Abstract
Surgery is a crucial intervention in most cancer patients, but the perioperative period is characterized by increased risks for future outbreak of pre-existing micrometastases and the initiation of new metastases – the major cause of cancer-related death. Here we argue that the short perioperative period is disproportionally critical in determining long-term recurrence rates, discuss the various underlying risk factors that act synergistically during this period, and assert that this timeframe presents an unexplored opportunity to reduce long-term cancer recurrence. We then address physiological mechanisms that underlie these risk factors, focusing on excess perioperative release of catecholamines and prostaglandins, which were recently shown to be prominent in facilitating cancer recurrence through directly impacting the malignant tissue and its microenvironment, and through suppressing anti-metastatic immunity. The involvement of the immune system is further discussed in light of accumulating evidence in cancer patients, and given the recent identification of endogenously activated unique leukocyte populations which, if not suppressed, can destroy autologous “immune resistant” tumor cells. We then review animal studies and human correlative findings suggesting the efficacy of blocking catecholamines and/or prostaglandins perioperatively, limiting metastasis and increasing survival rates. Finally, we propose a specific perioperative pharmacological intervention in cancer patients, based on simultaneous β-adrenergic blockade and COX2 inhibition, and discuss specific considerations for its application in clinical trials, including our approved protocol. In sum, we herein present the rationale for a new approach to reduce long-term cancer recurrence by employing a relatively safe, brief, and inexpensive intervention during the perioperative period.
Keywords: Cancer recurrence, Metastasis, Prostaglandins, Catecholamines, Perioperative period
1. The brief perioperative period is disproportionally critical for determining long-term cancer recurrence and presents an unexplored opportunity for effective interventions
Surgical excision of the primary tumor has been suspected to facilitate the progression of pre-existing micrometastases and the initiation of new metastases through several mechanisms, some of which have only recently been identified. Specifically, the unavoidable damage to the patients’ tissue, and the manipulations and excision of the primary tumor and its vascularization, were shown: (i) to increase shedding of tumor cells into the blood and lymphatic circulations (1); (ii) to naturally increase levels of growth factors which also act locally and systemically to facilitate the growth of minimal residual disease (2); and (iii) to decrease systemic levels of anti-angiogenic factors (e.g., endostatin) due to the removal of the primary tumor that induced their release (3), thus shifting the anti/pro-angiogenic balance in metastatic foci toward pro-angiogenesis.
Additionally, numerous soluble factors increase systemically during the perioperative period as a result of patients’ neuroendocrine and paracrine responses to (i) the presence of the primary tumor, to (ii) physiological and psychological stress, and (iii) to the surgical procedure itself and its accompanying anesthesia, analgesia, blood transfusion and other intra-operative procedures. These soluble compounds include catecholamines, prostaglandins, glucocorticoids, opioids, and a variety of administered anesthetic and analgesic agents. In recent years, it has become clear that in vitro, many of these factors act directly on malignant cells, activating several molecular processes that are critical for tumor metastatic activity, including tumor cell proliferation (4–5), adhesion (6), locomotion (7), extracellular matrix invasion capacity (4), resistance to apoptosis and anoikis (8–10), and secretion of pro-angiogenic factors (11–13). Additionally, in vitro and human and animal in vivo studies show that many of these soluble factors lead to suppression of anti-metastatic cell mediated immunity (CMI) (14–16), which is indeed a common perioperative phenomenon (17–21). As will be elaborated below, this suppression may significantly promote long-term cancer recurrence.
