our knowledge, only one study has examined effects of context on auditory perceptual organization in schizophrenia (Silverstein, Matteson, & Knight, 1996). In this study, participants listened to a list of numbers spoken by a human speaker followed by an animal sound that could be perceived as either originating from the human or an animal. When the final sound was perceived as originating from the male speaker, recall of the list of numbers worsened compared to when it was perceived as originating from an animal. By instructing participants that the suffix actually originated from a separate source (an animal), the interfering effects of the final sound were reduced. However, this contextual manipulation had no effect on schizophrenia participants, demonstrating impaired use of context by schizophrenia participants.
The aim of the current study is to examine schizophrenia participants during a streaming task, using behavioral and ERP measures obtained with a recently studied paradigm that is able to simultaneously reveal the presence of abnormalities in Δf-based segregation and the use of context (Snyder, Holder, et al., 2009). In this paradigm, a trial consists of two sequences of ABA-patterns: 1) a context sequence with a variable Δf, and 2) a test sequence with a fixed 6-semitone Δf (Figure 1a). Examining perception and ERPs during the context can provide information about the use of the Δf cue for streaming. Given the low-level auditory abnormalities described above, schizophrenia participants were expected to experience less of an increase in perception of two streams with increasing Δf compared to controls during the context, and to show a smaller effect of Δf on N1 and P2 response amplitude. Additionally, examining the impact of the prior context on later perception during the test can provide information about the operation of context effects, a high-level aspect of streaming. Given their reduced effects of context on auditory perceptual organization (Silverstein, Matteson, & Knight, 1996), schizophrenia participants were expected to experience less of a decrease in perception of two streams with increasing prior Δf compared to controls during the test. Schizophrenia participants were also expected to experience a reduced effect of prior perception such that, compared to controls, they less often report the same percept during the test as reported for the preceding context when the Δf between context and test was constant.
Methods
Participants
Twenty-one participants with schizophrenia and twenty-two healthy controls participated in the study. Table 1 contains demographic information for each group. As indicated in Table 1, groups did not significantly differ on age, gender, ethnicity, or handedness as measured by self-report. However, the schizophrenia group had significantly fewer years of education and significantly lower IQ. For the schizophrenia group, seventeen participants were taking atypical antipsychotics, two participants were taking an additional typical antipsychotic, and two were not taking any antipsychotics. The behavioral data from two schizophrenia participants and one healthy control were not included in the present report because these participants were unable to sufficiently understand the behavioral task. However, their ERP data displayed normal trends. Furthermore, previous work has shown that attention and other high-level factors do not greatly affect the ERP modulations we examined (Gutschalk et al., 2005; Snyder et al., 2006); rather the modulation of N1 and P2 are largely driven by Δf and thus reflect early frequency-based segregation of tones in auditory cortex. Thus, it is unlikely that the ERP data were compromised by poor task performance so the data were retained in the analysis. Demographic characteristics between groups did not change appreciably when these subjects were excluded. All participants exhibited normal hearing for their age (<30 dB HL from 250 to 1000 Hz, <40 dB HL from 2000 to 8000 Hz).
Table 1. Demographic characteristics for the schizophrenia and healthy control groups.
| Schizophrenia (n=21) |
Normal control (n=22) |
Between Group Differences |
|
|---|---|---|---|
| Demographic Information | |||
| Age (SD) | 45.2 (12.1) | 39.8 (12.0) | t=1.47, p=.15 |
| % Females | 38 | 50 | x2=.62, p=.43 |
| % Right Handed | 81 | 100 | x2=4.62, p=.10 |
| Education (SD) | 11.8 (2.9) | 14.6 (2.7) | t=-3.82, p<.001 |
| IQ (SD) | 79.8 (13.2) | 100.8 (12.0) | t=-5.42, p<.001 |
| Ethnic Distribution | x2=10.4, p=.10 | ||
| % Caucasian | 47.6 | 63.6 | |
| % African American | 33.3 | 9.0 | |
| % Hispanic / Latino | 4.8 | 13.6 | |
| % Pacific Islander | 4.8 | 0 | |
| % American Indian | 4.8 | 0 | |
| % Bi-racial | 4.8 | 0 | |
| % Other | 0 | 13.6 | |
| Current Psychiatric Medication | |||
| % Unmedicated | 9.5% | ||
| % Antipsychotics (typical) | 9.5% | ||
| % Antipsychotics (atypical) | 81.0% | ||
| Current Treatment (n=17)* | |||
| % Outpatient | 76.2% | ||
| % No Treatment | 4.8% | ||
| Other Patient Information | |||
| Age at Onset (SD) (n=19)* | 20.8 (8.0) | ||
| Duration of Illness (SD) (n=19)* | 25.1 (13.7) | ||
| # Hospitalizations (SD) (n=16)* | 3.6 (1.6) |
=value of n represents the number of schizophrenia participants with endorsed information
Schizophrenia participants were primarily recruited through an outpatient community mental health center, although two participants were recruited from the community. All control participants were recruited from the community. To be included in the study, all participants had to be between the ages of 18 and 65 years and have normal hearing. Additional exclusion criteria included history of electro-convulsive therapy; neurological disorder or a medical condition with known effects on CNS function; or diagnosis of alcohol or drug abuse or dependence within the last 12 months, alcohol or drug use within the last 24 hours, or use of medications that would affect the electroencephalogram (EEG), neurological or cognitive function, other than those medications that were prescribed to treat schizophrenia. Additionally, healthy controls were excluded if they reported during a standardized interview that they had a first- or second-degree relative diagnosed with a psychotic or affective disorder.
Diagnosis of schizophrenia was established using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2002), review of medical records, and information provided by mental health professionals providing treatment for the participants. Severity of positive, negative and general psychiatric symptoms were assessed using the Schedule for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984) and Negative Symptoms (SANS) (Andreasen, 1984), Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, 1962), and Calgary Depression Rating Scale (Addington, Addington, & Schissel, 1990). Vocabulary and Block Design subtests from the Wechsler Adult Intelligence Scale 3rd edition (WAIS-III) (Wechsler, 1997) were used to estimate current IQ (Ringe, Saine, Lacritz, Hynan, & Cullum, 2002). After complete description of the study to participants, written informed consent was obtained. An Institutional Review Board at the University of Nevada, Las Vegas approved the study’s protocol, which is consistent with the Declaration of Helsinki.
