Abstract
Photothermal ablation (PTA) is an emerging technique that uses near-infrared laser light-generated heat to destroy tumor cells. However, complete tumor eradication by PTA therapy alone is difficult because heterogeneous heat distribution can lead to sub-lethal thermal dose in some areas of the tumor. Successful PTA therapy requires selective delivery of photothermal conducting nanoparticles to mediate effective PTA of tumor cells, and the ability to combine PTA with other therapy modalities. Here, we synthesized multifunctional doxorubicin (DOX)-loaded hollow gold nanospheres (DOX@HAuNS) that target EphB4, a member of the Eph family of receptor tyrosine kinases overexpressed on the cell membrane of multiple tumors and angiogenic blood vessels. Increased uptake of targeted nanoparticles T-DOX@HAuNS was observed in three EphB4-positive tumors both in vitro and in vivo. In vivo release of DOX from DOX@HAuNS, triggered by near-infrared laser, was confirmed by dual radiotracer technique. Treatment with T-DOX@HAuNS followed by near-infrared laser irradiation resulted in significantly decreased tumor growth when compared to treatments with non-targeted DOX@HAuNS plus laser or HAuNS plus laser. The tumors in six of the eight mice treated with T-DOX@HAuNS plus laser regressed completely with only residual scar tissue by 22 days following injection, and none of the treatment groups experienced a loss in body weight. Together, our findings demonstrate that concerted chemo-photothermal therapy with a single nanodevice capable of mediating simultaneous PTA and local drug release may have promise as a new anticancer therapy.
Keywords: Hollow Gold Nanospheres, EphB4 receptors, Targeting, Doxorubicin, Multimodal Therapy
Introduction
Photothermal ablation (PTA) therapy is a recently developed technique that uses near-infrared (NIR) laser light-generated heat to destroy tumor cells. PTA has gained popularity recently because a specific amount of photo-energy is delivered directly into the tumor mass without causing systemic effects, thus promising minimally invasive intervention as an alternative to surgery (1, 2). However, PTA therapy alone is unlikely to kill all tumor cells because the heat distribution is non-uniform, especially in areas peripheral to large blood vessels where heat can be rapidly dissipated by circulating blood. To improve the efficacy and tumor selectivity of laser-induced PTA, light-absorbing photothermal conducting nanoparticles are introduced. In principle, NIR laser-modulated photothermal effects can not only enable PTA of tumor cells but also trigger release of anticancer agents. Such a multimodal approach, which permits simultaneous PTA therapy and chemotherapy, should provide an opportunity for complete eradication of tumor cells.
Hollow gold nanospheres (HAuNS) are a novel gold nanostructure ideally suited for PTA because of their unique combination of small size (30–50 nm), biocompatibility, and strong and tunable absorption in the entire NIR region (3, 4). Owing to their hollow interior, exceptionally high doxorubicin (DOX) payload to HAuNS (up to 60% by weight, 4-fold of the payload achievable with solid gold nanoparticles of the similar size) could be achieved with HAuNS (5). To maximize the therapeutic efficacy of DOX-loaded HAuNS (DOX@HAuNS), it is essential that DOX@HAuNS is selectively delivered to the tumor. The Eph receptors constitute the largest known family of receptor tyrosine kinases and have been reported to control various pathological processes, including tumor progression and angiogenesis, chronic pain following tissue damage, inhibition of nerve regeneration after spinal cord injury, and human congenital malformations (6–15). Overexpression of EphB4 has also been observed in numerous tumor types, including prostate, breast, bladder, lung, colon, gastric, and ovarian cancers (6–8, 14–18). Knocking down EphB4 can result in inhibition of tumor growth, induction of apoptosis, and reduction in tumor vascularity (18). Therefore, EphB4 is a particularly promising target for tumor-specific delivery of DOX@HAuNS.
We have recently reported the first micro-positron emission tomography (PET) imaging agent for detection of EphB4 in cancer cells (19). This PET imaging agent was developed on the basis of a 14-mer peptide identified by phage display technology (15). The parent peptide TNYLFSPNGPIARAW (designated as TNYL-RAW) and its derivatives displayed high binding affinity to EphB4, with KD values of 1.98 to 23 nM (19). Cyclic peptide c(TNYL-RAW) is a second-generation EphB4-binding antagonist with improved plasma stability and high receptor binding affinity. Here, we show for the first time that using c(TNYL-RAW) as a homing ligand, DOX@HAuNS were selectively targeted to EphB4-positive tumors, and that concerted chemo-photothermal therapy mediated by EphB4-targeting DOX@HAuNS induced remarkable antitumor efficacy with reduced systemic toxicity. Our results support the concept of integrating multiple functions into a single nanodevice to mediate simultaneous PTA and local drug release.
Materials and Methods
Synthesis of SH-PEG-c(TNYL-RAW)
The heterofunctional PEG linker SATA-PEG-CONHS containing a protected sulfohydryl group (S-acetylthioacetate, SATA) and NHS activated ester on each end of the PEG chain was first synthesized. Briefly, NH2-PEG-COOH (0.5 g, 0.25 mmol) and 1.2 equivalent N-succinimidyl-S-acetylthioacetate (SATA) were dissolved in 2 ml anhydrous DCM, and then 3 equivalents of dry DIPEA was added dropwise. The reaction solution was stirred at room temperature overnight. After all organic solvent was removed under a vacuum, the crude product was purified by gel filtration with PD-10 column. The collected aqueous solution was lyophilized to yield SATA-PEG-COOH, which was further activated with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) and NHS to make SATA-PEG-CONHS. c(TNYL-RAW) was synthesized on solid support (Supporting Information). The peptide was conjugated to SATA-PEG5000-CONHS in a 1:1 molar ratio in DCM to obtain SATA-PEG-c(TNYL-RAW). The product was purified by PD-10 column and then treated with 0.5 M NH2OH to obtain SH-PEG-c(TNYL-RAW) before conjugation with HAuNS.