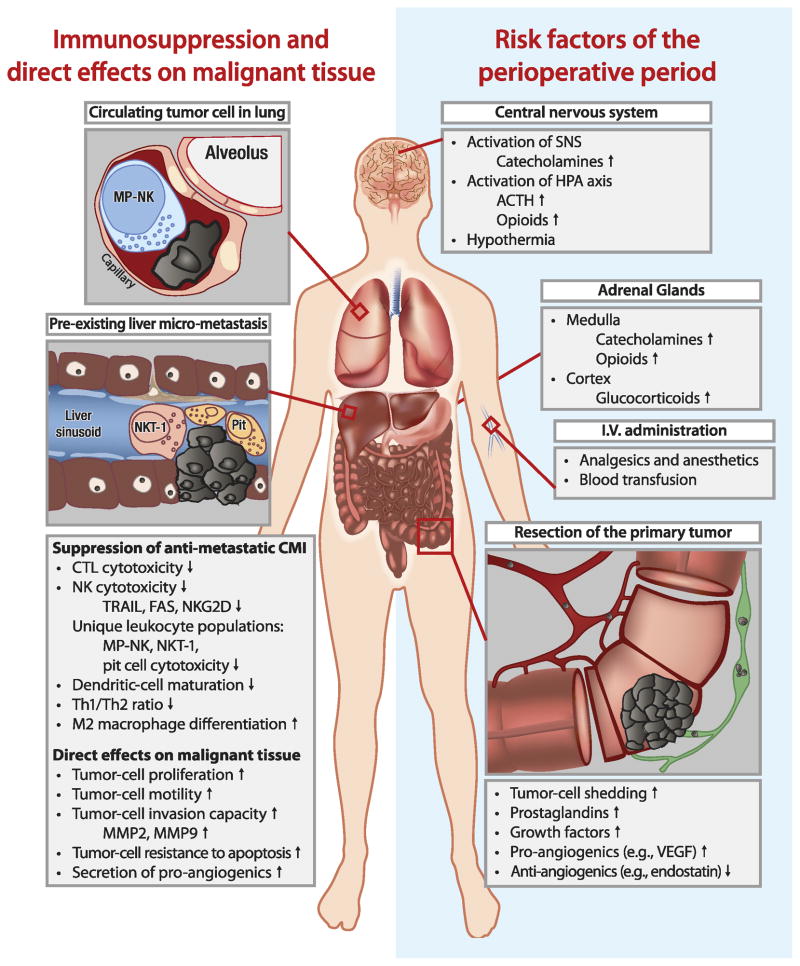
Most importantly, all of the above pro-metastatic processes occur simultaneously during the brief perioperative period, and thus may have a synergistic impact in promoting long-term cancer recurrence. For example, the drop in anti-angiogenic compounds, simultaneously with the rise in growth factors and in pro-angiogenic factors released by malignant and injured tissue, may turn on the “angiogenic switch”, causing dormant metastatic foci to initiate their growth (22). Simultaneously, excess release of malignant cells into the blood or lymphatic circulation, combined with suppressed anti-metastatic immunity, may render the patient markedly susceptible to metastatic dissemination and outbreak – a process which could have been prevented if such multiple deleterious processes had not been initiated (See Fig. 1).
Figure 1. Risk factors and processes occurring during the perioperative period, facilitating metastatic progression.
Risk factors that characterize the perioperative period, including those associated with resection of the primary tumor and the neuroendocrine responses to stress and surgery, are shown on the right side of this figure. As shown on the left side of this figure, these risk factors exert deleterious effects on cell-mediated anti-metastatic immunity, and directly promote progression of residual malignant tissue, including circulating tumor cells and pre-existing micro-metastases.
On the other hand, were it possible to circumvent at least some of these deleterious processes, the perioperative period would then theoretically also present an opportunity to eradicate cancer or successfully arrest its progression. Specifically, removal of the primary tumor reduces the potential immunosuppressive effects of the large malignant mass and its microenvironment (23), and eliminates the ongoing release of metastasizing malignant cells. Minimal residual disease is apparently more easily controlled than the primary tumor, and may thus be eliminated by a now more effective CMI.
2. Excess perioperative catecholamines and prostaglandins are key factors in promoting tumor metastasis by directly impacting the malignant tissue and by suppressing anti-metastatic CMI
Catecholamine and prostaglandin levels are commonly elevated perioperatively. Many tumors release prostaglandins, or recruit macrophages to do so (23), presumably as an immune-escape mechanism or to promote tumor vascularization. Catecholamines are abundantly released throughout the perioperative period, due to patients’ anxiety and fear of the disease, the medical procedures, cancer recurrence, and death. Additionally, tissue damage directly induces the local release of prostaglandins (24), and catecholamine secretion is a prominent neuroendocrine response to tissue damage and its accompanied inflammation, nociception, and pain (25). Interestingly, many human malignancies express receptors for catecholamines (26) and prostaglandins (27), and almost all leukocytes express both receptor systems (28–29). Thus, it is reasonable to assume that both immune and tumor cells are affected by catecholamines and prostaglandins, which are simultaneously elevated during the perioperative period (17).
