Abstract
Long-term dopamine replacement therapy with L-DOPA in Parkinson’s disease often leads to the development of abnormal involuntary movements known as L-DOPA-induced dyskinesia. Growing evidence suggests that following dopamine cell loss, serotonin neurons acting as surrogates for dopaminergic processes, take up L-DOPA, convert it to dopamine and release it in an unregulated fashion that precipitates dyskinesia. While most studies have focused on serotonin 5-HT1 receptor stimulation as an anti-dyskinetic strategy, targeting serotonin transporter modulation of dopamine activity has been overlooked. Therefore, in the current study, selective serotonin reuptake inhibitors were tested for their ability to reduce L-DOPA- and apomorphine-induced dyskinesia. In experiments 1 and 2, hemi-parkinsonian rats were primed with L-DOPA until stable dyskinesia developed. Rats in experiment 1 were administered the selective serotonin reuptake inhibitors paroxetine, citalopram or fluoxetine, followed by L-DOPA. Abnormal involuntary movements and forepaw adjusting steps were recorded to determine the effects of these compounds on dyskinesia and motor performance, respectively. Brains were collected on the final test day, after which striatal and raphe monoamines were examined via high performance liquid chromatography. In experiment 2, dyskinesias were measured after selective serotonin reuptake inhibitors and apomorphine. Serotonin reuptake inhibitors dose-dependently attenuated L-DOPA- but not apomorphine-induced dyskinesia, while preserving L-DOPA efficacy. Neurochemically, serotonin transporter inhibition enhanced striatal and raphe serotonin levels and reduced its turnover, indicating a potential mechanism of action. The present results support targeting serotonin transporters to improve Parkinson’s disease treatment and provide further evidence for the role of the serotonin system in L-DOPA’s effects.
Keywords: Abnormal involuntary movements, Dopamine, Parkinson’s disease, Selective serotonin reuptake inhibitors
INTRODUCTION
Since its discovery in the 1960’s, dopamine (DA) replacement therapy with L-DOPA has remained the gold-standard symptomatic treatment for Parkinson’s disease (PD) (Birkmayer and Hornykiewicz, 1961; Smith et al., 2012). Unfortunately, its chronic administration leads to debilitating abnormal involuntary movements (AIMs) termed L-DOPA-induced dyskinesias (LID) that occur in nearly 90% of PD patients within a decade (Ahlskog and Muenter, 2001). The preponderance of LID in late PD has led to a concerted effort to identify potential adjuncts with favorable anti-dyskinetic profiles that also maintain L-DOPA’s anti-parkinsonian efficacy.
Several pre- and post-synaptic mechanisms likely contribute to LID development and expression, including abnormal motor learning in corticostriatal afferents, fluctuations in striatal DA and enhanced striatal DA receptor signaling (de la Fuente-Fernandez et al., 2004; Lindgren et al., 2010; Feyder et al., 2011; Huang et al., 2011). Interestingly, compelling evidence has implicated raphestriatal serotonin (5-HT) neurons as a major source of striatal DA in the parkinsonian brain, as they contain the requisite transport and enzymatic machinery to take up, convert and release L-DOPA-derived DA (Arai et al., 1995; Maeda et al., 2005). Unfortunately, these neurons also lack DA transporters and D2 autoreceptors and are thus incapable of regulating exaggerated DA efflux that portends LID (Carta et al., 2007; Eskow et al., 2009; Navailles et al., 2010).
As such, current 5-HT anti-dyskinetic strategies have focused on stimulation of 5-HT1A and/or 5-HT1B receptors to moderate release of L-DOPA-derived DA (Munoz et al., 2008; Zhang et al., 2008; Bishop et al., 2009; Eskow et al., 2009). However, accumulating evidence points to a potential role for the 5-HT transporter (SERT) in DA transmission (Yamato et al., 2001; Kannari et al., 2006; Navailles et al., 2010; Larsen et al., 2011), implicating a mechanism by which LID might be modulated. Indeed, Rylander et al., 2011 recently reported that SERT is upregulated in striata of dyskinetic rats, monkeys, and humans, while others have demonstrated that removal of SERT-expressing terminals from raphestriatal projections reduces LID (Carta et al., 2007; Eskow et al., 2009). Despite preliminary support for the hypothesis that selective 5-HT reuptake inhibitors (SSRI) may alter LID (Durif et al., 1995; Chung et al., 2005; Dekundy et al., 2007; Kuan et al,. 2008) this has not been systematically addressed.