Stimuli
Auditory stimuli were generated in Matlab (The MathWorks, Inc., Natick, MA) and consisted of pure tones (50 ms in duration, including 10 ms rise/fall time) presented binaurally through ER3A headphones (Etymotic Research, Inc., Elk Grove Village, IL) at 70 dB SPL. Trials consisted of a 6.72 s context sequence, a 1.44 s silent interval, and a 6.72 s test sequence (Figure 1a). The intertrial interval was 3 s. Context and test sequences both consisted of 14 repeating ABA-patterns where A represents a low tone, B a high tone, and - a silence. The stimulus onset asynchrony between A and B tones within an ABA- cycle was 120 ms. The Δf between the low and high tones during the test sequence was always 6 semitones where A=300 Hz and B=424 Hz. During the context sequence, four of the following conditions could occur: (1) A=300 Hz, B=357 Hz (Δf=3 semitones), (2) A=300 Hz, B=424 Hz (Δf=6 semitones), (3) A=300 Hz, B=600 Hz (Δf=12 semitones), or (4) silence. Each context sequence was paired with the test sequence to make a total of 4 trial types. Each trial type occurred 40 times across 5 blocks. Between blocks, participants could break for as long as they liked. Similar paradigms have been successfully used to study auditory stream segregation in healthy controls (Snyder, Holder, et al., 2009).
Procedure
EEG signals were digitized continuously (512-Hz sampling rate, 104-Hz bandwidth) using a Biosemi ActiveTwo system (http://www.biosemi.com). EEG data were recorded on an array of 72 Ag-AgCl electrodes (512-Hz A/D rate), with a Common Mode Sense (CMS) active electrode and a Driven Right Leg (DRL) passive electrode serving as grounds (see http://www.biosemi.com/faq/cms&drl.htm), placed at 64 points based on a 10/20 system in a Biosemi electrode cap and 8 points below the hair line. Before EEG recording, conducting gel was applied to each electrode site with the cap on, and sintered Ag-AgCl pin-type electrodes were fit into place at each site in the cap. For the 8 points below the hair line, Ag-AgCl flat-type electrodes were attached using adhesive stickers. No abrading of the skin was performed. Voltage offsets were below 40 mV prior to recoding, and the resting EEG was checked for any problematic electrodes prior to and throughout recording.
During the experiment, participants were seated comfortably in a single-walled sound-attenuated room (Industrial Acoustic Corp, Bronx, NY) and were asked to maintain fixation on a white cross, centered on a black background on a computer screen, throughout the experiment. Participants were asked to listen to the stimuli during EEG recording and to avoid moving their eyes, head, or any other body parts while the stimuli were being presented. At the end of each context and test sequence, participants indicated by pressing one of two buttons whether they heard a galloping rhythm (one stream) for the entire sequence or two even metronomes (two streams) at any point during the sequence (Figure 1b). Participants were told to let their perception occur naturally and to not bias their perception one way or the other. Prior to beginning the main experimental trials, participants completed a practice block that was typically 8 trials long but lasted until they could sufficiently distinguish between the two percepts. Similar procedures have been successfully used to study the ERP correlates of the effect of Δf in auditory stream segregation in healthy controls (Snyder, Holder, et al., 2009).
Behavioral Data Analysis
To examine the effect of context Δf on perception during the context and test and whether this effect differed between groups, we calculated the proportion of trials that each participant reported hearing two streams during the context and test sequences separately for each trial type. The proportions of hearing two streams during the context were entered into a mixed-design ANOVA with context Δf (3, 6, 12 semitones) as a within-subjects factor and group (schizophrenia, control) as a between-subjects factor. A separate mixed-design ANOVA tested the effect of context Δf on perception during the test, with prior Δf (3, 6, 12 semitones) as a within-subjects factor and group as a between-subjects factor. A separate independent-samples t-test tested whether perception during test sequences following silent-context trials differed between groups.
To examine the effect of prior perception on later perception and whether this effect differed between groups, we examined only the stimulus condition in which the context and test had a Δf of 6 semitones. We calculated the proportion of trials in which participants reported hearing the same perception during the context and test (i.e., perceptual stabilization), separately for trials which context perception was reported as one stream or two streams and separately for the schizophrenia group and control group. These proportions were entered into one-sample t-tests to evaluate whether perceptual stabilization had occurred, defined by being significantly higher than 0.5 (i.e., more than 50% of the time). To examine whether the effect of prior perception was different between groups, the proportions were also entered into a mixed-model ANOVA with prior perception (one stream, two streams) as a within-subjects factor and group as a between-subjects factor. Given that the schizophrenia group heard two streams less frequently overall, portion of streaming during the test sequences following silent-context trials was added as a covariate. For all ANOVAs, the degrees of freedom were adjusted using the Greenhouse-Geisser ε and all reported probability estimates were based on the reduced degrees of freedom.
EEG Data Analysis
ERPs during the context were measured by averaging EEG epochs for each stimulus condition and electrode site separately, and rereferenced to the average of all electrodes not adjacent to the eyes. Epochs contaminated by artifacts (amplitude > 120 μV, gradient > 75 μV, low signal < .10 μV) were automatically rejected prior to averaging. Ocular artifacts (blinks, saccades, smooth movements) were corrected automatically with a Principal Component Analysis based method (Ille, Berg, & Scherg, 2002). Epochs were digitally bandpass filtered to attenuate frequencies below 1Hz (6 dB/octave attenuation, forward) and above 30Hz (24 dB/octave attenuation, zero phase). The number of epochs retained for analysis (on average 325 and 392 per condition for the schizophrenia and control groups, respectively) did not significantly differ between groups, F(1,42)=3.06, p>.05. ERPs during the test are not reported because there was no stimulus manipulation during the test and the effects of the context were unreliable.
To examine the effect of Δf and whether this differed between groups, ERPs were baseline corrected by subtracting the mean of the portion 30 ms prior to the B-tone from each point in the epoch. Previous ERP research in healthy participants has shown that Δf-modulations in P1-N1-P2 amplitude are time locked to the B-tone of an ABA-pattern (Snyder & Alain, 2007a; Snyder et al., 2006; Snyder, Holder, et al., 2009). Furthermore, this period is short enough that it does not overlap with the preceding A-tone. We then calculated grand-averaged ERP difference waves between conditions of interest during the context sequences. These difference waves were only used to choose latency windows in the original waveforms for analysis that showed maximal difference between groups. Indeed, as shown below, the latency windows chosen were sufficient to reveal both Δf-related ERP modulations and robust group differences. The first ABA-pattern of each sequence was not analyzed because of the large onset response. The last ABA-pattern of each sequence was also not analyzed because the ERP data may have been contaminated by muscle-related or movement-related activity as participants were getting ready to make a response. All analyses included the following electrodes: FC3, C3, FC4, and C4 because these electrodes are typically used to measure auditory ERPs arising from the superior temporal plane of STG and also because they allowed us to compare the effects of hemisphere, which were apparent upon visual examination of the ERPs. It is unlikely that Δf-related ERP modulations measured at these sites reflect motor preparation activity given that behavioral responses were required for all non-silent sequences. Thus, motor preparation activity should be similar across conditions. Additionally, behavioral responses were not made until the end of a sequence following ERP measurements. Therefore, differences in Δf-related ERP modulations across conditions are unlikely to reflect differences in motor preparation. Mean amplitudes of the original ERP waves were averaged across electrode sites in the same hemisphere for each participant and submitted to a mixed-design ANOVA with group as a between-subjects factor and hemisphere (left, right) and Δf (3, 6, 12 semitones) as within-subjects factors. For all ANOVAs, the degrees of freedom were adjusted using the Greenhouse-Geisser ε and all reported probability estimates were based on the reduced degrees of freedom.