Indeed, catecholamines were recently shown to directly act on human and animal malignant cells, changing their cellular characteristics to those favoring metastatic spread and growth. Specifically, in vitro and animal in vivo studies employing a variety of human tumor lines indicated that activation of tumor β-adrenoceptors can (i) enhance the production of several metastasis-promoting factors by tumor cells, including VEGF, MMP2 and MMP9, IL-6, and IL-8, and (ii) facilitate tumor angiogenesis, survival, migration, proliferation, and resistance to anoikis – effects that were all blocked by β-antagonists (5, 7, 10–11, 13, 30–31). Additionally, blockade of β-adrenergic receptors was shown to induce apoptosis of several human and animal carcinoma cell lines (32–33). Similarly, prostaglandins were repeatedly implicated in promoting neoplastic progression in human and animal in vitro and in vivo studies. Prostagladin-E2 (PGE2) administration was shown to facilitate macrophage differentiation toward the pro-tumoral M2 phenotype, contributing to cervical carcinoma tumor angiogenesis (34). In colorectal cancer patients, tumor COX2 expression levels were associated with tumor size, stage, blood vascularization, depth of invasion, lymph node metastasis, recurrence, and overall survival rates (35). Blocking the COX2 pathway in patients or animals was shown to promote tumor cell apoptosis (9, 36), to reduce levels of pro-angiogenic agents (12), and to decrease tumor microvascular density (9).
In addition to their aforementioned direct effects, both catecholamines and prostaglandins have been repeatedly shown in vitro to suppress most aspects of CMI, including CTL, macrophage, dendritic, and Natural Killer (NK) cell activity (37–41). These effects are mainly mediated through activation of leukocyte membrane receptors for these ligands, and intracellular initiation of the cAMP-PKA cascade common to both receptor systems (42–43). Catecholamines and prostaglandins are also known to shift the Th1/Th2 cytokine balance toward the anti-CMI Th2 dominance (15–16), and to increase ACTH and glucocorticoid levels (44), potentially suppressing additional aspects of CMI through these responses. Our in vivo animal studies indicated that administration of catecholamines (45–46) or prostaglandins (47), or exposure to behavioral stress and activation of the sympathetic-nervous-system (48), can each suppress NK activity in vivo; Additionally, our findings show that this immune suppression can compromise resistance to experimental metastasis (46–47) and to leukemia progression (14).
Therefore, surgery-related responses can potentially promote the metastatic process through the release of catecholamines and prostaglandins, which can act directly on the malignant tissue and its microenvironment and simultaneously suppress anti-metastatic CMI. See figure 1. Indeed, animal studies indicated that the use of a β-adrenergic blocker or a COX2 inhibitor, attenuated or abrogated the tumor-promoting effects of surgery, which were mediated through suppression of NK activity (19–20, 49), and through other mechanisms (50), including direct effects on the malignancies (51). Furthermore, in two models of spontaneous metastasis, in which a primary orthotopic metastasizing tumor was removed surgically, the simultaneous blockade of the two factors doubled long-term survival rates. Noteworthy, the blockade of any of these factors alone did not increase survival rates (20), nor did it prevent postoperative suppression of NK activity (19). We believe that the inefficiency of each blocker alone is ascribed to the fact that catecholamines and prostaglandins are simultaneously elevated during the perioperative period, causing redundant effects of these factors on immunocytes and on malignant cells.
In sum, we believe that catecholamines and prostaglandins are key mediators of the harmful effects of surgery on metastatic progression, as they (i) directly impact cells of the immune system and of the malignant tissue as detailed above, and (ii) induce other humoral responses that have similar effects on immunocytes and malignant tissue, including proinflammatory responses, activation of the HPA axis, and the shift toward Th2 cytokine dominance. The notion that excess perioperative catecholamine and prostaglandin secretions in cancer patients may increase long-term recurrence rates warrants the study of pharmacological interventions targeting these compounds (see below); Additionally, it supports the claim that adequate anxiety and pain control in this context may have beneficial effects on cancer prognosis.