Therefore, in the current series of studies, we examined the effects of the well-known and oft employed antidepressant SSRIs: paroxetine, citalopram, and fluoxetine against L-DOPA-induced AIMs, their potential modulation of L-DOPA’s anti-parkinsonian efficacy and their effects on DA agonist-induced dyskinesia with apomorphine. Finally, tissue levels of striatal and raphe monoamines were measured by high performance liquid chromatography (HPLC) to examine neurochemical correlates to behavior.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats were used (225–250 g upon arrival; Taconic Farms, NY, USA). Animals were housed in plastic cages (22 cm high, 45 cm deep and 23 cm wide) and had free access to standard lab chow (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony room was maintained on a 12/12 hr light/dark cycle (lights on at 0700 hrs) at a temperature of 22–23° C. Animals were maintained and protocols were approved in accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academic Press; NIH publication number 85–23, revised 1996).
6-Hydroxydopamine lesion surgeries
One week after arrival, all rats (N=38) received unilateral 6-hydroxydopamine (6-OHDA; Sigma, St. Louis, MO, USA) lesions of the left medial forebrain bundle to destroy DA neurons (Bishop et al. 2009). Desipramine HCl (25 mg/kg, ip; Sigma) was given 30 min prior to 6-OHDA injection to protect the loss of NE neurons. Rats were anesthetized with inhalant isoflurane (2–3%; Sigma) in oxygen (2.5 L/min), and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The coordinates for 6-OHDA injections were AP: -1.8 mm, ML: +2.0 mm, DV: -8.6 mm relative to bregma with the incisor bar positioned 3.3 mm below the interaural line (Paxinos & Watson, 1998). Using a 10 µl Hamilton syringe attached to a 26 gauge needle, 6-OHDA (12 µg) dissolved in 0.9% NaCl + 0.1% ascorbic acid was infused through a small burr hole in the skull at a rate of 2 µl/min for a total volume of 4 µl. The needle was withdrawn 5 min later. Following surgery, all rats were placed in clean cages on a warming pad for recovery, after which they were returned to group housing (2 rats/cage). Soft chow was provided as needed to facilitate recovery during the first week after surgery. To minimize pain, rats were injected with buprenorphine (0.03 mg/kg) prior to surgery, 6 hrs after surgery, and the following morning. All rats were allowed to recover for 3 weeks before testing commenced.
Pharmacological Treatments and Design
L-DOPA priming
Three weeks after lesion surgery, all rats were administered L-DOPA methyl ester (12 mg/kg, s.c.; Sigma) + DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride (benserazide; 15 mg/kg, s.c.; Sigma), hereafter denoted as L-DOPA, once daily for 7 days to induce stable and reliable LID (Putterman et al., 2007; Bishop et al. 2009). L-DOPA and benserazide were dissolved in Vehicle (0.9% NaCl containing 0.1% ascorbic acid) and administered at a volume of 1 mL/kg. All lesioned rats displaying a cumulative axial, limb, and orolingual AIMs (ALO AIMs) score of >30 on the 7th day of priming were used in the current study (29 of 38). Experiments outlined below continued on day 8 and testing occurred every 3rd or 4th day until studies were completed.
Experiment 1
Effects of SSRIs on L-DOPA-induced AIMs and rotations
To determine whether 5-HT transporter inhibition modified L-DOPA-induced ALO AIMs and rotations, one group of rats (n=12) received the various counterbalanced doses of the SSRIs citalopram (vehicle, 1, 3 and 5 mg/kg, i.p.) and then paroxetine (vehicle, 0.3, 0.5 and 1.25 mg/kg, i.p.) in a within subjects design. Another group of rats (n=11) received various counterbalanced doses of fluoxetine (vehicle, 5, 10 and 20 mg/kg, i.p.) in a within subjects design. During test days, 30 min after SSRI treatments, L-DOPA (6 mg/kg + 15 mg/kg benserazide, s.c.) was administered and AIMs and rotations were scored every 10 min for 3 h.
Effects of SSRIs on L-DOPA efficacy
To determine whether the anti-parkinsonian efficacy of L-DOPA was compromised by SERT inhibition, rats in the aforementioned groups were also tested for alterations in motor performance. To do so, one group received counterbalanced doses of citalopram (vehicle, 3 and 5 mg/kg, i.p.) and then, 1 week later, to allow a washout period, paroxetine (vehicle, 0.5 or 1.25 mg/kg, i.p.). The other group received counterbalanced doses of fluoxetine (vehicle, 10, 20 mg/kg, i.p.) 30 min prior to 6 mg/kg L-DOPA 60 min after which L-DOPA efficacy was examined using the forepaw adjusting steps test (FAS; Bishop, et al. 2009; Chang et al. 1999).
