Abstract
The Hedgehog (Hh) signaling pathway regulates embryonic development and may be aberrantly activated in a wide variety of human cancers. Efforts to target pathogenic Hh signaling have steadily progressed from the laboratory to the clinic, and the recent approval of the Hh pathway inhibitor vismodegib for patients with advanced basal cell carcinoma (BCC) represents an important milestone. On the other hand, Hh pathway antagonists have failed to demonstrate significant clinical activity in other solid tumors. The reasons for these negative results are not precisely understood, but it is possible that the impact of Hh pathway inhibition has not been adequately measured by the clinical endpoints used thus far or that aberrancies in Hh signal transduction limit the activity of currently available pathway antagonists. Further basic and correlative studies to better understand Hh signaling in human tumors and validate putative anti-tumor mechanisms in the clinical setting may ultimately improve the success of Hh pathway inhibition to other tumor types.
Keywords: Hedgehog, Smoothened antagonists, basal cell carcinoma, clinical trials
Background
The Hedgehog (Hh) signaling pathway is highly conserved from flies to humans and is essential for development of the normal embryo (1, 2). In mammals, Hh signaling regulates both patterning and polarity events during early embryogenesis and the morphogenesis of specific organs and tissues. The pathway is subsequently silenced in most adult tissues but can be reactivated following injury to promote repair and regeneration. Furthermore, aberrant Hh signaling has been detected in many human cancers suggesting a broad role in carcinogenesis.
Hh signal transduction
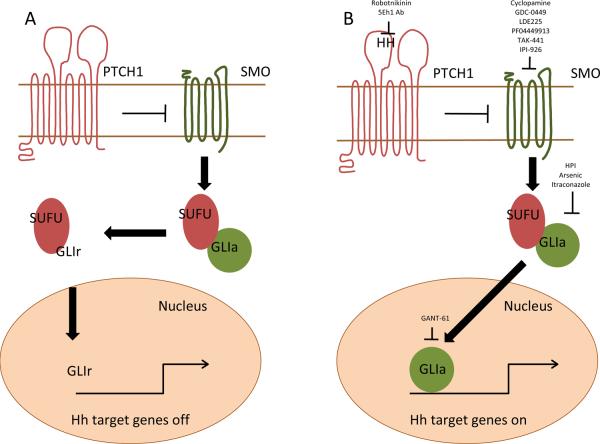
Hh signaling is initiated by binding of one of the three soluble and lipid modified HH ligands (Sonic, Indian, or Desert HH) found in vertebrates to the twelve-pass transmembrane receptor Patched (PTCH1, Figure). In the absence of ligand, PTCH1 represses the seven-pass transmembrane G-protein coupled receptor (GPCR)-like protein Smoothened (SMO). Ligand binding relieves this inhibition and allows SMO to modulate a cytoplasmic complex containing Suppressor of Fused (SUFU) that modifies the three Glioma associated (GLI) transcriptional regulators through phosphorylation, sumoylation, and selective proteolysis (3). GLI1 induces and GLI3 represses Hh target genes that include GLI1, PTCH1, Cyclin D1, c-Myc, and Bcl-2, whereas GLI2 can act in either a positive or negative manner depending on post-transcriptional and post-translational processing events (4). Vertebrate Hh signaling is further regulated by the translocation of signaling components between the cytoplasm, plasma membrane, nucleus, and primary cilium that acts as a sensor to monitor the extracellular environment (5). PTCH1 is initially located in the primary cilium and SMO within cytoplasmic vesicles. Following ligand binding to PTCH1, SMO moves to the primary cilium where it interacts with the GLI processing complex that eventually results in the nuclear translocation of the GLI transcriptional regulators (6).
figure 1.