Synthesis of DOX-loaded, c(TNYL-RAW)-conjugated HAuNS
HAuNS were synthesized according to a previously reported method (5). Briefly, cobalt nanoparticles were first synthesized by deoxygenating deionized water containing 4.5 ml of 1 M sodium borohydride, 2.8 ml of 0.1 M sodium citrate, and 1.0 ml of 0.4 M cobalt chloride. After chloroauric acid was added into the solution containing cobalt nanoparticles, the cobalt immediately reduced the gold ions onto the surface of the nanoparticles and was simultaneously oxidized to cobalt oxide. Any remaining cobalt was further oxidized by air, resulting in the final product, HAuNS. The size of the HAuNS was determined using dynamic light scattering on a Brookhaven 90Plus particle size analyzer (Holtsville, NY). The UV-visible spectra were recorded on a Beckman Coulter DU-800 UV-visible spectrometer (Brea, CA). The morphology of the HAuNS was examined using a JEM 1010 transmission electron microscope (JEOL USA, Peabody, MA).
To obtain c(TNYL-RAW)-conjugated HAuNS, SH-PEG-c(TNYL-RAW) (50 nmol) was mixed with 1.0 ml HAuNS solution (200 optical density [OD], 5.0 mg/ml) and stirred overnight. Then, SH-PEG (3 μmol) was added to the solution. After another overnight stirring, c(TNYL-RAW)-conjugated HAuNS was purified on a PD-10 column.
For DOX loading, free DOX (5 mg) in water (5 ml) was added to c(TNYL-RAW)-HAuNS (40 OD) in 5 ml of 2.8 mM citrate solution, and the mixture was stirred at room temperature for 24 h. The resulting DOX-loaded c(TNYL-RAW)-HAuNS (T-DOX@HAuNS) were purified by 3 repeated centrifugation and washing steps.
Cell uptake of HAuNS
Cells were transferred and cultured onto 20-mm glass cover slips in a 24-well plate and allowed to grow for 2 days. The medium was replaced with 1 ml of fresh culture medium containing T-DOX@HAuNS. After incubation for 2 h, cell nuclei were stained with DAPI. The cell monolayer on the cover slip was removed, repeatedly rinsed with PBS, and then mounted for microscopic examination. The cellular fluorescence and dark-field light scattering images were examined under a Zeiss Axio Observer Z1 fluorescence microscope equipped with a dark-field condenser.
Quantitative cellular uptake was determined using 111In-labeled nanoparticles. Hey and A549 cells (~1×106/dish) were cultured in 60 mm×15 mm dishes. Cells were incubated with 111In-labeled T-DOX@HAuNS or 111In-labeled DOX@HAuNS (1.5 μCi /dish) for 3 h at room temperature in culture medium lacking fetal bovine serum and phenol red. For the blocking experiments, cells were incubated with free c(TNYL-RAW) (1.0 μM) for 0.5 h and then with 111In-labeled T-DOX@HAuNS or 111In-labeled DOX@HAuNS under the same conditions. Cells were rinsed five times with PBS (pH 7.4). After the PBS was removed, the cells were scraped off the dish, suspended in PBS, and the radioactivity of the cell suspension was then measured with a gamma counter. Protein concentration in cell suspension was quantified using the Bio-Rad protein assay kit (Richmond, CA). The data are expressed as radioactivity (dpm per μg protein).
Cytotoxicity
Cytotoxicity was measured using an MTT assay according to the manufacturer’s suggested procedures. EphB4-positive A2780 cells and EphB4-negative A549 cells were exposed to free DOX, DOX@HAuNS, or T-DOX@-HAuNS for 72 h. The data are expressed as the percentage of surviving cells and are reported as the mean values of three measurements.
Pharmacokinetics and biodistribution
All animal studies were carried out under Institutional Animal Care and Use Committee-approved protocols. For the pharmacokinetic analysis, 8 healthy female Swiss mice (22–25 g; Charles River Laboratories, Wilmington, MA) for each group were each injected intravenously with a dose of either 111In-labeled T-DOX@-HAuNS or 111In-labeled DOX@HAuNS (both 20 μCi, 1.0 mg/ml [40 OD] in 0.2 ml citrate buffered solution). At predetermined intervals up to 48 h, blood samples (10 μL) were taken from the tail vein, and the radioactivity of each sample was measured with a gamma counter. The percentage of the injected dose per gram of blood (%ID/g) was calculated. The blood pharmacokinetic parameters for the radiotracer were analyzed using a noncompartmental model with WinNonlin 5.0.1 software (Pharsight, Sunnyvale, CA).
To investigate the in vivo distribution of T-DOX@-HAuNS, we intravenously injected female nude mice (Harlan, Indianapolis, IN) bearing 6–8 mm subcutaneous A2780, MDA-MB-231, or Hey tumors with 111In-labeled T-DOX@-HAuNS or 111In-labeled DOX@HAuNS (20 μCi/mouse in 0.2 ml). For the blocking studies, the mice were intravenously injected with a mixture of 111In-labeled T-DOX@-HAuNS (20 μCi/mouse in 0.2 ml) and an excess of free c(TNYL-RAW) (0.3 μmol). Mice were killed at 24 h after injection. Various tissues, including tumors, were collected and weighted. The radioactivity for each sample was measured with a gamma counter. Uptake of nanoparticles in various tissues was calculated as %ID/g.
Single photon emission computed tomography (SPECT) imaging
Mice bearing subcutaneous Hey tumors were intravenously injected with 111In-labeled T-DOX@HAuNS or DOX@HAuNS (8.0 mCi/kg, 0.525 mg/ml [25 OD]). For the blocking studies, mice were injected intravenously with a mixture of 111In-labeled DOX@c(TNYL-RAW)-HAuNS and free c(TNYL-RAW) (0.3 μmol). The mice were placed in a prone position and anesthetized with 0.5–2.0% isoflurane gas (Iso-Thesia, Rockville, NY) in oxygen. SPECT images were generated at 24 h after injection. After imaging, the mice were killed, and their tumors were removed. The tumors were snap-frozen and cut into 5-μm slices that were then used for autoradiography analysis on a Fujifilm FLA-5100 imaging system (Stamford, CT).
NIR laser-triggered DOX release in vivo
For measuring the photothermal effect of T-DOX@HAuNS, mice bearing Hey tumors were injected intravenously with saline (5.0 ml/kg) or T-DOX@-HAuNS (5.0 ml/kg of 50 OD HAuNS). At 24 h after injection, the tumor was irradiated with an NIR laser (3W/cm2) for 5 min; Diomed 15 Plus, UK) through the skin surface. Temperature was measured with two thermocouples inserted into the tumor. Care was taken to ensure the thermocouple was not directly exposed to the laser beam.