Correlation Analysis
To determine whether there was a relationship between behavioral responses and ERP mean amplitudes, we first calculated the correlation between behavioral reports of streaming and ERP mean amplitudes for each individual participant. The mean behavioral responses at each Δf were correlated with the corresponding ERP mean amplitudes for each Δf, separately for each participant. This enabled us to evaluate whether on average the correlations across subjects were different than 0. Correlations were then entered into a one-sample t-test evaluating the hypothesis that the correlations were on average different from zero. This analysis was conducted for both groups together and separately and for both hemispheres together and separately. For negative-voltage ERPs, we expected to find a negative correlation such that as response mean amplitudes decreased (“increase in negativity”) proportion of streaming would increase. Likewise, for positive ERPs, we expected to find a positive correlation.
Next, we determined whether any of our group differences measured during test (prior Δf and prior perception) could be accounted for by impaired processing of Δf during context. To do this, we examined whether the effect of Δf correlated with the effect of prior Δf or prior perception in separate correlation analyses. For each participant, the effect of Δf was calculated as the slope of streaming measured during context as a function of Δf. The effect of prior Δf was calculated as the slope of streaming measured during test as a function of context Δf. The effect of prior perception was calculated as the difference between the proportion of stabilized trials and trials in which perception changed, for both context perceptions (one stream, two streams) separately. The differences for both context perceptions were then collapsed to give one measure of prior perception.
To determine whether there was a relationship between behavioral or ERP responses and symptom ratings on the BPRS, SAPS, or SANS for the schizophrenia group we first calculated slopes of ERP mean amplitudes as a function of Δf in addition to the behavioral slope. Slopes and symptom ratings were then entered into a correlation analysis. To avoid inflating the rate of Type I errors, only symptoms of particular interest were considered that included both global and individual item scores. Symptoms measures from the BPRS included the following: hallucinations and total score. Symptom measures from the SAPS included the following: auditory hallucinations, voices commenting, voices conversing, tactile hallucinations, olfactory hallucinations, visual hallucinations, global rating of hallucinations, global rating of delusions, and global rating of formal thought disorder. Symptom measures from the SANS included the following: global rating of affective flattening, global rating of avolition, global rating of anhedonia-associality, and global rating of attention. For brevity, only significant symptom rating correlations are reported. Finally, to determine whether any group differences in our behavioral or ERP measures could be explained by years of education or IQ, we calculated correlations between behavioral or ERP slopes and years of education or current IQ.
Results
Demographic Analyses
Given the differences between the schizophrenia and healthy control groups on years of education and current IQ, correlations were calculated between these variables and the behavioral and ERP measures. Neither years of education nor current IQ were significantly correlated with any of our behavioral or ERP measures, even without correcting for multiple comparisons, suggesting that behavioral nor ERP group differences were not accounted for by the observed differences in education or IQ (see Table 2).
Table 2. Correlation matrix for schizophrenia and control groups separately displaying Pearson Correlation (r) and corresponding p-value (p) for correlations between Education or IQ and the effect of current Δf on behavioral responses (Δf), N1 ERP amplitudes (N1), P2 ERP amplitudes (P2), the effect of prior Δf on behavioral responses (Prior Δf), or the effect of prior perception on behavioral responses (Prior Perception).
| Schizophrenia | |||||
|---|---|---|---|---|---|
| Δf/ | N1 | P2 | Prior Δf | Prior Perception |
|
| Education [r, (p)] | .201 (.410) | .269 (.238) | −.347 (.124) | −.326 (.173) | −.296 (.219) |
| IQ [r, (p)] | −.174 (.475) | .082 (.722) | −.120 (.604) | .022 (.929) | .042 (.864) |
| Control | |||||
|---|---|---|---|---|---|
|
| |||||
| Δ f | N1 | P2 | Prior Δf | Prior Perception |
|
| Education [r, (p)] | .031 (.894) | −.150 (.515) | −.329 (.145) | −.065 (.780) | .224 (.330) |
| IQ [r, (p)] | .086 (.711) | −.098 (.673) | −.165 (.474) | −.105 (.651) | .203 (.379) |
Effects of Current Δf
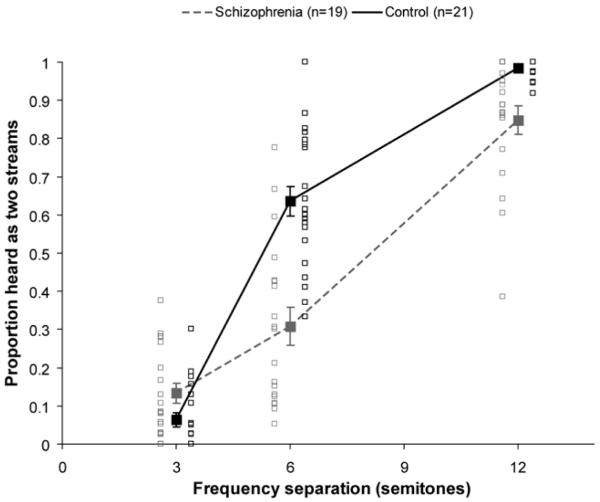
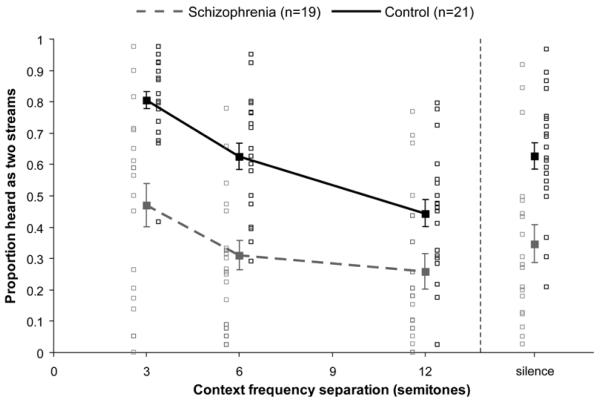
As shown in Figure 2, as Δf increased there was an increase in the likelihood of hearing two streams for both groups, F(2,76)=359.43, p<.001, ε=.92, η2=.904; however, the schizophrenia group was overall less likely to perceive two streams compared to controls, F(1,38)=24.44, p<.001, η2=.391. Given that the schizophrenia group reported hearing two streams more often with increasing Δf suggests they understood the task and performed appropriately, and were not for example simply reporting the presence of two different frequencies within the same stream, which would likely have resulted in very high proportions of reporting two sounds even for the 3-semitone condition (cf. Javitt et al., 1997).
Figure 2.
Behavioral effects of current Δf. Effects of Δf on proportion of streaming. For both groups, there was a significant effect of Δf (p<.001); however, the effect of Δf was smaller in the schizophrenia group than the control group (p<.001). Grey boxes represent individual schizophrenia participants. Black boxes represent individual control participants. Note that boxes have been spaced apart on the x-axis for visual comparison purposes only. Error bars represent ±1 SE.