The Hedgehog signaling pathway. Positive and negative regulatory components are depicted in green and red, respectively. A. In the absence of HH ligand, PTCH1 inhibits SMO allowing the GLI processing complex containing SUFU to generate GLIr transcriptional repressors. B. HH ligand binding to PTCH depresses SMO and generates GLIa nuclear factors that induce the expression of Hh target genes. Clinical and preclinical inhibitors of pathway signaling are listed at their sites of pathway activity.
The Hh pathway and cancer
A role for Hh signaling in cancer was initially provided by studies in Gorlin syndrome, an autosomal dominant disorder characterized by craniofacial and skeletal abnormalities and a markedly increased risk of advanced basal cell carcinoma (BCC) and medulloblastoma (7, 8). The discovery of PTCH1 mutations as the cause of Gorlin syndrome suggested that dysregulated Hh pathway activity was responsible for the development of these cancers (9, 10), and these findings were substantiated by the identification of PTCH1, SMO, and to a lesser extent SUFU mutations in approximately 90% and 15–30% of spontaneously arising BCCs and medulloblastomas, respectively (11, 12). Furthermore, the recapitulation of BCC and medulloblastoma in transgenic mouse models has provided definitive proof that PTCH1 and SMO mutations are a causal factor in these tumor types.
Aberrant Hh pathway activity is also a feature of many other human cancers. However, activating mutations in pathway components are uncommon and over-expression of HH ligands is thought to drive increased pathway activity. In these “ligand-dependent” tumors, several types of Hh signaling have been described. Autocrine and juxtacrine signaling in which tumor cells both secrete and respond to HH ligands has been reported in many cancers including small cell lung, pancreas, colorectal, and metastatic prostate carcinomas as well as melanoma and glioblastoma (13–18). Paracrine signaling in which the cells secreting ligands are distinct from those responding with pathway activation has also been described in lymphoma and multiple myeloma in which HH ligands produced by stromal cells in the local microenvironment induce pathway activity in tumor cells (19). Alternatively, studies in epithelial cancers have found that paracrine Hh signaling is reversed with tumor cells secreting HH ligands that activate signaling within stromal cells to produce secondary factors supporting angiogenesis and tumor cell proliferation and survival (20, 21).
The Hh pathway can also regulate cancer stem cells (CSCs) with enhanced tumor initiating and self-renewal potential. In multiple myeloma, Hh pathway activation induces the expansion of CSCs whereas pathway inhibition results in terminal differentiation, loss of self-renewal, and exhaustion of the malignant clone (22). Studies in chronic myeloid leukemia (CML) and breast cancer have similarly found that Hh pathway inhibition limits tumorigenic potential and self-renewal (23–25). Emerging data suggest that CSCs in solid tumors are involved in metastatic disease progression (26), and the Hh pathway has been found to regulate the epithelial-mesenchymal transition and dissemination of CSCs in pancreatic and colorectal carcinoma (15, 27). Therefore, the Hh signaling pathway may specify CSC fate decisions similar to its role in development.
Most studies have focused on canonical Hh signaling events, but GLI-independent effects have been identified in normal cells that may contribute to its pathogenic role in cancer. For example, SMO has been found to activate the RhoA and Rac1 GTPases to induce cytoskeletal remodeling, fibroblast migration, and endothelial tubulogenesis (28, 29). In addition, PTCH1 has been found to act as a dependence receptor that directly triggers apoptosis in the absence of ligand, whereas ligand binding induces canonical target gene expression (30). Therefore, non-canonical effects should be further studied in human cancers and, along with variations in the mode of canonical pathway activation, must be considered when developing clinical targeting strategies.
Clinical-Translational Advances
The development of strategies targeting the Hh signaling pathway began with the discovery that cyclopamine, a steroidal alkaloid derived from Veratrum californicum, inhibits SMO (31, 32). Cyclopamine has been extensively used to study Hh signaling and found to inhibit tumor growth in multiple in vitro and in vivo models. Efforts to improve the specificity, potency, and pharmacologic profile of cyclopamine have led to the synthesis of novel derivatives (IPI-926) (33). In addition, large-scale chemical library screens have been undertaken to identify inhibitors of Hh signaling and have generated novel SMO antagonists (GDC-0449, LDE225, PF04449913, TAK-441) (34–37). All of these novel agents have initiated clinical testing.