Effects of DA lesions, L-DOPA and SSRIs on striatal and raphe monoamines
In order to better understand the neurochemical mechanism(s) by which SSRIs may be modifying behavior, rats from experiment 1 were divided in 3 equally dyskinetic groups (n=7–8/group) and were randomly assigned to treatment with either vehicle + vehicle, vehicle + L-DOPA (6 mg/kg, s.c.) or citalopram (5 mg/kg, i.p.) + L-DOPA (6 mg/kg, s.c.) with injections 1 and 2 spaced 30 min apart. Sixty min after the second injection, rats were decapitated, the left and right striata and the dorsal raphe were dissected, and tissue was immediately flash frozen for subsequent analysis of monoamine and metabolite content by high performance liquid chromatography (HPLC).
Experiment 2
Dose-dependent effects of apomorphine on AIMs and rotations
Apomorphine, a non-selective DA receptor agonist has been shown to induce dyskinesia in animal models of PD and PD patients by direct DA receptor stimulation (Di Chiara and Gessa, 1978; Verhagen Metman, 1997; Fox et al., 2001; Papathanou et al., 2009). To establish a dose response for apomorphine, L-DOPA primed rats (n=6) were injected with various doses of the compound (vehicle, 0.025, 0.05, 0.1 mg/kg, s.c) in a counterbalanced within subjects design. On test days, AIMs and rotations were scored every 10 min for 90 min.
Effects of SSRIs on apomorphine-induced AIMs and rotations
In order to examine whether the anti-dyskinetic effects of SSRIs were acting pre- or post-synaptically, doses of paroxetine (0.5) citalopram (3) and fluoxetine (10) shown to reduce L-DOPA-induced AIMs in experiment 1 were tested against apomorphine. Rats from the aforementioned dose-response study received SSRIs 30 min prior to apomorphine (0.05 mg/kg, s.c.) in a counterbalanced, within subjects design. On test days, AIMs and rotations were scored every 10 min for 90 min.
Behavioral Analyses
AIMs and Rotations
Rats were monitored for AIMs using a procedure described previously (Lindenbach et al., 2011) and similar to that initially depicted by Lundblad et al. (2002) with slight modifications. On test days (0900–1400 hrs), rats were individually placed in plexiglass cylinders (22.2 cm diameter, 25.4 cm height; Thermo Fisher Scientific, Rochester, NY, USA) 5 min prior to pretreatments. Following L-DOPA injection, a trained observer blind to treatment condition assessed each rat for exhibition of axial, limb, and orolingual AIMs. Each new rater was trained for a minimum of 3 sessions and then correlated with a well-trained instructor. A correlation of ≥ 90% with the instructor is required before new raters can score AIMs. In addition, contralateral rotations, defined as complete 360 degree turns away from the lesioned side of the brain, were tallied. Dystonic posturing of the neck and torso, involving positioning of the neck and torso in a twisted manner directed toward the side of the body contralateral to the lesion, were referred to as “axial” AIMs. “Forelimb” AIMs were defined as rapid, purposeless movements of the forelimb located on the side of the body contralateral to the lesion. “Orolingual” AIMs were composed of repetitive openings and closings of the jaw and tongue protrusions. The movements are considered abnormal since they occur at times when the rats are not chewing or gnawing on food or other objects. For the L-DOPA dose response experiment, ALO AIMs and rotations were recorded for 1 min, every 20th min for 2 hrs. For all other experiments, AIMs and rotations were recorded for 1 min, every 10th min for 3 hrs. During the AIMs observation period a severity score of 0–4 was assigned for each AIMs category: 0= not present, 1= present for less than 50% of the observation period (i.e., 1–29 sec), 2= present for more than 50% or more of the observation period (i.e., 30–59 sec), 3 = present for the entire observation period (i.e., 60 sec) and interrupted by a loud stimulus (a tap on the cylinder), or 4 = present for the entire observation period but not interrupted by a loud stimulus. For each AIMs category, the scores were summed for the entire 2 or 3 hr period.