Analyses also showed that the groups were significantly different for all Δf levels. For the 3-semitone condition the schizophrenia group was slightly more likely to perceive two streams overall compared to controls, t(38)=2.17, p<.05. This could reflect a small degree of response bias to report hearing two streams. However, for the 6- and 12-semitone conditions the schizophrenia group was less likely to perceive two streams overall compared to controls [Δf=6: t(38)=5.36, p<.001; Δf=12: t(38)=3.84, p<.002]. Importantly, there was a group x Δf interaction, F(2,76)=19.88, p<.001, ε=.92, η2=.343, such that the effect of Δf was smaller in the schizophrenia group compared to controls. Although there were quadratic trends in the data of both groups, the interaction between the linear components was significant, F(1,38)=13.24, p<.001, η2=.258.
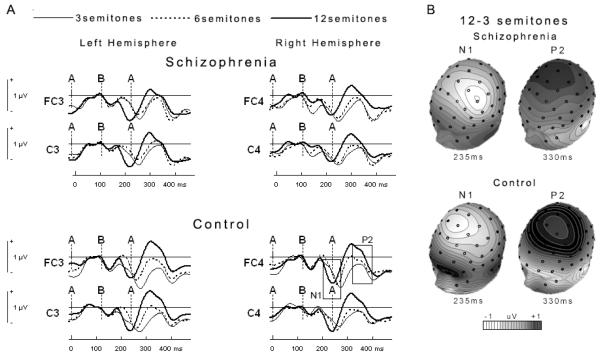
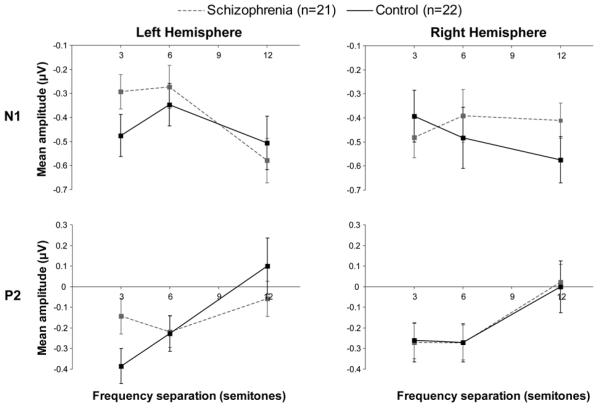
For both groups, as Δf increased there was a marginally increased negativity in the ERP response to the ABA- pattern, likely to be an N1 component 80-150 ms after the B tone, F(2,82)=3.01, p=.06, ε=.915, η2=.068. There was also an increased positivity, likely to be a P2 component 200-280 ms after the B tone, F(2,82)=11.46, p<.001, ε=.919, η2=.218 (Figure 3 and Figure 4). For both components, we found no main effect of group, [N1: F(1,41)=.32, p>.50, η2=.008; P2: F(1,41)=.03, p>.80, η2=.001] nor did we find a Δf x group interaction [N1: F(2,82)=.06, p>.90, ε=.915, η2=.001; P2: F(2,82)=1.01, p>.30, ε=.919, η2=.024]. However, for both N1 and P2 mean amplitudes there was a significant hemisphere x Δf x group interaction [N1: F(2,82)=4.57, p<.02, ε=.859, η2=.10; P2: F(2,82)=3.66, p<.05, ε=.803, η2=.082] such that the effect of Δf on ERP mean amplitude was smaller in the schizophrenia group in the right hemisphere for the N1 and smaller in the left hemisphere for the P2 (Figure 4). For both components, there was no main effect of hemisphere, [N1: F(1,41)=1.40, p>.20, η2=.033; P2: F(1,41)=.42, p>.50, η2=.01] nor was there a significant hemisphere x Δf interaction [N1: F(2,82)=2.25, p>.10, ε=.859, η2=.052; P2: F(2,82)=.19, p>.70, ε=.803, η2=.005].
Figure 3.
Event-related potential (ERP) results. Effect of Δf on ERPs time-locked to the B tone. For both groups, there was a marginally increased negativity (p=.06), likely to be an N1 component 80-150 ms after the B tone and a significantly increased positivity (p<.001), likely to be a P2 component 200-280 ms after the B tone. N1 and P2 components have been referenced in the bottom right panel. Boxed frames highlight the latency windows chosen for statistical analysis. These latency windows were chosen as they showed the largest difference between conditions and groups. Vertical lines with labels indicate tone onsets in the ABA pattern.
Figure 4.
(A) Event-related potential (ERP) results. Graph representation of the data presented in Figure 3 for the N1 and P2 ERPs separated by hemisphere. The effect of Δf was smaller in the schizophrenia group in the right hemisphere for the N1 (p<.02) and smaller in the left hemisphere for P2 (p<.05). Data for the N1 are mean amplitudes 80-150 ms after the B tone. Data for the P2 are mean amplitudes 200-280 ms after the B tone. Note the different mean amplitude scales for each component. Left hemisphere includes FC3 and C3 electrodes. Right hemisphere includes FC4 and C4 electrodes. Error bars represent ±1 SE. (B) Topographies of difference waves for ERPs elicited when Δf is 12-3 semitones. Isopotential contours reflect 0.05 μV/step.
The following correlations assess the relationship between cortical Δf encoding and perception of streaming (see Figure 5). When combining all participants from both groups, the correlations between proportion of hearing two streams and size of P2 mean amplitude were significantly greater than zero (mean r=0.39, t(39)=3.69, p<.001), indicating a moderate relation between low-level ERPs and perception of streaming. For the schizophrenia group separately there was a non-significant trend for the P2 correlations to be greater than zero, r=0.25, t(18)=1.87, p=.078. For the control group separately, the P2 correlations were significantly greater than zero, r=0.51, t(20)=3.25, p<.005. Results were similar when hemispheres were examined separately. For N1, no mean correlations were significantly different from zero (p>.05) for both groups together or separately and for both hemispheres together or separately, although the trends were in the expected direction.
Figure 5.
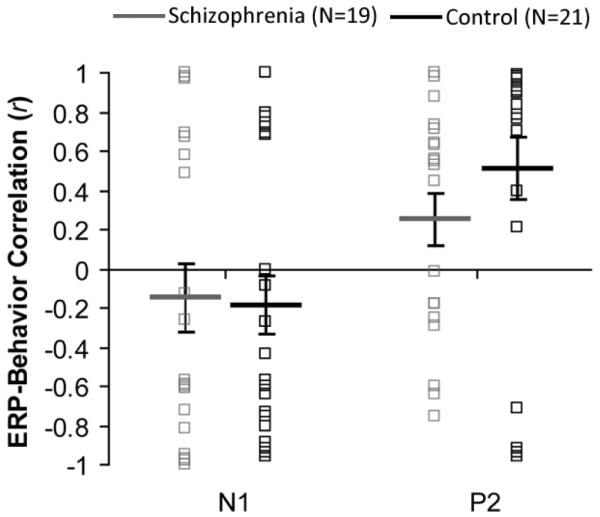
Brain-behavior correlations. Average correlations between participants’ ERP mean amplitude modulations due to increased Δf and behavioral modulations of reporting two streams due to increased Δf, for the N1 and P2, respectively. Solid lines and boxes represent group averages and individual participants, respectively. Error bars represent ±1 SE.