3. New evidence for the significance of anti-metastatic immunity in the perioperative context
The claim that suppression of CMI promotes the metastatic process relies on the assumption that CMI encompasses anti-metastatic capacities. Animal studies provided ample evidence supporting this immune-surveillance hypothesis, but were justifiably criticized for not simulating many aspects of human cancer progression (17). However, evidence from cancer patients clearly indicates that the immune system extensively interacts with developing primary tumors, metastasizing cells, and established metastases, recognizing and killing many malignant cells, but eventually sparing tumor foci that have adopted efficient immune-escape mechanisms – a process that is now termed “immunoediting” (52). Attesting to these processes in cancer patients, and to the significance of immunosuppression are: the numerous immune-escape mechanisms revealed in human malignancies (23); the finding that in vitro mixed lymphocyte response against excised autologous breast tumors predicts long-term survival rates better than tumor stage and grade (53); the increased frequency of certain malignancies, and the dramatic rise in metastatic development in immunocompromised patients of various etiologies (54–55); and the recent promising outcomes of immune-based therapies, including the CTLA-4 receptor blocker ipilimumab, which enhances T-cell mediated anti-tumor immunity and increases survival (56).
Importantly, several new leukocyte populations that were recently identified in vivo in rodents and some also in humans, exhibit a unique ability to kill autologous tumor cells that were traditionally considered “immune-resistant”. These populations include type-1 NKT cells (57); marginating-pulmonary (MP)-leukocytes and their subpopulation of activated NK cells (49, 58); liver pit cells, which are activated NK cells residing in hepatic sinusoids (59); dendritic-epidermal T cells (60); and killer-dendritic cells (61) (see Figure 1). These populations resemble in vitro activated lymphocytes in terms of their cytotoxic activity and gene expression profile, but exist endogenously without immune stimulation. Additionally, most of these populations are strategically located in capillaries or other structures that filter all circulating blood and foster close contacts with circulating malignant cells, enabling efficient recognition and destruction of aberrant cells. Lastly, most of these unique leukocyte populations have already been shown to be suppressed by catecholamines and/or prostaglandins, including marginating-pulmonary leukocytes (19, 49, 62–63), type-1 NKTs (64), and dendritic-epidermal T cells (65).
4. Blockade of catecholamines or prostaglandins during the perioperative period in cancer patients
COX2 inhibitors have been extensively studied as long-term chemo-preventers of numerous malignancies (e.g., gastro-intestinal tract, breast, and skin tumors), and showed promising outcomes (66); However, this chronic approach focuses on prophylaxis and utilizes minimal doses, which cannot be expected to effectively antagonize the massive release of prostaglandins in the perioperative context. Similarly, a few epidemiological studies have also examined the impact of a chronic use of β-blockers on breast cancer initiation and progression, and indicated a modest but significant reduction in cancer rates (67–68).
To this day, only a few randomized controlled trials (RCT) or retrospective cohort studies have examined the impact of a focused acute perioperative treatment with either a β-blocker or a COX-inhibitor on long-term cancer outcomes. Specifically, in gastric or esophageal cancer patients, a very low daily dose of aspirin (25–50mg/day) during the first postoperative year significantly improved five-year survival rate from 41% to 51.2%, but only in low-stage, non-disseminated malignancies (T2N0M0) (69). Additionally, three RCTs studied the effects of a two - four-week pre-surgical treatment with 400–800 mg/day of the COX2 inhibitor celecoxib, on tumor characteristics in patients with stage I/II primary breast cancer (70), invasive transitional-cell carcinoma (71), or prostate cancer (72). The first two studies exhibited a modest increase in tumor-cell apoptosis; the last study also indicated a reduction in tumor cell proliferation, microvessel density, angiogenesis and HIF-1α expression.
We could not locate any RCT that had directly tested the impact of perioperative treatment with a β-blocker on long-term survival. However, several retrospective epidemiological studies had found associations between these variables. Specifically, in triple-negative breast cancer patients, the use of β-blockers for several months prior to surgery, along with neoadjuvant therapy, was associated with an improved recurrence-free survival (73); moreover, in malignant melanoma patients, a β-blocker treatment significantly predicted a reduced cancer-related and all-cause mortality, even when initiated less than 90 days before diagnosis and surgery. Interestingly, and similarly to other studies described above, the β-adrenergic blockade was effective only in non-metastasized (stage I/II) melanomas (74). This suggests that the treatment is effective in controlling the initial stages of the metastatic process, rather than established malignant foci.
The above human studies present only initial indications for positive effects ofβ-blockers or of COX-inhibitors in the context of oncological surgery. To date, no study had utilized a combined perioperative regimen of β-blockers and COX-inhibitors in cancer patients. Considering the common intra-cellular pathways that are activated by membrane receptors for catecholamines and prostaglandins (42–43), and the simultaneous excess release of both factors during the perioperative period, it is reasonable that only a combined blockade during this critical timeframe could result in a substantial improvement in long-term recurrence-free survival. In our animal studies, we directly compared each drug alone to a combined use, and found the combined treatment to be either significantly more effective or the only effective intervention in increasing survival rates in mice undergoing excision of a spontaneously metastasizing primary tumor (20).