Forepaw Adjusting Steps
The FAS test is a measure of forelimb akinesia and rats with >80% unilateral DA depletion perform poorly with the lesioned forepaw (Chang et al. 1999). L-DOPA reduces this deficit so the test can be used to determine if an L-DOPA adjunct is interfering with the relief of PD symptoms provided by L-DOPA (Eskow et al. 2007). To do so, an experimenter held the rat’s hindlimbs and one forelimb such that the free forelimb was forced to bear the rat’s body weight. Rats were then moved laterally for 90cm over 10 sec across a marked surface while another experimenter counted the number of steps taken in the forehand direction (defined as movement toward the rat’s midline) and backhand direction (movement distal to the rat’s midline). Each FAS test consisted of 3 backhand and 3 forehand trials with each limb, for a total of 12 trials per rat. The score for percent intact stepping was derived by taking the sum of the total steps with the lesioned forepaw dividing that number by total steps with the unlesioned forepaw and multiplying the outcome by 100. Lower percent intact scores indicate greater forelimb akinesia. Prior to FAS testing with the compounds of interest, all rats received 3 separate acclimation periods to reduce potential practice effects.
High performance liquid chromatography
To determine the cerebral levels of monoamines, their metabolites, and monoamine turnover (a ratio of metabolite/monoamine), tissue samples were prepared for high performance liquid chromatography (HPLC). Each sample was homogenized in a solution of ice-cold perchloric acid (0.1 M), 1% ethanol, and 0.02% EDTA. Subsequent homogenates were spun for 30 min at 14,400 g with the temperature maintained at 4°C. Aliquots of supernatant were then analyzed for abundance of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-hydroxyindole-3-acetic acid (5-HIAA) using reverse-phase HPLC coupled to electrochemical detection according to the protocol of Kilpatrick et al. (1986). The system employed consisted of an ESA autoinjector (Model 542), an ESA solvent delivery system (Model 582), an ESA Guard-Pak column, a C-18 (100×4.6mm, 5 μm packing) column (ESA), a Coulochem III electrochemical detector (ESA) connected to an analytical cell (ESA model 5011A) located immediately after the column, an ESA Model 5020 guard cell positioned prior to the autoinjector, and an external pulse dampener (ESA). Samples were chromatographically separated using a mobile phase composed of 90 mM sodium dihydrogen phosphate (monobasic, anhydrous), 0.05 mM EDTA, 1.7 mM octane sulfonic acid, 10% (v:v) acetonitrile, and adjusted to pH 3.0 with o-phosphoric acid. The first electrode of the analytical cell was set to a potential of −100 mV and the second electrode to +250 mV. The guard cell had a potential of 350 mV. As monoamines and metabolites eluted from the column and passed through the analytical cell, they were oxidized at the second electrode and a spike in current was generated. This signal was recorded as a trace of electrode current versus time by EZChrom Elite software via a Scientific Software, Inc. (SS420χ) module. Each compound was detected as a peak in this trace, and the EZChrom Elite software was used to calculate peak areas. Peak areas were converted to picograms (pg) of compound using a standard curve made from samples of known monoamine and metabolite concentrations (1e-6 M to 1e-9 M), normalized to striatal and raphel tissue weights, and expressed as picograms (pg) of compound per milligrams (mg) of tissue (mean ± standard error of the mean; S.E.M.).
Statistical Analyses
All parametric data (HPLC, rotations and FAS) were expressed as mean ± standard error of the mean (S.E.M.). Non-parametric ALO AIMs data were expressed as medians ± median absolute deviation (M.A.D.). HPLC-derived striatal and raphe monoamine and metabolite levels and turnover ratios were analyzed by a 2-factor (Side × Treatment) and 1-factor (Treatment) analyses of variance (ANOVA), respectively. ALO AIMs were analyzed by nonparametric Friedman ANOVAs at each time point or over the 3 hr period. When appropriate, parametric Fisher LSD or nonparametric Wilcoxon post hoc tests were employed. All statistical analyses were performed with the use of Statistica software version 10 (Statsoft Inc., Tulsa, OK). Alpha was set at 0.05.