Effects of Prior Δf
As shown in Figure 6, as prior Δf increased there was a decrease in the likelihood of hearing two streams during a later test sequence for both groups, F(2,76)=42.01, p<.001, ε=.778, η2=.525, as demonstrated in previous studies (Snyder, Carter, et al., 2009; Snyder, et al., 2008; Snyder, Holder, et al., 2009; Snyder & Weintraub, 2011). Consistent with the data from the context pattern presented above, the schizophrenia group was less likely to perceive two streams overall compared to controls, F(1,38)=23.27, p<.001, η2=.38. Most importantly, there was a group x prior Δf interaction, F(2,76)=3.81, p<.05, ε=.778, η2=.091, such that the effect of prior Δf was smaller in the schizophrenia group compared to controls. Although there were quadratic trends for Δf in both groups, the interaction involving the linear component was significant, F(1,38)=4.28, p<.05, η2=.101. The effect of prior Δf was not significantly correlated with the behavioral effect of current Δf measured during the context, suggesting that the abnormal prior Δf effect cannot be simply attributed to impaired processing of Δf during the context sequences. Finally, the schizophrenia group reported less streaming compared to controls for test sequences following silent-context trials, t(38)=3.73, p<.001, consistent with the overall main effects of group reported above.
Figure 6.
Behavioral effects of prior Δf. For both groups, there was a significant effect of prior Δf (p<.001), with less perception of two streams when the prior Δf was larger; however, the effect of prior Δf was smaller in the schizophrenia group compared to controls (p<.05). Additionally, the schizophrenia group reported less streaming compared to controls for test sequences following silent-context trials (p<.001). Gray boxes represent individual schizophrenia participants. Black boxes represent individual control participants. Note that boxes have been spaced apart on the x-axis for visual comparison purposes only. Error bars represent ±1 SE.
Effects of Prior Perception
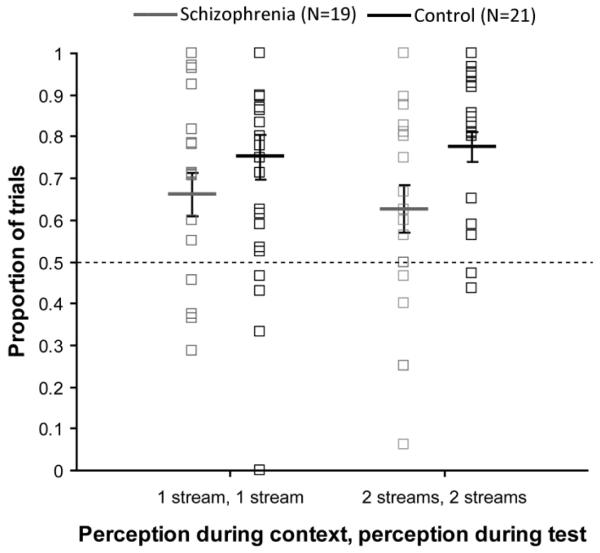
As shown in Figure 7, both groups showed a significant likelihood of hearing one stream during the test sequence when the previous context sequence was also heard as one stream [schizophrenia: t(18)=4.26, p<.001; control: t(20)=3.47, p<.003]. A similar perceptual stabilization occurred when the prior perception was two streams, however, this was only marginally so for the schizophrenia group [schizophrenia: t(18)=1.70, p=.10; control: t(20)=8.39, p<.001]. Importantly, there was a significant main effect of group, F(1,37)=4.80, p<.05, η2=.115, such that the effect of prior perception was smaller in the schizophrenia group compared to controls. There was no significant prior perception x group interaction, F(1,37)=.25, p>.60, η2=.007, suggesting that the group difference reported above was the same whether the prior percept was one or two streams. This effect is solely attributable to the prior perception and not aspects of the prior stimulus because this effect was only examined for trials in which both the context and test had Δf values of 6 semitones. Additionally, the effect of prior perception was not significantly correlated with the behavioral effect of current Δf measured during the context, suggesting that the abnormal prior perception effect cannot be attributed to impaired processing of Δf during the context sequences
Figure 7.
Behavioral effects of prior perception. For both groups, there was a significant effect of prior perception (p<.001), with a likelihood greater than chance of perceiving the same organization for consecutive patterns; however, the effect of prior perception was smaller in the schizophrenia group compared to controls (p<.04). Solid lines and boxes represent group averages and individual participants, respectively. Averages reflect adjusted means from the ANCOVA analysis. Dotted horizontal line indicates the 50% proportion. Error bars represent ±1 SE.
Symptom Correlations
For the schizophrenia group, proportion of hearing two streams was negatively correlated with commenting hallucinations, r(17)=−.62, p<.01, tactile hallucinations, r(17)=−.59, p<.01, and global ratings of attention, r(17)=−.63, p<.01, as measured from the SAPS and SANS. There was a marginal correlation between slope of P2 mean amplitudes and global ratings of attention, r(19)=.42, p=.056. One study has shown that attention to a stream alters its neural representation, as reflected by a sharpened tuning curve for neurons responding to the frequency of attended sounds during a stream segregation task (Yin, Ma, Elhilali, Fritz, & Shamma, 2007). However, it is unlikely that the correlations with attention reflect a failure to attend to the sequences given the P2 modulations observed here are not affected by attention (Snyder et al., 2006), and all participants responded to most trials on time (within a 1.44 s window) and in the expected direction (minus the two participants who failed to understand the task). Finally, smaller prior Δf effects on perception were associated with larger avolition ratings, r(17)=.47, p<.05, as measured from the SAPS/SANS.
Discussion
We found that schizophrenia participants were less likely to report hearing two streams with increasing Δf, compared to controls. This was coupled with a reduced effect of Δf in the right-hemisphere N1 and left-hemisphere P2 ERPs time locked to the B (high) tone. Given that N1 and P2 arise from sources in the superior temporal plane, it is likely that the observed group differences are due at least in part to sensory-level impairments in auditory cortical areas in schizophrenia participants that are critical for organization of sound based on frequency. Furthermore, the fact that we found no main effect of group differences in our ERP measures and instead found impairments that were lateralized to either hemisphere suggests that our results are not due to other factors within our schizophrenia group such as medication effects, inattention, fatigue, etc. Instead, our results likely do reflect a true sensory processing impairment. Such impairment has clear implications for how auditory cortex abnormalities negatively affect normal functioning in day-to-day life, which we discuss in more detail below.