5. Rationale and specific considerations for RCTs testing a perioperative blockade of catecholamines and prostaglandins in cancer patients
We believe that cancer patients will benefit from a brief perioperative treatment that is based on a β-blocker and a COX2-inhibitor, given: (i) the disproportional impact of the short perioperative period in determining long-term cancer outcomes; (ii) the recent discovery of leukocyte populations that can control tumor progression, provided protection from immunosuppression; (iii) the recent findings that excess catecholamine and prostaglandin secretions underlie many of the perioperative risk factors for long-term recurrence, through immune suppression and through non-immunological mechanisms; and (iv) the few positive findings in animal and human studies employing each drug alone (69–74), and the promising results from our animal studies employing the combined blockade of catecholamines and prostaglandins (19–20). To the best of our knowledge, this new approach for reducing cancer recurrence has never been clinically tested in cancer patients. Therefore, we hereby propose specific considerations for such RCTs.
Drug schedule
We propose to test a two-four-week perioperative treatment with a combination of a non-selective β-adrenergic blocker (e.g., propranolol) and a selective COX2 inhibitor (e.g., etodolac), beginning a few days before surgery. Based on the suggested synergistic impact of propranolol and etodolac, we do not recommend testing each of these drugs separately without testing their combination. Both drugs are in routine clinical use and are not reciprocally contraindicated; accordingly, we propose simultaneous intermediate doses that are considered safe and effective for other indications (e.g., pain and hypertension). To reduce potential adverse effects, propranolol should be given at escalating, peak, and withdrawal doses, ranging from 40 to 160 (or more) mg/day (peaking on the day of surgery). Propranolol is preferred over selective β1-blockers, as immunocytes predominantly express β2 over β1 adrenoceptors, and both have been implicated in immune-suppression (45, 75–76). In low doses, propranolol is also anxiolytic (77). Etodolac should be given at a dose of 600–1200 mg/day. Etodolac is preferred over other COX-inhibitors, given its relative selectivity toward COX2 inhibition (78) and few known side effects compared to more selective COX2 inhibitors.
The drug treatment should commence a few days prior to surgery, as psychological distress and malignant tissue (and its surrounding stromal and immune cells) are known to induce the release of catecholamines and prostaglandins, respectively, even before surgery when immune-suppression has been reported (18, 21). It may be paradoxically destructive to initiate drug treatment long before surgery, as enhancing immune anti-tumor activity without tumor removal may theoretically cause harmful immunoediting, selecting immune-resistant tumor cells. Drug treatment should be terminated approximately one-three weeks postoperatively, as we expect major immune and endocrine perturbations to dissipate by this time (21, 79), and as adverse effects of COX-inhibitors accumulate over time.
Patients
We recommend that only patients with a single operable tumor without apparent metastatic disease will be included in such RCT. We also recommend excluding patients with a history of previous malignancies, patients in whom surgical resection is planned without curative intent, and patients with contraindications for these drugs (e.g., cardiac conduction defects or renal failure).
Cancer type and surgery type
All cancers with metastasizing potential could be considered for this kind of RCT. While it may be hypothesized that more extensive surgeries would bear greater deleterious consequences, this notion has been ascertained only with respect to some immunological indices, and less so with respect to tumor progression and survival rates (80), including in colorectal cancer patients (81). Our animal studies indicated that adding a laparotomy to a minor surgery did not significantly increase long-term mortality rates, and that the proposed drug intervention was similarly effective in minor and more severe procedures (20). These findings suggest that cancer patients undergoing minor or major surgeries may all benefit from the treatment.
Outcomes
The primary outcomes of such a potential RCT should include long-term cancer recurrence, cancer-related mortality, and all-cause mortality. Secondary/interim outcomes could include: (i) pre- and post-surgical immune indices, including a complete differential blood count, NK cytotoxicity, and pro- and anti-inflammatory cytokine levels (e.g., IL-6, IL-12, interferon-γ); (ii) pre- and post-surgical general markers of angiogenesis, tumor invasion, and surgical stress responses such as plasma levels of VEGF, sVEGFR-1, MMP-2, MMP-9, cortisol and CRP; (iii) post-excisional histological tumor expression levels of pro-metastatic compounds (e.g., VEGF, PGE2), and tumor receptors for catecholamines, prostaglandins, and growth-factors; and (iv) post-surgical complications and daily amounts of morphine and other narcotics used over the postoperative period. We hypothesize that the primary and most interim outcomes will be improved in the experimental group, and that, as suggested by the literature, the interim measures will predict long-term outcome and will suggest specific underlying mechanisms.