The DOX release mediated by the photothermal effect in the Hey tumors was studied using 111In- and 3H-labeled T-DOX@-HAuNS, in which the HAuNS were labeled with 111In and the DOX with 3H. Tumors were irradiated by NIR laser light (3W/cm2 for 5 min) at 1 h after intratumoral injection of the dual-labeled nanoparticles (3H: 10 μCi; 111In: 20 μCi) into the center of the tumor. The mice were killed 5 min after laser irradiation, and the tumors were removed, snap-frozen, and sliced into 10-μm sections. Injected tumors that did not receive NIR laser treatment were similarly prepared and used as controls.
The radioactive signals from 111In and 3H were detected using a Fujifilm FLA-5100 imaging system. Briefly, the sections of the tumors were exposed to phosphorous screen film (an SR imaging plate) for 15 min at −10°C, and the 111In autoradiograph was obtained by scanning the film. After the 111In was completely decayed (stored at −80°C for 5 weeks), the same sections were exposed to phosphorous screen film (a TR imaging plate) for 3 days at −10°C, and the 3H autoradiograph was obtained by scanning the film. Concurrently, control specimens from tumors that were not laser-treated were subjected to the same procedures. The autoradiographic distribution of 111In-HAuNS and 3H-DOX was compared by overlaying the two autoradiograms.
Antitumor activity in vivo
Hey tumors were generated by subcutaneous injection of Hey cells (5.0×106 cells/mouse). When the mean tumor volume reached ~200 mm3, mice were divided into four groups consisting of 6–8 mice each. Mice in groups 1 through 4 were injected intravenously with saline (n = 6, 5.0 ml/kg), HAuNS (n = 6, 5.0 ml/kg of 1.25 mg HAuNS/ml solution), DOX@HAuNS (n = 7, 10 mg equivalent DOX/kg, 5.0 ml/kg of 1.25 mg HAuNS/ml solution), and T-DOX@HAuNS (n = 8, 10 mg equivalent DOX/kg, 5.0 mL/kg of 1.25 mg HAuNS/ml solution), respectively. All mice received NIR laser irradiation through the skin surface at 24 h postinjection (2.0 W/cm2 for 3 min). The tumor dimensions were measured with a caliper, and the tumor volume was calculated according to the equation: volume = (tumor length) × (tumor width)2/2. At the end of the experiment (when the tumor size reached >1500 mm3 or 22 days after initial injection, whichever came first), mice were killed by CO2 asphyxiation, and the tumors were collected and weighed. Parts of the tumors were fixed in formalin and cut into 5-μm slices for hematoxylin and eosin staining. Body weight was measured weekly to assess systemic toxicity.
Statistics
Pharmacokinetic data were analyzed using non-compartmental WinNonlin method. Statistical analysis was performed using the SYSTAT program. P values were obtained using the two-sample t-test. For cellular uptake, biodistribution, and antitumor activity data, statistic analyses were performed by ANOVA, with P<0.05 considered to be statistically significant.
Results
Synthesis and characterization of EphB4-targeted T-DOX@HAuNS
Prior to carrying out efficacy studies, we performed a number of experiments to fully characterize the targeted DOX@HAuNS system for in vivo delivery. The average diameter of the HAuNS was 36.8 ± 1.6 nm, as determined by dynamic light scattering. Transmission electron microscopy (TEM) confirmed that the HAuNS consisted of a thin gold shell (~4 nm thickness) with a hollow interior. The extinction spectrum showed that the plasma resonance peak for HAuNS was ~ 800 nm (fig. S1).
The targeting ligand cyclic peptide c(TNYL-RAW) is a second-generation EphB4-binding antagonist. The peptide had an equilibrium dissociation constant (Kd) of 4.4 nM as determined by surface plasmon resonance sensorgram (fig. S2a). No degradation of 64Cu-labeled c(TNYL-RAW) was observed by high-performance liquid chromatography after incubation of the peptide in mouse plasma over a period of 24 h, whereas 64Cu-labeled linear TNYL-RAW was degraded as soon as 2 h after incubation (fig. S2b). c(TNYL-RAW) was linked to SATA-PEG-NHS through an activated ester. After deprotection of the SH group, SH-PEG-c(TNYL-RAW) was conjugated to HAuNS in an aqueous solution via S-Au bonding (Fig. 1). The amount of c(TNYL-RAW) conjugated to the HAuNS was determined by quantitative amino acid analysis after complete dissolution of c(TNYL-RAW)-conjugated HAuNS. The conjugation efficiency was 13.7% and there were about 880 molecules of c(TNYL-RAW) on each HAuNS nanoparticle. DOX was readily loaded into c(TNYL-RAW)-conjugated HAuNS using a previously reported method to give T-DOX@HAuNS (5). DOX loading efficiency was over 90%, and DOX content was 30% (w/w).
Fig. 1.
Reaction scheme for the synthesis of SH-PEG-c(TNYL-RAW) and its conjugation to HAuNS.
In vitro uptake in cancer cells
Next, we evaluated the selectivity of uptake of c(TNYL-RAW)-conjugated DOX@HAuNS in EphB4-positive tumor cells. Western blotting analysis indicated high expression levels of EphB4 receptor in MBA-MD-231, A2780, and Hey cells, but only low expression level in A549 cells (fig. S3A). Immunostaining using anti-EphB4 antibody confirmed strong EphB4 signals from A2780, MDA-MB-231, and Hey cells but weak signal from A549 cells (fig. S3B). On the basis of these findings, we selected the Hey tumor for subsequent efficacy studies.