SMO inhibitors: early success
SMO inhibitors have been studied as anti-cancer agents in over 50 clinical trials across a wide range of tumor types (38). The earliest reported clinical data involved a phase I trial of vismodegib (Erivedge, GDC-0449, Genentech and Curis) in refractory solid tumor patients (39). Early activity was observed in patents with locally advanced or metastatic BCC, presumably because of the high incidence of Hh pathway activating mutations, and this study was expanded to specifically study BCC (40). Of 33 advanced BCC patients receiving vismodegib, 55% of patients experienced clinical responses, including 2 complete responses. Serious grade 3 or 4 toxicities were infrequent (21% of patients) and consisted of fatigue, nausea, dysgeusia, and muscle cramps that have been similarly observed with other SMO inhibitors. A subsequent open label, single-arm phase II trial involving 96 BCC patients (ERIVANCE BCC) demonstrated overall response rates of 43% and 30% in patients with locally advanced and metastatic disease, respectively, and a median duration of response of 7.6 months (41). These results led to the approval of vismodegib by the FDA as a treatment for advanced or metastatic BCC in January 2012.
The SMO antagonist PF-04449913 (Pfizer) has also provided encouraging early results in patients with hematologic malignancies (42). In this dose-escalation phase I trial, 32 patients with a variety of diseases including acute myeloid leukemia, myelodysplastic syndrome, myelofibrosis, CML, and chronic myelomonocytic leukemia (CMMoL) received PF-04449913 as a single agent. Responses were observed across all diseases as evidenced by decreased leukemic blast counts and/or improvements in normal hematopoiesis, and a patient with transformed CMMoL achieved a complete remission.
Mechanisms of clinical resistance have also been reported in a patient with metastatic medulloblastoma who became unresponsive to vismodegib after three months of therapy (43). Serial tumor biopsies identified a novel SMO mutation at relapse that interferes with vismodegib binding, and subsequent studies showed that a similar SMO mutation could be generated in mouse medulloblastoma cells gaining in vivo resistance (44). Given that the various SMO inhibitors in development are chemically distinct, it is possible that these other agents may not display cross-resistance, but data thus far are limited.
Smo antagonists: negative clinical data
While SMO antagonists are active in BCC, clinical results in other solid tumors have been less encouraging. A phase II, randomized, double-blind, placebo-controlled trial of single-agent vismodegib in ovarian cancer has recently been reported (45). In this trial, 104 women received vismodegib or placebo as maintenance therapy following second or third complete remission. At a median follow-up of 5.7 months and analysis of 57 progression-free survival (PFS) events, the median PFS was 5.8 months for patients receiving placebo vs. 7.5 months in the vismodegib arm (HR=0.79 (95% CI: 0.46, 1.35; p=0.39)). A second phase II, randomized, double-blind, placebo-controlled trial compared the addition of vismodegib or placebo to standard first-line chemotherapy in 199 patients with metastatic colorectal carcinoma (46). Compared to placebo, the addition of vismodegib to FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) + bevacizumab or FOLFIRI (5-fluorouracil, leucovorin, irinotecan) + bevacizumab failed to significantly improve PFS compared to chemotherapy + bevacizumab alone (HR stratified by chemotherapy regimen=1.24 (95% CI: 0.83, 1.87; p=0.30)). In metastatic pancreatic cancer, the cyclopamine analogue saridegib (IPI-926, Infinity Pharmaceuticals) was studied in combination with gemcitabine in a phase II, randomized, placebo-controlled trial. A total of 122 patients were studied, but the trial was halted prematurely after patients in the saridegib + gemcitabine arm were found to have a higher rate of progressive disease and lower median survival than those receiving gemcitabine alone.