RESULTS
Experiment 1
SSRIs dose dependently attenuate L-DOPA-induced ALO AIMs and rotations
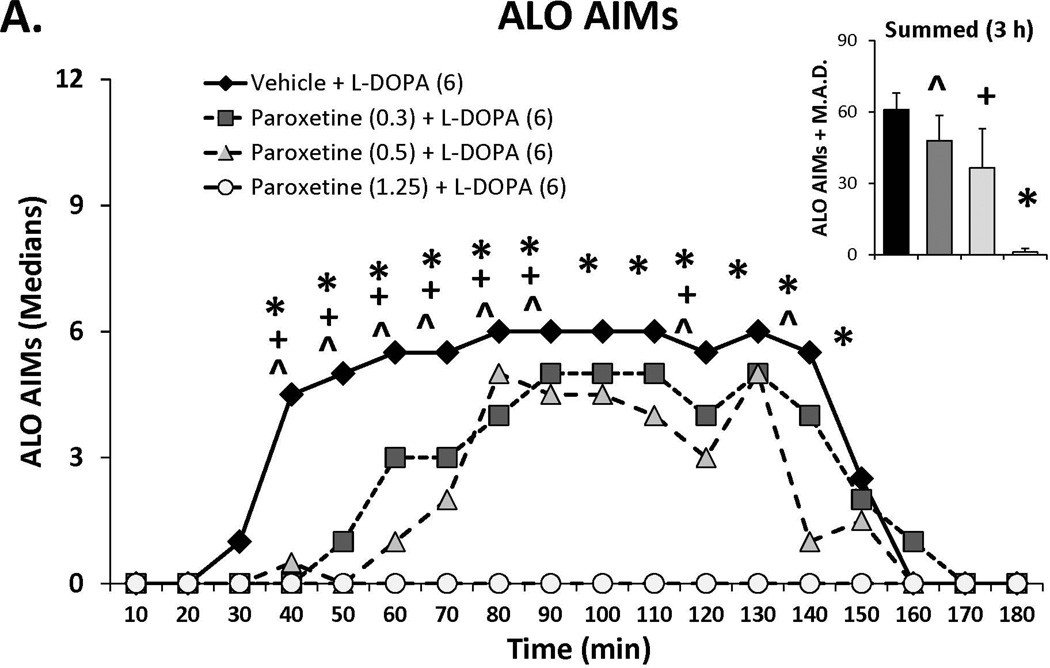