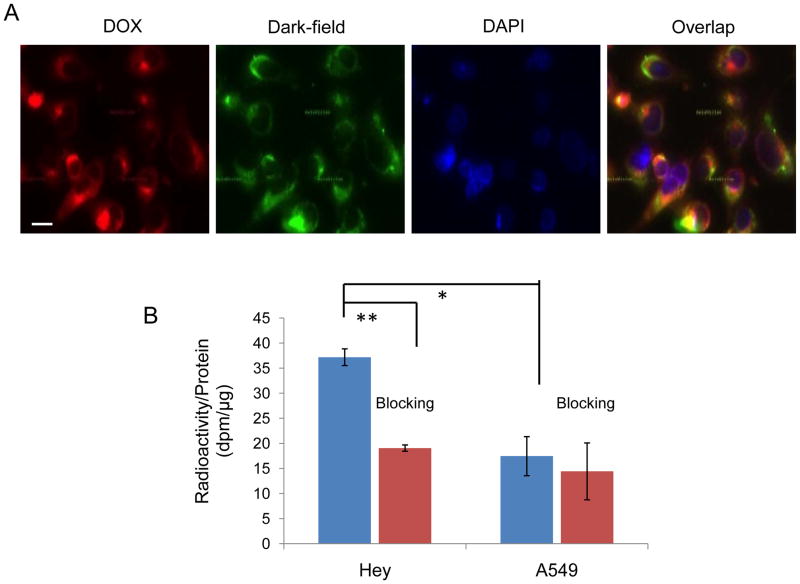
Figure 2A shows representative photomicrographs of fluorescence and dark-field images of Hey cells incubated with T-DOX@HAuNS. The nanoparticles were readily taken up by the tumor cells. The fluorescence signal from the DOX was colocalized with the signal from the HAuNS, indicating that the DOX remained associated with the HAuNS after T-DOX@HAuNS were internalized. Under the same conditions, significantly more T-DOX@HAuNS was internalized in the cells with high EphB4 receptor expression (Hey) than in the cells with low EphB4 receptor expression (A549) (P<0.05, Fig. 2B). The addition of free c(TNYL-RAW) peptide into the culture medium (Hey cells) induced a significant decrease in the cellular uptake of the nanoparticles (P<0.005) but did not cause a change in uptake in the A549 cells that had low EphB4 receptor expression (Fig. 2B). These data indicate that T-DOX@HAuNS was taken up by EphB4-positive cells via receptor-mediated endocytosis.
Fig. 2.
Cellular uptake of T-DOX@HAuNS. (A) Representative photomicrographs of Hey cells after incubation with T-DOX@HAuNS for 2 h. The scattering signal from the HAuNS was visualized using a dark-field condenser (green), and red fluorescence was from DOX. Cell nuclei were counterstained with DAPI (blue). Bar, 20 μm. (B) Quantitative cellular uptake of nanoparticles in Hey and A549 cells after 3 h incubation with 111In-labeled T-DOX@HAuNS and T-DOX@HAuNS plus free c(TNYL-RAW) (blocking). **,P<0.005*; P<0.05.
Cytotoxicity
For cells with low EphB4 expression (A549), the difference in cytotoxicity between T-DOX@HAuNS and non-targeted DOX@HAuNS was minimal, demonstrating the importance of EphB4-mediated uptake (fig. S4A). Free DOX exhibited higher toxicity in A549 cells than both T-DOX@HAuNS and DOX@HAuNS. The lower cytotoxic potency of T-DOX@HAuNS and DOX@HAuNS can be attributed to relatively stable complexes formed between DOX and HAuNS and delayed DOX release inside cells. However, significantly higher toxicity was shown for T-DOX@HAuNS in A2780 cells than either free DOX or DOX@HAuNS (fig. S4B). The higher cellular cytotoxicity with targeted nanoparticles can be attributed to the increased uptake of T-DOX@HAuNS in the target cells.
Pharmacokinetics, biodistribution, and SPECT imaging
Figure 3A shows the mean blood activity time profile of 111In-labeled T-DOX@HAuNS and DOX@HAuNS. The pharmacokinetic parameters are summarized in Table 1. DOX@HAuNS had moderately higher area under the blood drug concentration-time curve extrapolated to infinite time (AUC0-∞, 322.3 ± 72 %ID h/ml) than T-DOX@HAuNS (242.8 ± 46 %ID h/ml, P=0.046). However, there was no significant difference between the two HAuNS formulations in any other pharmacokinetic parameters (P≥0.05), suggesting that the conjugation of c(TNYL-RAW) to HAuNS did not significantly change the pharmacokinetic properties of the nanoparticles. Both types of nanoparticles (T-DOX@HAuNS and DOX@HAuNS) were almost completely eliminated from the blood at 48 h after injection. Figure 3B shows the biodistribution of T-DOX@HAuNS, T-DOX@HAuNS with blocking by free c(TNYL-RAW), and DOX@HAuNS in nude mice at 24 h after injection. Most nanoparticles were taken up by the liver, spleen, and kidney. Interestingly, significantly less T-DOX@HAuNS than DOX@HAuNS accumulated in the liver (p=0.0009) and the spleen (p=0.0006). Conversely, T-DOX@HAuNS had significantly higher uptake in the kidney than DOX@HAuNS did (P = 0.004). With the exception of kidney uptake, co-injection with an excess of c(TNYL-RAW) did not affect the biodistribution pattern of T-DOX@HAuNS. c(TNYL-RAW) blocking reduced the kidney uptake of T-DOX@HAuNS from 17.2 %ID/g to 12.9 %ID/g (P = 0.02). This finding may be reflective of EphB4 expression in venous endothelium of the kidney (20).
Fig. 3.
T-DOX@HAuNS pharmacokinetics, biodistribution, and tumor uptake. (A) Activity-time profiles of 111In-labeled T-DOX@HAuNS and DOX@HAuNS in Swiss mice. The data are expressed as percentage of the injected dose per gram of blood (%ID/g) and are presented as mean ± standard deviation (n = 8). (B) Biodistribution of 111In-labeled T-DOX@HAuNS, T-DOX@HAuNS with blocking, and DOX@HAuNS in nude mice at 24 h after injection. (C) Comparison of nanoparticle uptake in Hey, A2780, and MDA-MB-231 tumors at 24 h postinjection. The data are expressed as percentage of the injected dose per gram of tissue (%ID/g) and are presented as mean ± standard deviation (n = 6). **:P<0.005; *:P<0.05.
Table 1.