Potential explanations and future directions
The reasons for negative results in solid tumors other than BCC are unclear but may include the postulated anti-tumor effects of SMO inhibition, clinical trial designs, the broad applicability of pathway inhibition in a specific tumor type, and aberrancies in Hh signal transduction. Since clinical activity is detected through endpoints that are dependent on specific anti-tumor effects, efficacy may not be observed if either the proposed mechanism of action or the choice of endpoints is incorrect. For example, if Hh pathway inhibition primarily impacts tumor cell proliferation and survival, then response rates that reflect changes in tumor burden or PFS should be affected even with the use of SMO antagonists as single agents as in BCC. Alternatively, if SMO antagonists primarily modulate the microenvironment and sensitize tumors to chemotherapy, positive effects on response rates and PFS should be observed only when they are given concomitantly with other therapies. Finally, if Hh signaling primarily regulates rare CSCs responsible for disease relapse and metastatic progression, significant changes in response rates are unlikely to be observed but relapse or metastasis-free survival may be prolonged. Since multiple effects have been ascribed to the Hh in pancreatic and colorectal cancer in the preclinical setting (14, 15, 20, 27), it is possible that PFS was not the endpoint that optimally reflects the actual clinical anti-tumor effects of SMO inhibition. Correlative studies may be able to determine whether Hh signaling predominantly occurs within a specific cell compartment and what cellular effects actually take place in response to SMO inhibition. These insights would be invaluable in guiding the design of subsequent trials.
It is also possible that the Hh pathway is not uniformly active or pathogenic in all cases of a specific tumor type, and clinical efficacy was limited to a specific subset of patients in these clinical trials. Predictive biomarkers have been identified for a number of therapies that can be used to select patients with enhanced responses, such as overexpression of Her2/neu and treatment with trastuzumab in breast cancer. However, biomarkers of response to SMO antagonists have not been established in any tumor type, but may allow patient selection during clinical testing. Thus far, changes in GLI1 and/or PTCH1 expression have been used as pharmacodynamic markers in normal tissues, such as hair follicles, to provide evidence that SMO antagonists actually inhibit Hh signaling in vivo (39), but it is not known whether the expression of these genes within tumors can identify patients with tumors responsive to SMO antagonists. It is likely that the diverse modes of pathway activation and aberrant signaling events in cancer will further complicate the discovery of predictive biomarkers. Moreover, it is possible that these biomarkers will need to be assessed in specific cell compartments, such as stromal cells or CSCs. Given the success of SMO inhibition, further correlative studies in BCC and preclinical studies in other tumor types may identify specific biomarkers that improve patient selection.
Mammalian Hh signal transduction has been largely deciphered by studying normal embryonic fibroblasts, and it is possible that aberrant signaling events within the context of specific cancers impact the efficacy of SMO antagonists. For example, SUFU mutations in medulloblastoma or the direct induction of GLI1 expression by the EWS-FLI fusion protein in Ewing sarcoma may result in pathway activation downstream of SMO (47–49). Similarly, the absence of the primary cilium that is required for Hh signaling in normal cells may lead to tumor formation by activated GLI2 or allow SMO-independent pathway activation through the loss of GLI3 repressor function (50, 51). Although potential aberrancies in Hh signal transduction may prevent the utility of SMO antagonists, alternative targeting strategies have emerged that may ultimately be useful as anti-cancer agents (Figure). To this end, several preclinical strategies have been developed that target Hh ligand-patched interactions (robotnikinin), the intracellular processing and translocation of pathway components (HPIs, arsenic, itraconazole), GLI1 function (GANT-61), or primary cilia formation (HPI) (52–56). Further studies using relevant models and specific tumor types may provide valuable mechanistic data that not only suggest clinical potential but also specify the design of clinical trials.