In conclusion, we herein present and rationalize a new approach to reducing long-term cancer recurrence in patients, through studying the use of a brief pharmacological intervention to mitigate excess perioperative effects of catecholamines and prostaglandins. This unexplored approach is also advantageous in using commonly administered medications that are relatively safe, accessible, and inexpensive.
Statement of translational relevance.
Metastasis is the common cause of morbidity and death in cancer patients. Ample evidence from animal and human studies has long indicated that surgery and other perioperative processes can promote metastasis. Recent research has identified several underlying perioperative neuro-endocrine mediators, of which excess catecholamines and prostaglandins play a pivotal role. These compounds can act directly by stimulating pro-metastatic capacities of the malignant tissue and its microenvironment, and/or through suppressing cell-mediated immunity. Recent animal studies targeting catecholamines, prostaglandins, or both, in the perioperative context, have shown reduced metastasis and increased survival rates, and human correlative findings concurred with these outcomes. Here we discuss and analyze findings from different fields of study, suggesting the above mediating mechanisms, supporting the rationale for a brief perioperative clinical intervention, simultaneously targeting catecholamines and prostaglandins. We conclude by suggesting specific considerations for randomized controlled trials in cancer patients aimed at reducing recurrence rates through this intervention.
Acknowledgments
Funding: This work was supported by National Cancer Institute at the National Institute of Health (CA125456 to S.B.E.), a grant from the Israeli Science Foundation (to S.B.E.), and a grant from the Israel-USA bi-national Science Foundation (2005331 to S.B.E.).
Footnotes
The authors declare no conflict of interest
References
- 1.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer. 1999;79:1392–8. doi: 10.1038/sj.bjc.6690223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 4.Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–67. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernabe DG, Tamae AC, Biasoli ER, Oliveira SH. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun. 2011;25:574–83. doi: 10.1016/j.bbi.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. 2009;249:727–34. doi: 10.1097/SLA.0b013e3181a3ddbd. [DOI] [PubMed] [Google Scholar]
- 7.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9. [PubMed] [Google Scholar]
- 8.Kerros C, Brood I, Sola B, Jauzac P, Allouche S. Reduction of cell proliferation and potentiation of Fas-induced apoptosis by the selective kappa-opioid receptor agonist U50 488 in the multiple myeloma LP-1 cells. J Neuroimmunol. 2010;220:69–78. doi: 10.1016/j.jneuroim.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Roche-Nagle G, Connolly EM, Eng M, Bouchier-Hayes DJ, Harmey JH. Antimetastatic activity of a cyclooxygenase-2 inhibitor. Br J Cancer. 2004;91:359–65. doi: 10.1038/sj.bjc.6601967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120:1515–23. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 12.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–8. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 13.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–75. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6:e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972–92. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Bartal I, Melamed R, Greenfeld K, Atzil S, Glasner A, Domankevich V, et al. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun. 2010;24:376–86. doi: 10.1016/j.bbi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–52. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–57. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 21.Greenfeld K, Avraham R, Benish M, Goldfarb Y, Rosenne E, Shapira Y, et al. Immune suppression while awaiting surgery and following it: Dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21:503–13. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Naumov GN, Folkman J, Straume O, Akslen LA. Tumor-vascular interactions and tumor dormancy. APMIS. 2008;116:569–85. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 24.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–10. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Traynor C, Hall GM. Endocrine and metabolic changes during surgery: anaesthetic implications. Br J Anaesth. 1981;53:153–60. doi: 10.1093/bja/53.2.153. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Diz PG, Gandara Rey JM, Garcia-Garcia A. Beta-adrenergic receptors in cancer: therapeutic implications. Oncol Res. 2010;19:45–54. doi: 10.3727/096504010x12828372551867. [DOI] [PubMed] [Google Scholar]