Pharmacokinetic parameters of DOX@HAuNS and T-DOX@HAuNS after intravenous injection in female Swiss mice. Analysis of variance showed no differences between the two HAuNS formulations. Cmax, maximum blood concentration; AUC0-∞, area under the blood drug concentration-time curve extrapolated to infinite time; CL, systemic clearance; Vd, volume of distribution; Vss, volume of distribution at steady-state; MRT, mean residence time.
| PK Parameters | DOX@HAuNS | T-DOX@HAuNS | P Value |
|---|---|---|---|
| Elimination half-life (h) | 11.9 ± 3.8 | 9.75 ± 1.7 | 0.229 |
| Cmax (%ID/ml) | 28.9 ± 4.9 | 30.8 ± 3.7 | 0.459 |
| AUC0-∞ (%ID h/ml) | 322.3 ± 72 | 242.8 ± 46 | 0.046 |
| CL (ml/h) | 0.323 ± 0.068 | 0.426 ± 0.092 | 0.050 |
| Vd (ml) | 5.30 ± 0.81 | 5.85 ± 0.69 | 0.239 |
| Vss (ml) | 4.94 ± 0.70 | 5.25 ± 0.61 | 0.439 |
| MRT (h) | 16.0 ± 5.0 | 12.6 ± 1.8 | 0.137 |
To demonstrate efficiency of tumor targeting, we next carried out biodistribution studies in tumor-bearing mice. T-DOX@HAuNS displayed significantly higher accumulation than nontargeted DOX@HAuNS in tumors with high EphB4 receptor expression, with 3.0-, 2.1-, and 1.6-fold mean increases in Hey (P = 0.0016), A2780 (P = 0.0005), and MDA-MB-231 tumors (P = 0.019), respectively (Fig. 3C). Furthermore, the addition of free c(TNYL-RAW) peptide significantly reduced the uptake of T-DOX@HAuNS in these tumors, with 2.3-, 1.5-, and 2.0-fold decreases, respectively (Fig. 3C). These results indicated that T-DOX@HAuNS was actively targeted to tumors expressing EphB4.
SPECT images showed significant blood activity in the liver and spleen for both 111In-labeled T-DOX@HAuNS and DOX@HAuNS (Fig. 4). By 24 h after injection, accumulation of T-DOX@HAuNS in the tumor was clearly visualized (Fig. 4A). The uptake of T-DOX@HAuNS in the Hey tumors was blocked by an excess of free c(TNYL-RAW) (Fig. 4B). Similarly, tumor uptake of then non-targeted DOX@HAuNS was barely visible (Fig. 4C). Autoradiographs of all 3 tumors (Hey, A2780, and MDA-MB-231) showed stronger 111In radioactivity signals with T-DOX@HAuNS than with T-DOX@HAuNS plus free c(TNYL-RAW) or with DOX@HAuNS (Fig. 4D).
Fig. 4.
microSPECT/CT imaging and autoradiography of 111In-labeled T-DOX@HAuNS and DOX@HAuNS. (A–C) Representative SPECT images of nude mice bearing Hey tumors at 24 h after intravenous injection of 111In-labeled T-DOX@HAuNS, 111In-labeled T-DOX@HAuNS plus free c(TNYL-RAW), and 111In-labeled DOX@HAuNS. Arrows indicate tumors. (D) Representative autoradiographs of slices sectioned from Hey, A2780, and MDA-MB-231 tumors at 24 h after injection. Stronger signals were visualized for tumors imaged after the injection of 111In-labeled T-DOX@HAuNS than of T-DOX@HAuNS plus free c(TNYL-HAuNS) or DOX@HAuNS.
In vivo antitumor activity
The temperature measured by the thermocouple within the tumor reached ~53°C after 5 min of NIR laser exposure on the tumor surface at an output power of 3 W/cm2 in mice injected with T-DOX@HAuNS (Fig. 5A). No change in temperature was noted under the same conditions in the tumors of control mice. Dual-tracer autoradiography showed that immediately after NIR laser irradiation, 3H-DOX was released and dispersed into the area surrounding the site where T-DOX@HAuNS was introduced. Conversely, 3H-DOX was mostly colocalized with 111In-HAuNS in mice that did not undergo NIR laser treatment (Fig. 5B).
Fig. 5.
In vivo release of DOX upon NIR treatment. (A) NIR-induced temperature change in tumors injected with HAuNS. (B) DOX release from T-DOX@HAuNS in tumors treated with NIR laser irradiation. The white broken circle indicates the tumor outline. Green (111In-HAuNS) and red (3H-DOX) signals indicate the location of the HAuNS and DOX, respectively.