Summary
The development of Hh pathway inhibitors has been marked by success and failure. The association between PTCH1 mutations in Gorlin syndrome and aberrant pathway activity in BCC along with the discovery of cyclopamine as a naturally occurring SMO antagonist and the development and approval of vismodegib provides an exceptional example of successful translational research. On the other hand, negative clinical results question whether Hh pathway inhibition will actually be effective in tumors that typically lack activating mutations. Aberrant Hh signaling has been found to impact cancers in multiple and diverse ways, but it is unclear which of these is clinically relevant. However, better understanding these mechanisms in the clinical setting should dictate the choice of clinical trial endpoints. Correlative studies from completed and ongoing trials may provide critical insights in this regard and should include examining the effects of SMO antagonists on tumor cells, stromal cells, and CSCs. Moreover, studies to determine why many advanced BCC patients do not respond to SMO antagonists despite likely harboring activating mutations may reveal specific mechanisms responsible for the lack of efficacy in other tumor types. Finally, continued basic studies of the Hh pathway as a regulator of embryonic development may provide a reference point to further understand aberrancies in signal transduction that occur in cancer and lead to the development of novel targeting strategies as well as define predictive biomarkers capable of identifying responsive cases. Therefore, correlative and basic studies of Hh signaling within the context of human cancers coupled with clinical trial designs and endpoints capable of evaluating its precise role in a specific cancer may expand the utility of pathway antagonists beyond BCC.
Acknowledgments
We thank Akil Merchant for helpful comments and critical review of the manuscript.
Grant Support National Institutes of Health R01CA127574, R21CA155733, the Gabrielle's Angel Foundation for Cancer Research, the Sidney Kimmel Foundation for Cancer Research, the Multiple Myeloma Research Foundation and the Leukemia and Lymphoma Society.
Footnotes
Disclosure of Potential Conflicts of Interest W. Matsui, patent interest, Infinity Pharmaceuticals; consultant, Bristol-Myers Squibb, Pfizer.
References
- 1.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and rinciples. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 3.Hui C, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27 doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–24. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 5.Hassounah NB, Bunch TA, McDermott KM. Molecular Pathways: The Role of Primary Cilia in Cancer Progression and Therapeutics with a Focus on Hedgehog Signaling. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA. 2009;106:3196–201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorlin RJ, Goltz RW. Multiple Nevoid Basal-Cell Epithelioma, Jaw Cysts and Bifid Rib. New Engl J Med. 1960;262:908–12. doi: 10.1056/NEJM196005052621803. [DOI] [PubMed] [Google Scholar]
- 8.Kimonis VE, Goldstein AM, Pastakia B, Yang ML, Kase R, DiGiovanna JJ, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. American Journal of Medical Genetics. 1997;69:299–308. [PubMed] [Google Scholar]
- 9.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 11.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–54. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated Genomics Identifies Five Medulloblastoma Subtypes with Distinct Genetic Profiles, Pathway Signatures and Clinicopathological Features. PloS one. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–7. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 14.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R. Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 15.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. Embo Mol Med. 2009;1 doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 17.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895–900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo G-R, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–51. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- 20.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 21.Gallinari P, Filocamo G, Jones P, Pazzaglia S, Steinkühler C. Smoothened antagonists: a promising new class of antitumor agents. doi: 10.1517/17460440902852686. wwwexpertopincom/edc. [DOI] [PubMed]