- 27.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers:an update on the molecular mechanisms. Cancer Lett. 2010;295:7–16. doi: 10.1016/j.canlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Landmann R. Beta-adrenergic receptors in human leukocyte subpopulations. European Journal of Clinical Investigation. 1992;1:30–6. [PubMed] [Google Scholar]
- 29.Uotila P. The role of cyclic AMP and oxygen intermediates in the inhibition of cellular immunity in cancer. Cancer Immunol Immunother. 1996;43:1–9. doi: 10.1007/BF03354243. [DOI] [PubMed] [Google Scholar]
- 30.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–64. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 31.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao X, Che X, Zhao W, Zhang D, Bi T, Wang G. The beta-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor kappaB signaling. Oncol Rep. 2010;24:1669–76. doi: 10.3892/or_00001032. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, Ma Q, Shen S, Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist’s anticancer effect in pancreatic cancer cell. Pancreas. 2009;38:94–100. doi: 10.1097/MPA.0b013e318184f50c. [DOI] [PubMed] [Google Scholar]
- 34.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011;187:1157–65. doi: 10.4049/jimmunol.1100889. [DOI] [PubMed] [Google Scholar]
- 35.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Cao W, Yue P, Hao C, Khuri FR, Sun SY. Celecoxib promotes c-FLIP degradation through Akt-independent inhibition of GSK3. Cancer Res. 2011;71:6270–81. doi: 10.1158/0008-5472.CAN-11-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellstrand K, Hermodsson S. An immunopharmacological analysis of adrenaline-induced suppression of human natural killer cell cytotoxicity. International Archives of Allergy and Applied Immunology. 1989;89:334–41. doi: 10.1159/000234972. [DOI] [PubMed] [Google Scholar]
- 38.Koren HS, Leung KH. Modulation of human NK cells by interferon and prostaglandin E2. Mol Immunol. 1982;19:1341–6. doi: 10.1016/0161-5890(82)90302-9. [DOI] [PubMed] [Google Scholar]
- 39.Broug-Holub E, Persoons JH, Schornagel K, Mastbergen SC, Kraal G. Effects of stress on alveolar macrophages: a role for the sympathetic nervous system. Am J Respir Cell Mol Biol. 1998;19:842–8. doi: 10.1165/ajrcmb.19.5.3103. [DOI] [PubMed] [Google Scholar]
- 40.Seiffert K, Granstein RD. Neuroendocrine regulation of skin dendritic cells. Ann N Y Acad Sci. 2006;1088:195–206. doi: 10.1196/annals.1366.011. [DOI] [PubMed] [Google Scholar]
- 41.Khan MM, Sansoni P, Engleman EG, Melmon KL. Pharmacologic effects of autacoids on subsets of T cells. Regulation of expression/function of histamine-2 receptors by a subset of suppressor cells. J Clin Invest. 1985;75:1578–83. doi: 10.1172/JCI111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochemical Journal. 1990;272:327–31. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997;272:5495–500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- 44.Giguere V, Labrie F. Additive effects of epinephrine and corticotropin-releasing factor (CRF) on adrenocorticotropin release in rat anterior pituitary cells. Biochem Biophys Res Commun. 1983;110:456–62. doi: 10.1016/0006-291x(83)91171-3. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–64. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 46.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. Journal of Immunology. 1998;160:3251–8. [PubMed] [Google Scholar]
- 47.Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10:469–79. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and beta-adrenergic mechanisms. Physiol Behav. 1996;60:277–82. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 49.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 51.Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–6. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 53.McCoy JL, Rucker R, Petros JA. Cell-mediated immunity to tumor-associated antigens is a better predictor of survival in early stage breast cancer than stage, grade or lymph node status. Breast Cancer Res Treat. 2000;60:227–34. doi: 10.1023/a:1006405504158. [DOI] [PubMed] [Google Scholar]
- 54.Detry O, Honore P, Meurisse M, Jacquet N. Cancer in transplant recipients. Transplant Proc. 2000;32:127. doi: 10.1016/s0041-1345(99)00908-2. [DOI] [PubMed] [Google Scholar]