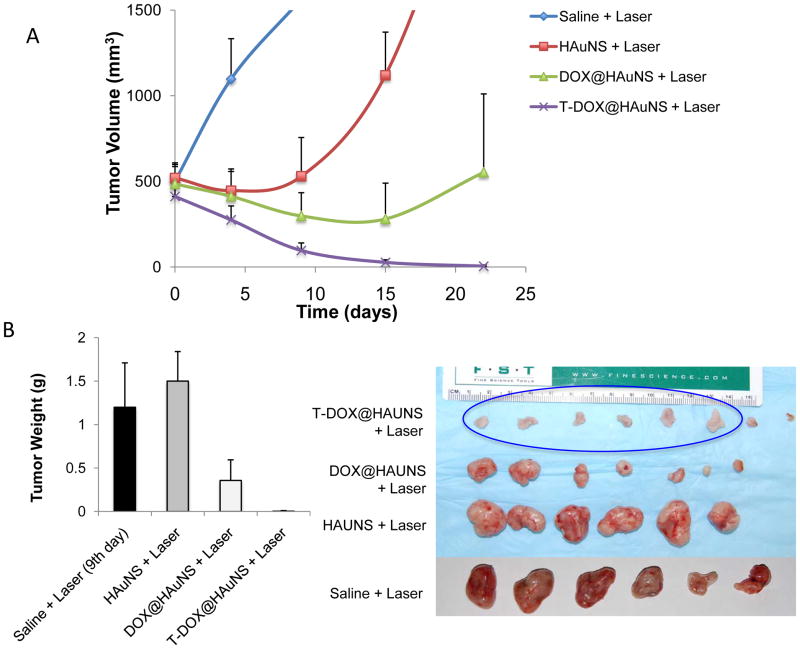
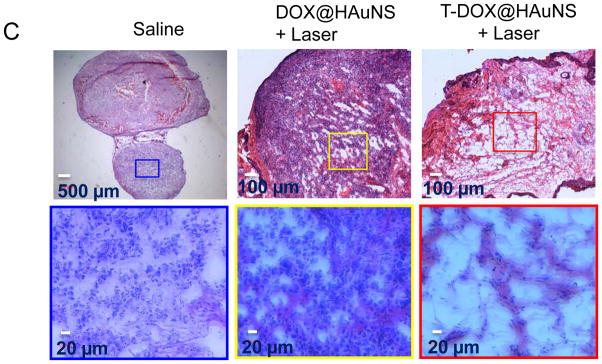
Figure 6A shows the Hey tumor growth curves after intravenous injections of saline, HAuNS (5.0 ml/kg of 1.25 mg HAuNS/ml saline [50 OD], no DOX, no targeting), DOX@HAuNS (10 mg equivalent DOX/kg, 5.0 ml/kg of 1.25 mg HAuNS/ml), and T-DOX@HAuNS (10 mg equivalent DOX/kg, 5.0 ml/kg of 1.25 mg HAuNS/ml). Mice in each group received NIR laser treatment (2.0 W/cm2 for 3 min) 24 h after injection. The mice in the saline-plus-laser group were killed on day 9 after injection because most of the tumors in this group were ~1500 mm3 at that time. Mice in the other three groups were killed on day 22. Treatment with T-DOX@HAuNS-plus-laser showed significantly enhanced antitumor activity compared with saline-plus-laser, HAuNS-plus-laser, and DOX@HAuNS-plus-laser. The mean tumor weight in T-DOX@HAuNS-plus-laser group on day 22 after treatment was 0.0038 ± 0.0007 g (n = 8), which was significantly smaller than that of the saline-plus-laser (1.2 ± 0.51 g on day 9, n = 6; P<0.0001), HAuNS-plus-laser (1.5 ± 0.34 g on day 22, n=6; P<0.0001), and DOX@HAuNS-plus-laser (0.36 ± 0.24 g on day 22, n = 7; P<0.001) groups (Fig. 6B). The tumors in this group of mice became whitish immediately after treatment, suggesting disruption of blood perfusion. Remarkably, the tumors in six of the eight mice treated with T-DOX@HAuNS-plus-laser regressed completely and became scar tissue by 22 days after the injection. Histological analysis showed the presence of scar tissue and a lack of residual tumor cells in mice treated with T-DOX@HAuNS-plus-laser (Fig. 6C). Tumors in the nontargeted DOX@HAuNS-plus-laser group became loose and discrete, suggesting damage to tumor cells. However, tumors could not be completely eradicated without targeting ligand. Tumors in the saline-plus-laser group appeared to be intact. In the saline-plus-laser and DOX@HAuNS-plus-laser treatment groups, viable tumor cells were found throughout the tumor volumes (Fig. 6C). No apparent signs of toxicity were observed throughout the course of the study. The body weight of mice in all treatment groups steady increased during the study period (fig. S5).
Fig. 6.
Antitumor activity of various treatments against Hey tumors. (A) Tumor growth curves for mice treated with saline plus laser (n = 6), HAuNS plus laser (n = 6), DOX@HAuNS plus laser (n = 7), and T-DOX@HAuNS plus laser (n = 8). NIR laser irradiation (2.0 W/cm2 for 3 min) was commenced at 24 h after injection. The photographs (right panel) were taken on day 22 after nanoparticle injection. (B) Average tumor weights (left panel) and photographs (right panel) of tumors from different treatment groups. Tumors were removed on day 22 for all groups except the saline-plus-laser group, in which tumors were removed on day 9. Six tumors in the T-DOX@HAuNS group regressed completely by day 22 after treatment, and only the scar tissues at the site of tumor inoculation were removed and photographed (blue circle, right panel). (C) Representative photomicrographs of hematoxylin and eosin-stained slides from scar tissue from a mouse treated with T-DOX@HAuNS-plus-laser or tumors from mice treated with saline or DOX@HAuNS-plus-laser on day 22 after treatment.
Discussion
In this study, we showed that T-DOX@HAuNS had significantly greater cytotoxic effects in tumor cells with high EphB4 receptor expression than DOX@HAuNS and free DOX did in vitro. Moreover, T-DOX@HAuNS demonstrated enhanced antitumor activity when combined with NIR laser irradiation than DOX@HAuNS plus laser treatment in vivo. These findings indicate that c(TNYL-RAW) peptide is highly effective for selective delivery of T-DOX@HAuNS to tumors with high expression of EphB4. The significantly enhanced antitumor activity of T-DOX@HAuNS could be attributed to 1) increased accumulation of the nanoparticles in tumors, 2) controlled release of DOX mediated by NIR laser irradiation, and 3) synergistic interaction between chemotherapy and PTA therapy, both of which were activated concurrently by NIR laser.
In our nanoconstruct, c(TNYL-RAW) peptide with high EphB4 binding affinity and high plasma stability was conjugated to HAuNS through a PEG linker, which ensured availability of the peptide to the target receptor (Fig. 1). This strategy has been successfully used for the formulation of other targeted nanoparticles by us and others (4, 21–24). Additional monofunctional SH-PEG chains were introduced together with SH-PEG-c(TNYL-RAW) to ensure that all available gold surface was covered by PEG. This process was used to create DOX@HAuNS and ligand conjugated T-DOX@HAuNS, both of which had high colloidal stability.
Both in vitro and in vivo data showed that tumor uptake of T-DOX@HAuNS could be partially blocked by free c(TNYL-RAW) peptide, confirming that cell uptake of T-DOX@HAuNS was mediated by EphB4 (Figs. 2–4). In vivo, T-DOX@HAuNS displayed significantly higher accumulation than nontargeted DOX@HAuNS in all three tumor models evaluated. Because DOX@HAuNS and T-DOX@HAuNS exhibited similar pharmacokinetic behaviors (Fig. 3A, Table 1), the difference in tumor uptake between targeted and nontargeted HAuNS is unlikely a result of enhanced permeability and retention effect. Hence, our data support successful EphB4 receptor-mediated targeted delivery of T-DOX@HAuNS after intravenous injection.