This is the first study to provide evidence that auditory stream segregation is impaired in schizophrenia, in contrast with a previous study (Bourdet et al., 2003). It is possible that we obtained different results because of differences in stimuli and tasks. For example, their study used very large Δf levels, which may have been unsuitable for revealing robust group differences. Indeed, the largest group difference reported in our study was at the intermediate Δf. Also, they indirectly measured streaming using a temporal-irregularity detection task, whereas our study measured streaming more directly by asking participants to report their perceptual experience. However, this latter difference is less likely to be responsible for the different findings because indirect and direct measures of streaming typically lead to similar findings (Micheyl & Oxenham, 2010a).
The present results can be interpreted in terms of a place model of stream segregation (Hartmann & Johnson, 1991), which posits that alternating tone sequences with a small Δf will activate overlapping frequency-selective neuronal populations and consequently be perceived as one auditory object; likewise, sequences with a large Δf will activate non-overlapping frequency-selective neuronal populations and consequently be perceived as two auditory objects. As a consequence of this spatial distinctiveness in the brain, neural populations will interact less such that suppression (e.g., adaptation, forward suppression, lateral inhibition) of the ERP response to the B (high) tone caused by the preceding A (low) tone is reduced. The enhanced ERP mean amplitudes with increasing Δf in the present study is therefore likely due to distinct neural populations being activated by the A and B tones, respectively, leading to more summed activity at the scalp. Thus, the schizophrenia group’s reduced N1 and P2 mean amplitude with increasing Δf likely reflects more overlap in frequency-selective neurons compared to controls. This is consistent with studies of schizophrenia participants showing abnormal tonotopic organization and impaired processing of multiple frequencies in MEG responses (Rojas et al., 2002; Rojas et al., 2007). The significant correlation between behavioral performance and P2 mean amplitude reported here is consistent with our previous work (Snyder & Alain, 2007a; Snyder et al., 2006), demonstrating a link between auditory cortical ERPs and perception of stream segregation. The results further suggest that the reduced effect of Δf on perception of streaming in schizophrenia is related to abnormalities in place coding in auditory cortex rather than higher-level factors. This interpretation also explains why the perceptual group differences were most evident for the 6-semitone condition and did not increase linearly. That is, the 3- and 12-semitone conditions used here might be unlikely to show large group differences because in both groups they might be expected to show similar amounts of overlap or separation, respectively. The 6-semitone condition, in contrast, might be more likely to show large group differences because of an intermediate amount of overlap at a neural level. Nevertheless, the possibility that the reduced effect of Δf on perception and ERPs in the schizophrenia group actually reflect more cognitive-level factors, such as an attentional impairment, cannot be definitively ruled out without further studies.
It is noteworthy that in the present study, N1 abnormalities in schizophrenia were right-hemisphere lateralized and P2 abnormalities were left-hemisphere lateralized. These results were probably not due to differences in handedness between the schizophrenia and control groups, given that the groups had similar proportions of left- and right-handed individuals. The results are also unlikely to be due to medication because the majority of schizophrenia participants were taking antipsychotic medicines, which do not seem to affect auditory ERPs (Umbricht et al., 1998, 1999). However, given the poor spatial resolution of ERPs and the inherent ambiguities in performing source analysis due to the inverse problem (Luck, 2005), these findings of lateralized ERP abnormalities require replication with methods that are better at revealing hemispheric differences in auditory cortex such as MEG. Nevertheless, the left-lateralized P2 abnormality is consistent with MRI studies showing left-lateralized STG gray matter volume reductions (McCarley et al., 1999; Shenton et al., 2001). Additionally, similar left-lateralized deficits have been shown in neurophysiological studies of auditory components M50 (an MEG form of P50) (Thoma et al., 2003), N200 (O’Donnell et al., 1993), and P300 (McCarley et al., 2002; McCarley et al., 1993). Previous right-lateralized deficits, similar to the N1 reduction reported here, have also been found in schizophrenia participants, in the form of reduced auditory steady-state response amplitudes to 40 Hz amplitude modulated noise (Hamm, Gilmore, Picchetti, Sponheim, & Clementz, 2011; Mulert, Kirsch, Pascual-Marqui, McCarley, & Spencer, 2011). Finally, a recent study showed N1 abnormalities were larger in anterior electrode sites compared to posterior sites. In contrast, P2 abnormalities in first-hospitalized schizophrenia participants were larger in posterior electrode sites than anterior sites (Salisbury, Collins, & McCarley, 2010). These findings provide additional evidence for topographical differences in N1 and P2 abnormalities in schizophrenia. Thus, our results of lateralized N1 and P2 impairments are consistent with previous studies showing that there may be distinct right- and left-lateralized deficits in the auditory cortices of schizophrenia participants.
In addition to low-level impairments in processing Δf in schizophrenia participants, we also observed reduced influences of immediate prior contexts compared to controls. These results are of interest because effects of context are necessary for understanding perception in naturalistic situations, such as when trying to interpret an utterance in the context of previous utterances by the same speaker. These reduced effects of prior Δf and prior perception in schizophrenia were unlikely to arise simply from lower-level impairments because the reductions did not correlate with reductions in effects of current Δf. This is consistent with other research suggesting that cognitive impairments during an auditory perception task do not arise from low-level impairments (van der Stelt, Frye, Lieberman, & Belger, 2004; but see Leitman et al., 2010). Instead, these impairments likely reflect abnormalities within relatively high-level auditory areas that are not finely tuned to frequency (Snyder, Carter, et al., 2009) but are sensitive to rhythmic pattern (Snyder & Weintraub, 2011). Our findings are also consistent with previous research using visual, auditory, and language processing paradigms, which also showed that schizophrenia participants show a reduced tendency to be influenced by prior context (visual studies: Silverstein, Bakshi, Chapman, & Nowlis, 1998; Silverstein, Knight, et al., 1996; Uhlhaas, Phillips, Mitchell, & Silverstein, 2006; Uhlhaas & Silverstein, 2005; auditory studies: Ford, 1999; Ford et al., 2010; Ford, White, Lim, & Pfefferbaum, 1994; Niwa et al., 1992; van der Stelt et al., 2004; language studies: Condray, Steinhauer, Cohen, van Kammen, & Kasparek, 1999; Ditman & Kuperberg, 2007; Kuperberg, Kreher, Goff, McGuire, & David, 2006; Kuperberg, McGuire, & David, 1998; Mitchell et al., 1991; Strandburg et al., 1997). And our results also extend one previous study showing a reduced tendency for prior contextual information to influence auditory perceptual organization (Silverstein, Matteson, et al., 1996). Thus, our findings strongly suggest that both low-level and high-level aspects of stream segregation are impaired in schizophrenia participants and therefore raise the important question of how these impairments affect functioning in real-world situations such as a noisy social setting. Our results further suggest that processing of contextual information is a general difficulty found in schizophrenia that is likely to impair their ability to make sense of complex real-world acoustic situations.