- 55.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, et al. Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: a multicenter study of 412 patients. World J Gastroenterol. 2006;12:7319–25. doi: 10.3748/wjg.v12.i45.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Postow M, Callahan MK, Wolchok JD. Beyond cancer vaccines: a reason for future optimism with immunomodulatory therapy. Cancer J. 2011;17:372–8. doi: 10.1097/PPO.0b013e31823261db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hegde S, Fox L, Wang X, Gumperz JE. Autoreactive natural killer T cells: promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells. Immunology. 2010;130:471–83. doi: 10.1111/j.1365-2567.2010.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melamed R, Rosenne E, Benish M, Goldfarb Y, Levi B, Ben-Eliyahu S. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33:16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo DZ, Vermijlen D, Ahishali B, Triantis V, Plakoutsi G, Braet F, et al. On the cell biology of pit cells, the liver-specificNK cells. World J Gastroenterol. 2000;6:1–11. doi: 10.3748/wjg.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macleod AS, Havran WL. Functions of skin-resident gammadelta T cells. Cell Mol Life Sci. 2011;68:2399–408. doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldfarb Y, Benish M, Rosenne E, Melamed R, Levi B, Glasner A, et al. CpG-C oligodeoxynucleotides limit the deleterious effects of beta-adrenoceptor stimulation on NK cytotoxicity and metastatic dissemination. J Immunother. 2009;32:280–91. doi: 10.1097/CJI.0b013e31819a2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levi B, Benish M, Goldfarb Y, Sorski L, Melamed R, Rosenne E, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain, Behavior, and Immunity. 2011;25:727–35. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27:693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 65.Martinet L, Poupot R, Fournie JJ. Pitfalls on the roadmap to gammadelta T cell-based cancer immunotherapies. Immunol Lett. 2009;124:1–8. doi: 10.1016/j.imlet.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012 doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 67.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–44. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 68.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–38. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu JF, Jamieson GG, Wu TC, Zhu GJ, Drew PA. A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol. 2009;16:1397–402. doi: 10.1245/s10434-009-0382-z. [DOI] [PubMed] [Google Scholar]
- 70.Martin LA, Davies GL, Weigel MT, Betambeau N, Hills MJ, Salter J, et al. Pre-surgical study of the biological effects of the selective cyclo-oxygenase-2 inhibitor celecoxib in patients with primary breast cancer. Breast Cancer Res Treat. 2010;123:829–36. doi: 10.1007/s10549-010-1100-z. [DOI] [PubMed] [Google Scholar]
- 71.Dhawan D, Craig BA, Cheng L, Snyder PW, Mohammed SI, Stewart JC, et al. Effects of short-term celecoxib treatment in patients with invasive transitional cell carcinoma of the urinary bladder. Mol Cancer Ther. 2010;9:1371–7. doi: 10.1158/1535-7163.MCT-10-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sooriakumaran P, Coley HM, Fox SB, Macanas-Pirard P, Lovell DP, Henderson A, et al. A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer. Anticancer Res. 2009;29:1483–8. [PubMed] [Google Scholar]
- 73.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–52. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:2273–9. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annual Review of Pharmacology Toxicology. 1995;35:417–48. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 76.Sanders VM, Kasprowicz DJ, Swanson-Mungerson MA, Podojil JR, Kohm AP. Adaptive immunity in mice lacking the beta(2)-adrenergic receptor. Brain Behav Immun. 2003;17:55–67. doi: 10.1016/s0889-1591(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 77.Oberbeck R, Schurmeyer T, Jacobs R, Benschop RJ, Sommer B, Schmidt RE, et al. Effects of beta-adrenoceptor-blockade on stress-induced adrenocorticotrophin release in humans. Eur J Appl Physiol Occup Physiol. 1998;77:523–6. doi: 10.1007/s004210050370. [DOI] [PubMed] [Google Scholar]
- 78.Glaser K, Sung ML, O’Neill K, Belfast M, Hartman D, Carlson R, et al. Etodolac selectively inhibits human prostaglandin G/H synthase 2 (PGHS-2) versus human PGHS-1. Eur J Pharmacol. 1995;281:107–11. doi: 10.1016/0014-2999(95)00302-2. [DOI] [PubMed] [Google Scholar]
- 79.Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126:338–42. doi: 10.1001/archsurg.1991.01410270082013. [DOI] [PubMed] [Google Scholar]
- 80.Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411–9. doi: 10.1007/s00464-003-8275-x. [DOI] [PubMed] [Google Scholar]
- 81.Liang Y, Li G, Chen P, Yu J. Laparoscopic versus open colorectal resection for cancer: a meta-analysis of results of randomized controlled trials on recurrence. Eur J Surg Oncol. 2008;34:1217–24. doi: 10.1016/j.ejso.2007.11.004. [DOI] [PubMed] [Google Scholar]