In addition to active targeting mediated by receptor ligands exemplified in this study, physiological and physical methods such as tumor priming, vascular disruption, degradation of the extracellular matrix, and vessel normalization may be used to improve tumor distribution of nanoparticles (25–29). Recent studies have shown that losartan, a clinically approved angiotensin II receptor antagonist, is an effective agent for normalizing tumor vasculature (29). losartan inhibited collagen I production by tumor-associated fibroblasts, which led to a dose-dependent reduction in stromal collagen in several human tumor xenograft models. As a result, the drug enhanced the delivery and efficacy of intravenously injected pegylated liposomal doxorubicin (Doxil). These studies suggest that targeted nanoparticles such as T-DOX@HAuNS may achieve further improvement in tumor deposition when combined with physiological and physical approaches.
The temperature in tumors of mice received intravenous injection of T-DOX@HAuNS reached ~53°C after 5 min of continuous-wave NIR laser exposure at 3 W/cm2 (Fig. 5A). This temperature is sufficient for causing irreversible damage to cancer cells (30). As expected, there was no temperature change in the tumors of mice that did not receive the nanoparticle injection followed by NIR irradiation. Therefore, T-DOX@HAuNS medicated efficient photothermal effect. We have previously shown that in aqueous solution, the release of DOX from DOX@HAuNS could be activated by NIR laser irradiation (5). To demonstrate DOX could be release in vivo upon NIR irradiation, we used an autoradiographic technique with dual-radiotracer labeling. 3H undergoes beta decay with a 12.3 year half-life, releasing 18.6 keV of energy in the process. On the other hand, 111In emits gamma radiation with a half-life of 2.81 days at much higher energy (171 KeV and 245 KeV). Taking advantage of these differences in the decay properties between 3H and 111In, we were able to separate signals from HAuNS and DOX in the tumors. Thus, immediately after NIR laser irradiation at 3 W/cm2, 3H-DOX was dissociated from 111In-HAuNS, whereas in the control tumor not exposed to NIR laser irradiation, signals from 3H-DOX colocalized perfectly with 111In-HAuNS (Fig. 6B). These data confirm that free DOX was released from T-DOX@HAuNS and then diffused away from the nanoparticles when tumors were exposed to the NIR laser.
The antitumor efficacy of free DOX, DOX@HAuNS, DOX@HAuNS-plus-NIR laser was investigated against MDA-MB-231 tumors in our previous work (31). There was no significant difference in tumor size between groups treated with DOX (15 mg/Kg, one injection) and DOX@HAuNS (15 mg equivalent DOX/kg per injection, 2 injections). However, DOX@HAuNS-plus-NIR laser (15 mg equivalent DOX/kg per injection, 2 injections) reduced tumor volume significantly more than the other 2 treatments (31). In these earlier studies, free DOX at a single dose of 15 mg/kg caused serious systemic toxicity and cardiotoxicity. Therefore, we did not use free DOX as a control in the current study. Instead, we used non-targeted DOX@HAuNS as a control and compared the antitumor studies of T-DOX@HAuNS against ovarian Hey tumors with that of DOX@HAuNS. Combined T-DOX@HAuNS-plus-laser treatments demonstrated significantly better antitumor activity than combined HAuNS-plus-laser (PTA only) and nontargeted DOX@HAuNS-plus-laser treatments (Fig. 6).
Significantly, this higher antitumor activity was achieved without causing significant systemic toxicity, as indicated by continued increase in body weight in mice in all treatment groups (fig. S5). In our previous work, we compared the toxicity of free DOX, liposomal DOX, and DOX@HAuNS (31). DOX@HAuNS after a single dose at 60 mg equivalent DOX/kg had no cardiotoxicity compared to liposomal DOX (two doses at a total dose of 30 mg DOX/kg) and free DOX (single dose of 15 mg/kg). In the heart, 100% of both liposomal DOX- and free DOX-treated mice had a vacuolar cardiomyopathy. However, for mice treated with DOX@HAuNS, the histopathologic features in the heart were similar to those observed in the saline-treated control mice and no abnormal features were observed. Because DOX@HAuNS and T-DOX@HAuNS had similar pharmacokinetic properties and there was no significant different in heart accumulation between the 2 agents, we expect that T-DOX@HAuNS will display toxicity profile similar to that of DOX@HAuNS. Further studies are required to document both short-term and long-term systemic toxicity of T-DOX@HAuNS after intravenous injection.
Our study has several limitations. First, in vivo release of DOX upon laser irradiation was performed after intratumoral injection, which may not accurately reflect DOX distribution that will be see after systemic administration followed by NIR treatment. However, the sole purpose of this experiment was to examine whether DOX release can be triggered by NIR laser irradiation under in vivo conditions, which was confirmed in the current study. Second, we did not analyze the distribution of DOX when DOX@HAuNS and T-DOX@HAuNS were given intravenously. Detailed DOX biodistribution in comparison to HAuNS after systemic administration of non-targeted DOX@HAuNS has been studied and published in our previous work using a dual radiotracer technique (31). It was found that DOX@HAuNS was stable in the blood during the first 6 h after injection. By 24 h after injection, there was disparity in biodistribution pattern between DOX and HAuNS, indicating gradual release of DOX from DOX@HAuNS.