Future studies should continue to examine the extent to which sound segregation is impaired in schizophrenia. For example, studies have shown useful cues for stream segregation besides pure-tone frequency, such as fundamental frequency of complex tones (Vliegen & Oxenham, 1999), amplitude modulation (Grimault, Bacon, & Micheyl, 2002), and interaural time difference (Hartmann & Johnson, 1991). Given that individuals with schizophrenia have difficulty processing auditory cues other than frequency, including intensity (Bach, Buxtorf, Strik, Neuhoff, & Seifritz, 2011) and interaural time difference (Matthews, Todd, Budd, Cooper, & Michie, 2007), they might show a generalized impairment in stream segregation. Another important question is whether sound segregation impairments generalize to sounds played concurrently (Micheyl & Oxenham, 2010b). Finally, future studies should use tasks that more closely match real-world environments such as using tests that assess the ability to recognize spoken sentences in the presence of background noise (Bilger, Nuetzel, Rabinowitz, & Rzeczkowski, 1984). Abnormalities on speech in noise tests would suggest that sound segregation impairments in schizophrenia generalize to more natural settings.
Acknowledgments
This work was supported by a President’s Research Award from the University of Nevada, Las Vegas and a National Institutes of Health grant [R21MH079987].
We would like to thank Jason Schwartz at Mojave Mental Health in Las Vegas, Nevada for his help in patient recruitment. These data were previously presented at the Society of Biological Psychiatry 65th Annual Meeting (2010), New Orleans, Louisiana.
Footnotes
David Weintraub, Erin M. Ramage, Griffin Sutton, Erik Ringdahl, Aaron Boren, Amanda Pasinski, Nick Thaler, Michael Haderlie, Daniel Allen, & Joel Snyder, Department of Psychology, University of Nevada Las Vegas.
References
- Addington D, Addington J, Schissel B. A depression rating-scale for schizophrenics. Schizophrenia Research. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale of the assessments of negative symptoms (SANS)/Scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City (IA): 1984. [Google Scholar]
- Bach DR, Buxtorf K, Strik WK, Neuhoff JG, Seifritz E. Evidence for impaired sound intensity processing in schizophrenia. Schizophrenia Bulletin. 2011;37:426–431. doi: 10.1093/schbul/sbp092. doi: 10.1093/schbul/sbp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger RC, Nuetzel JM, Rabinowitz WM, Rzeczkowski C. Standardization of a test of speech-perception in noise. Journal of Speech and Hearing Research. 1984;27:32–48. doi: 10.1044/jshr.2701.32. [DOI] [PubMed] [Google Scholar]
- Bourdet C, Brochard R, Rouillon F, Drake C. Auditory temporal processing in schizophrenia: High level rather than low level deficits? Cognitive Neuropsychiatry. 2003;8:89–106. doi: 10.1080/13546800244000238. doi: 10.1080/13546800244000238. [DOI] [PubMed] [Google Scholar]
- Cienfuegos A, March L, Shelley AM, Javitt DC. Impaired categorical perception of synthetic speech sounds in schizophrenia. Biological Psychiatry. 1999;45:82–88. doi: 10.1016/s0006-3223(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, Cohen JD, van Kammen DP, Kasparek A. Modulation of language processing in schizophrenia: Effects of context and haloperidol on the event-related potential. Biological Psychiatry. 1999;45:1336–1355. doi: 10.1016/s0006-3223(98)00317-5. [DOI] [PubMed] [Google Scholar]
- Ditman T, Kuperberg GR. The time course of building discourse coherence in schizophrenia: An ERP investigation. Psychophysiology. 2007;44:991–1001. doi: 10.1111/j.1469-8986.2007.00565.x. doi: 10.1111/j.1469-8986.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disoders: Research version, patient edition. (SCID-I/P) Biometric Research, New York State Psychiatric Institute; New York (NY): 2002. [Google Scholar]
- Ford JM. Schizophrenia: The broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it’s time for a change: Failures to track context in schizophrenia. International Journal of Psychophysiology. 2010;78:3–13. doi: 10.1016/j.ijpsycho.2010.05.005. doi: 10.1016/j.ijpsycho.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: A single-trial analysis. Biological Psychiatry. 1994;35:96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Grimault N, Bacon SP, Micheyl C. Auditory stream segregation on the basis of amplitude-modulation rate. Journal of the Acoustical Society of America. 2002;111:1340–1348. doi: 10.1121/1.1452740. doi: 10.1121/1.1452740. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Micheyl C, Melcher JR, Rupp A, Scherg M, Oxenham AJ. Neuromagnetic correlates of streaming in human auditory cortex. Journal of Neuroscience. 2005;25:5382–5388. doi: 10.1523/JNEUROSCI.0347-05.2005. doi: 10.1523/Jneurosci.0374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NAM, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biological Psychiatry. 2011;69:989–996. doi: 10.1016/j.biopsych.2010.11.021. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann WM, Johnson D. Stream segregation and peripheral channeling. Music Perception. 1991;9:155–184. [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clinical Neurophysiology. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. Journal of Abnormal Psychology. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Kreher DA, Goff D, McGuire PK, David AS. Building up linguistic context in schizophrenia: Evidence from self-paced reading. Neuropsychology. 2006;20:442–452. doi: 10.1037/0894-4105.20.4.442. doi: 10.1037/0894-4105.20.4.442. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, David AS. Reduced sensitivity to linguistic context in schizophrenic thought disorder: Evidence from on-line monitoring for words in linguistically anomalous sentences. Journal of Abnormal Psychology. 1998;107:423–434. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biological Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. American Journal of Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. MIT; Cambridge (MA): 2005. [Google Scholar]
- March L, Cienfuegos A, Goldbloom L, Ritter W, Cowan N, Javitt DC. Normal time course of auditory recognition in schizophrenia, despite impaired precision of the auditory sensory (“echoic”) memory code. Journal of Abnormal Psychology. 1999;108:69–75. doi: 10.1037//0021-843x.108.1.69. [DOI] [PubMed] [Google Scholar]
- Matthews N, Todd J, Budd TW, Cooper G, Michie PT. Auditory lateralization in schizophrenia: Mismatch negativity and behavioral evidence of a selective impairment in encoding interaural time cues. Clinical Neurophysiology. 2007;118:833–844. doi: 10.1016/j.clinph.2006.11.017. doi: 10.1016/j.clinph.2006.11.017. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: Their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophrenia Research. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Archives of General Psychiatry. 2002;59:321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Archives of General Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biological Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Oxenham AJ. Objective and subjective psychophysical measures of auditory stream integration and segregation. Journal of the Association for Research in Otolaryngology. 2010a;11:709–724. doi: 10.1007/s10162-010-0227-2. doi: 10.1007/s10162-010-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Oxenham AJ. Pitch, harmonicity and concurrent sound segregation: Psychoacoustical and neurophysiological findings. Hearing Research. 2010b;266:36–51. doi: 10.1016/j.heares.2009.09.012. doi: 10.1016/j.heares.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PF, Andrews S, Fox AM, Catts SV, Ward PB, McConaghy N. Active and passive attention in schizophrenia: An ERP study of information-processing in a linguistic task. Biological Psychology. 1991;32:101–124. doi: 10.1016/0301-0511(91)90004-z. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Gockel H. Factors influencing sequential stream segregation. Acta Acustica United with Acustica. 2002;88:320–333. [Google Scholar]
- Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. International Journal of Psychophysiology. 2011;79:55–63. doi: 10.1016/j.ijpsycho.2010.08.004. doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa SI, Hiramatsu KI, Saitoh O, Fukuda M, Kameyama T, Itoh K, Hayashida S. Information dysregulation and event-related potentials in schizophrenia. Schizophrenia Bulletin. 1992;18:95–105. doi: 10.1093/schbul/18.1.95. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Shenton ME, McCarley RW, Faux SF, Smith RS, Salisbury DF, Jolesz FA. The auditory N2 component in schizophrenia: Relationship to MRI temporal-lobe gray-matter and to other ERP abnormalities. Biological Psychiatry. 1993;34:26–40. doi: 10.1016/0006-3223(93)90253-a. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating-scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: Imprecision or distractibility? Archives of General Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Ringe WK, Saine KC, Lacritz LH, Hynan LS, Cullum CM. Dyadic short forms of the wechsler adult intelligence scale-III. Assessment. 2002;9:254–260. doi: 10.1177/1073191102009003004. doi: 10.1177/1073191102009003004. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Carlson JP, Arciniegas DB, Teale PD, Reite ML. Alterations in tonotopy and auditory cerebral asymmetry in schizophrenia. Biological Psychiatry. 2002;52:32–39. doi: 10.1016/s0006-3223(01)01365-8. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Slason E, Teale PD, Reite ML. Neuromagnetic evidence of broader auditory cortical tuning in schizophrenia. Schizophrenia Research. 2007;97:206–214. doi: 10.1016/j.schres.2007.08.011. doi: 10.1016/j.schres.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MM, Moore BC. The relationship between stream segregation and frequency discrimination in normally hearing and hearing-impaired subjects. Hear Res. 2005;204(1-2):16–28. doi: 10.1016/j.heares.2004.12.004. doi: 10.1016/j.heares.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophrenia Bulletin. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, Mcconaghy N. Mismatch negativity: An index of a preattentive processing deficit in schizophrenia. Biological Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. doi:10.1016/S0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Bakshi S, Chapman RM, Nowlis G. Perceptual organisation of configural and nonconfigural visual patterns in schizophrenia: Effects of repeated exposure. Cognitive Neuropsychiatry. 1998;3:209–223. [Google Scholar]
- Silverstein SM, Knight RA, Schwarzkopf SB, West LL, Osborn LM, Kamin D. Stimulus configuration and context effects in perceptual organization in schizophrenia. Journal of Abnormal Psychology. 1996;105:410–420. doi: 10.1037//0021-843x.105.3.410. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Matteson S, Knight RA. Reduced top-down influence in auditory perceptual organization in schizophrenia. Journal of Abnormal Psychology. 1996;105:663–667. doi: 10.1037//0021-843x.105.4.663. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Alain C. Sequential auditory scene analysis is preserved in normal aging adults. Cerebral Cortex. 2007a;17:501–512. doi: 10.1093/cercor/bhj175. doi: 10.1093/cercor/bhj175. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Alain C. Toward a neurophysiological theory of auditory stream segregation. Psychological Bulletin. 2007b;133:780–799. doi: 10.1037/0033-2909.133.5.780. doi: 10.1037/0033-2909.133.5.780. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Alain C, Picton TW. Effects of attention on neuroelectric correlates of auditory stream segregation. Journal of Cognitive Neuroscience. 2006;18:1–13. doi: 10.1162/089892906775250021. doi:10.1162/089892906775250021. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Carter OL, Hannon EE, Alain C. Adaptation reveals multiple levels of representation in auditory stream segregation. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1232–1244. doi: 10.1037/a0012741. doi: 10.1037/A0012741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Carter OL, Lee SK, Hannon EE, Alain C. Effects of context on auditory stream segregation. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:1007–1016. doi: 10.1037/0096-1523.34.4.1007. doi: 10.1037/0096-1523.34.4.1007. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Gregg MK, Weintraub DM, Alain C. Attention, awareness, and the perception of auditory scenes. Frontiers in Psychology. 2012;3:15. doi: 10.3389/fpsyg.2012.00015. doi: 10.3389/fpsyg.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Holder WT, Weintraub DM, Carter OL, Alain C. Effects of prior stimulus and prior perception on neural correlates of auditory stream segregation. Psychophysiology. 2009;46:1208–1215. doi: 10.1111/j.1469-8986.2009.00870.x. doi: 10.1111/j.1469-8986.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Weintraub DM. Pattern specificity in the effect of prior Δf on auditory stream segregation. Journal of Experimental Psychology: Human Perception and Performance. 2011;37:1649–1656. doi: 10.1037/a0023098. doi: 10.1037/A0023098. [DOI] [PubMed] [Google Scholar]
- Strandburg RJ, Marsh JT, Brown WS, Asarnow RF, Guthrie D, Harper R, Nuechterlein KH. Event-related potential correlates of linguistic information processing in schizophrenics. Biological Psychiatry. 1997;42:596–608. doi: 10.1016/S0006-3223(96)00410-6. [DOI] [PubMed] [Google Scholar]
- Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (echoic) memory dysfunction in schizophrenia. American Journal of Psychiatry. 1995;152:1517–1519. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang MX, Weisend MP, Canive JM. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM. Perceptual grouping in disorganized schizophrenia. Psychiatry Research. 2006;145:105–117. doi: 10.1016/j.psychres.2005.10.016. doi: 10.1016/j.psychres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Silverstein SM. Perceptual organization in schizophrenia spectrum disorders: Empirical research and theoretical implications. Psychological Bulletin. 2005;131:618–632. doi: 10.1037/0033-2909.131.4.618. doi: 10.1037/0033-2909.131.4.618. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J. Effects of clozapine on auditory event-related potentials in schizophrenia. Biological Psychiatry. 1998;44:716–725. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J. Effects of risperidone on auditory event-related potentials in schizophrenia. International Journal of Neuropsychopharmacology. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Archives of General Psychiatry. 2004;61:237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- Vliegen J, Oxenham AJ. Sequential stream segregation in the absence of spectral cues. Journal of the Acoustical Society of America. 1999;105:339–346. doi: 10.1121/1.424503. doi: 10.1121/1.424503. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale (WISC-III) third edition Psychological Corporation; New York (NY): 1997. [Google Scholar]
- Yin P, Ma L, Elhilali M, Fritz J, Shamma S. Primary auditory cortical responses while attending to different streams. In: Hollmeier B, Klump G, Hohmann V, Langemann U, Mauermann M, Uppenkamp S, Verhey J, editors. Hearing: From sensory processing to perception. Spring-Verlag; Berlin (Germany): 2007. pp. 257–265. [Google Scholar]