- 22.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA. 2007;104:4048–53. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dierks C, Beigi R, Guo G-R, Zirlik K, Stegert MR, Manley P, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–49. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Dontu G, Mantle ID, Patel S, Ahn N-s, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–51. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi Proteins Link Hedgehog Signaling to Activation of Rho Small GTPases to Promote Fibroblast Migration. J Biol Chem. 2011;286:19589–96. doi: 10.1074/jbc.M110.197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate proangiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–9. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 30.Mille F, Thibert C, Fombonne J, Rama N, Guix C, Hayashi H, et al. The Patched dependence receptor triggers apoptosis through a DRAL-caspase-9 complex. Nat Cell Biol. 2009;11:739–46. doi: 10.1038/ncb1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–7. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 32.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay MR, Nevalainen M, Nair SJ, Porter JR, Castro AC, Behnke ML, et al. Semisynthetic cyclopamine analogues as potent and orally bioavailable hedgehog pathway antagonists. J Med Chem. 2008;51:6646–9. doi: 10.1021/jm8008508. [DOI] [PubMed] [Google Scholar]
- 34.Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, et al. GDC-0449-a potent inhibitor of the Hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–81. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 35.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, et al. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Medicinal Chemistry Letters. 2010;1:130–4. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson-Fisher AJ, McMahon MJ, Lam J, Li C, Engstrom LD, Tsaparikos K, et al. Abstract 4504: PF-04449913, a small molecule inhibitor of Hedgehog signaling, is effective in inhibiting tumor growth in preclinical models. Cancer Res. 2011;71:4504. [Google Scholar]
- 37.Tojo H, Shibata S, Satoh Y, Kawamura M, Inazuka M, Yamakawa H, et al. Abstract 2823: TAK-441, a novel investigational small molecule hedgehog pathway inhibitor for use in cancer therapy. Cancer Res. 2011;71:2823. [Google Scholar]
- 38. http://www.clinicaltrials.gov.
- 39.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, et al. Phase I Trial of Hedgehog Pathway Inhibitor Vismodegib (GDC-0449) in Patients with Refractory, Locally Advanced or Metastatic Solid Tumors. Clin Cancer Res. 2011;17:2502–11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Hoff D, Lorusso P, Rudin C, Reddy J, Yauch R, Tibes R, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009 doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 41.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis K, Hainsworth JD, et al. CO14. A pivotal study evaluating efficacy and safety of the hedgehog pathway inhibitor (HPI) vismodegib (GDC-0449) in patients with locally advanced (la) or metastatic (m) basal cell carcinoma (BCC) Melanoma Research. 2011;21:e9. 10.1097/01.cmr.0000399448.65869.b7. [Google Scholar]
- 42.Jamieson C, Cortes JE, Oehler V, Baccarani M, Kantarjian HM, Papayannidis C, et al. Phase 1 Dose-Escalation Study of PF-04449913, An Oral Hedgehog (Hh) Inhibitor, in Patients with Select Hematologic Malignancies. ASH Annual Meeting Abstracts. 2011;118:424. [Google Scholar]
- 43.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yauch RL, Dijkgraaf GJP, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaye SB. A phase 2, randomized, placebo-controlled study of Hedgehog (HH) pathway inhibitor GDC-0449 as maintenance therapy in patients with ovarian cancer in 2nd or 3rd complete remission (CR) 2010 2010. [Google Scholar]
- 46.Berlin J. A phase 2, randomized, double-blind, placebo-controlled study of hedgehog pathway inhibitor (HPI) GDC-0449 in patients with previously untreated metastatic colorectal cancer (mCRC) 2010 2010. [Google Scholar]
- 47.Taylor MD, Liu L, Raffel C, Hui C-c, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–10. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 48.Zwerner JP, Joo J, Warner KL, Christensen L, Hu-Lieskovan S, Triche TJ, et al. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. 2008;27:3282–91. doi: 10.1038/sj.onc.1210991. [DOI] [PubMed] [Google Scholar]
- 49.Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–82. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong SY, Seol AD, So P-L, Ermilov AN, Bichakjian CK, Epstein EH, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–61. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang CF, Ramaswamy G, Serra R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:152–61. doi: 10.1016/j.joca.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–6. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci U S A. 2009;106:14132–7. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Lee J, Gardner D, Beachy P. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci U S A. 2010;107:13432. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Tang J, Gong R, Kim J, Lee J. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauth M, Bergström A, Shimokawa T, Toftgård R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]