A potential limitation of the proposed concurrent chemo-photothermal therapy strategy is penetration depth of near-infrared light. The current state-of-the-art enables NIR light to penetrate up to 1–2 cm in soft tissue (32). With the newer 10- to 40-mm-long quartz laser fibers and water-cooled laser application sheaths, ablative areas of 50–80 mm have been achieved (33–35). Deeper reach is also possible with various minimally invasive interventional techniques, such as endoscopic, intravascular, and percutaneous approaches. Currently, laser beam is delivered through laser fibers inserted into the tumors, as in the case of primary and metastatic liver cancer (33, 35, 36) and lung metastases (37). In our previous work, we evaluated the triggered release of paciltaxel via NIR laser irradiation and its antitumor efficacy by hepatic arterial administration of HAuNS- and paclitaxel-loaded microspheres into rabbits with liver carcinoma in situ (38). The results showed that NIR laser irradiation after the microspheres administration results in intratumoral heating. NIR laser irradiation can trigger release of paclitaxel from the microspheres, and can potentially increase the efficacy of treatment after hepatic arterial delivery of the microspheres in liver tumors. Thus, we believe that chemo-photothermal therapy approach entailed in this work is best suited for unresectable, locally advanced disease that ultimately causes patient death. It is envisioned that chemo-photothermal ablation therapy may also be used in conjunction with surgery, especially used in post-surgery setting to eradicate residual tumor cells. In such as case, the laser beam can be conveniently delivered through surface exposure.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants RC2 GM092599, Center of Cancer Nanotechnology Excellence grant (U54 CA151668), the John S. Dunn Foundation, and the National Nature Science Foundation of China (81001411). We also acknowledge the NCI Cancer Center Support Grant CA016672, which supports MD Anderson’s Small Animal Imaging Facility and TEM Core Facility.
Footnotes
Disclosure of Potential Conflict of Interest: No potential conflicts of interest were disclosed.
References
- 1.Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44:936–46. doi: 10.1021/ar200023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melancon M, Lu W, Li C. Gold-based magneto/optical nanostructures: Challenges for in vivo applications in cancer diagnostics and therapy. Mater Res Bull. 2009;34:415–21. doi: 10.1557/mrs2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ, Li C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther. 2008;7:1730–9. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin Cancer Res. 2009;15:876–86. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4:1033–41. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 7.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–9. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 8.Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc Res Tech. 2002;59:58–67. doi: 10.1002/jemt.10177. [DOI] [PubMed] [Google Scholar]
- 9.Walker-Daniels J, Hess AR, Hendrix MJ, Kinch MS. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–42. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037–49. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- 11.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci USA. 2004;101:5583–8. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battaglia AA, Sehayek K, Grist J, McMahon SB, Gavazzi I. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci. 2003;6:339–40. doi: 10.1038/nn1034. [DOI] [PubMed] [Google Scholar]
- 13.Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–73. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, Quinn DI, Weaver FA, Gill PS. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–80. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 15.Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with Ephrin-like affinity. J Biol Chem. 2005;280:17301–11. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- 16.Erber R, Eichelsbacher U, Powajbo V, Korn T, Djonov V, Lin JH, Hammes HP, Grobholz R, Ullrich A, Vajkoczy P. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. Embo J. 2006;25:628–41. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA, Beart RW, Singh G, Nguyen C, Kahn M, Gill PS. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–45. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- 18.Kumar SR, Singh J, Xia GB, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong C, Huang M, Zhang R, Song S, Lu W, Flores L, 2nd, Gelovani J, Li C. In vivo small-animal PET/CT of EphB4 receptors using 64Cu-labeled peptide. J Nucl Med. 2011;52:241–8. doi: 10.2967/jnumed.110.081943. [DOI] [PubMed] [Google Scholar]
- 20.Ozgur E, Heidenreich A, Dagtekin O, Engelmann U, Bloch W. Distribution of EphB4 and EphrinB2 in normal and malignant urogenital tissue. Urol Oncol. 29:78–84. doi: 10.1016/j.urolonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Qian X, Beitler JJ, Chen ZG, Khuri FR, Lewis MM, Shin HJ, Nie S, Shin DM. Detection of circulating tumor cells in human peripheral blood using surface-enhanced Raman scattering nanoparticles. Cancer Res. 2011;71:1526–32. doi: 10.1158/0008-5472.CAN-10-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Zhang G, Zhang R, Flores LG, II, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010;70:3177–88. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther. 2007;322:80–8. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 26.Melancon MP, Elliott A, Ji X, Shetty A, Yang Z, Tian M, Taylor B, Stafford RJ, Li C. Theranostics with multifunctional magnetic gold nanoshells: photothermal therapy and t2* magnetic resonance imaging. Invest Radiol. 2011;46:132–40. doi: 10.1097/RLI.0b013e3181f8e7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eikenes L, Tari M, Tufto I, Bruland OS, de Lange Davies C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81–8. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eikenes L, Bruland OS, Brekken C, de Davies CL. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–73. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 29.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–14. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc Chem Res. 2011;44:947–56. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS, Liang D, Wei W, Sood AK, Li C. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: A platform for near-infrared light-trigged drug release. J Control Release. 2012;158:319–28. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y, Chen WR, Xia M, Jeong SW, Liu H. Effect of photothermal therapy on breast tumor vascular contents: noninvasive monitoring by near-infrared spectroscopy. Photochem Photobiol. 2005;81:1002–9. doi: 10.1562/2004-09-05-RA-305. [DOI] [PubMed] [Google Scholar]
- 33.Gough-Palmer AL, Gedroyc WM. Laser ablation of hepatocellular carcinoma--a review. World J Gastroenterol. 2008;14:7170–4. doi: 10.3748/wjg.14.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muralidharan V, Malcontenti-Wilson C, Christophi C. Interstitial laser hyperthermia for colorectal liver metastases: the effect of thermal sensitization and the use of a cylindrical diffuser tip on tumor necrosis. J Clin Laser Med Surg. 2002;20:189–96. doi: 10.1089/104454702760230500. [DOI] [PubMed] [Google Scholar]
- 35.Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: experimental and clinical data. Int J Hyperthermia. 2004;20:713–24. doi: 10.1080/02656730400007212. [DOI] [PubMed] [Google Scholar]
- 36.Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;230:450–8. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg C, Puls R, Hegenscheid K, Kuehn J, Bollman T, Westerholt A, Weigel C, Hosten N. Laser ablation of metastatic lesions of the lung: long-term outcome. Am J Roentgenol. 2009;192:785–92. doi: 10.2214/AJR.08.1425. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Stafford RJ, Javadi S, Ozkan E, Ensor JE, Wright KC, Elliot AM, Jian Y, Serda RE, Dixon KA, Miller JJ, Klump S, Wallace MJ, Li C. Effects of near-infrared laser irradiation of biodegradable microspheres containing hollow gold nanospheres and paclitaxel administered intraarterially in a rabbit liver tumor model. J Vasc Interv Radiol. 2012;23:553–61. doi: 10.1016/j.jvir.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.