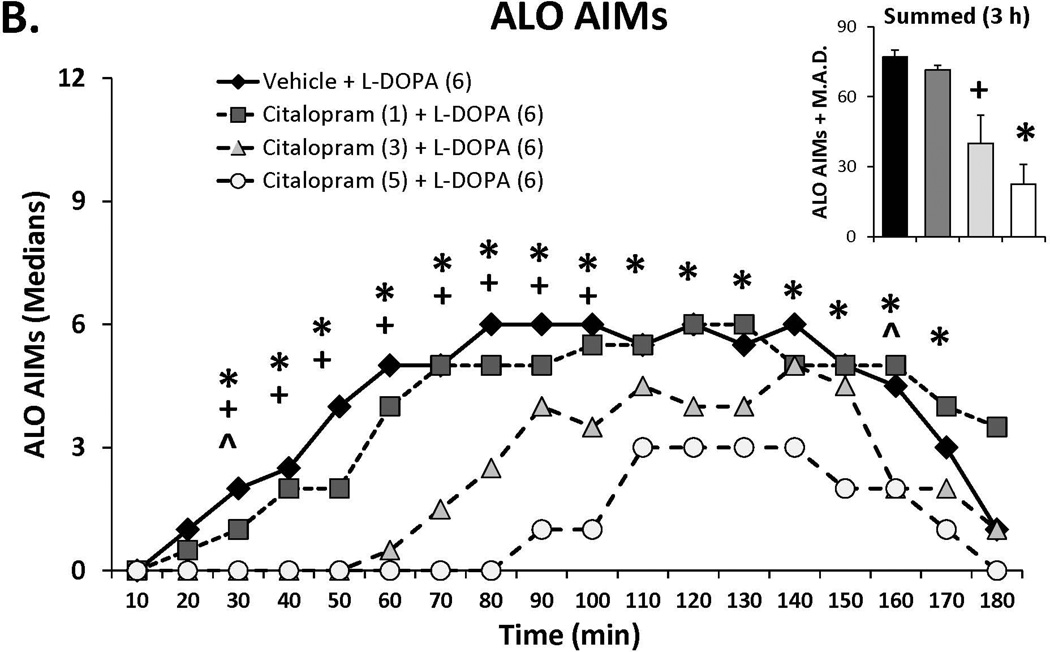
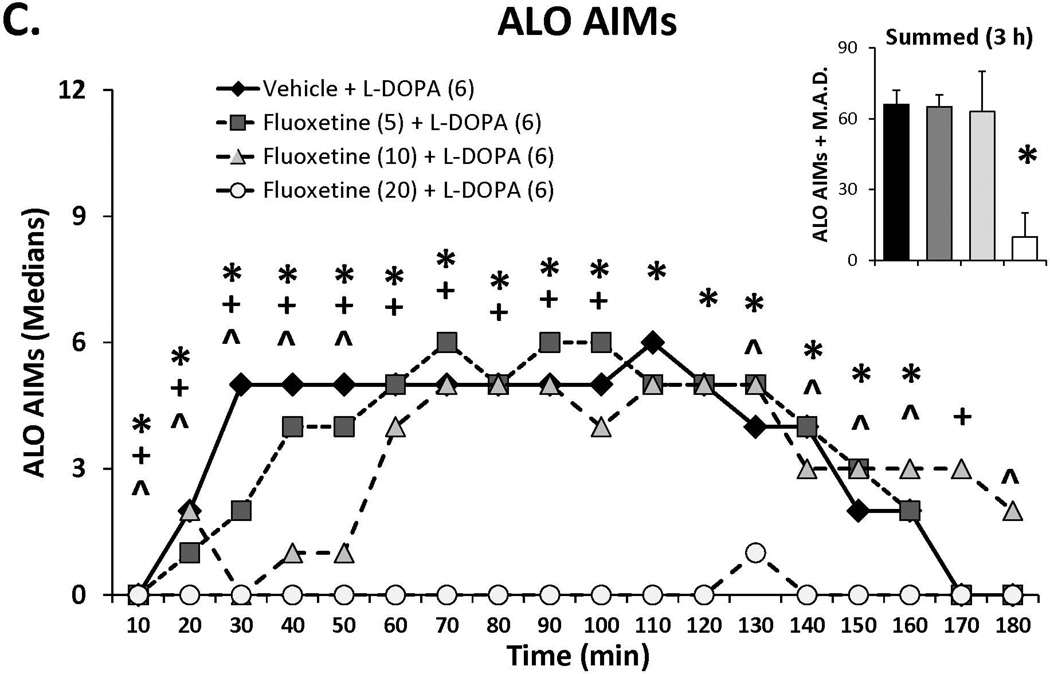
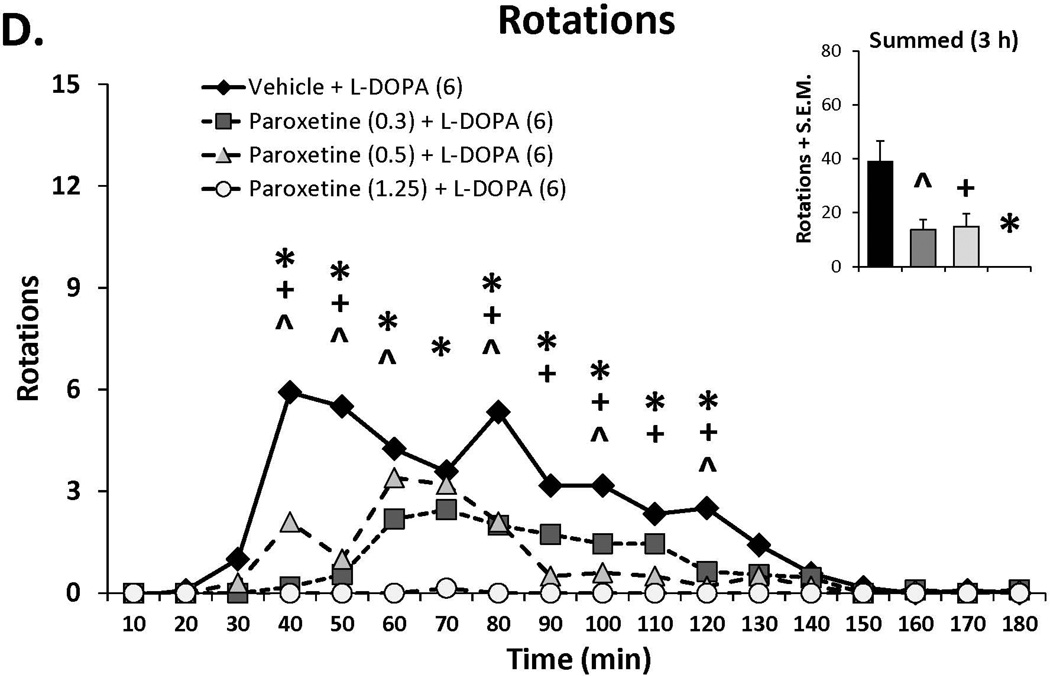
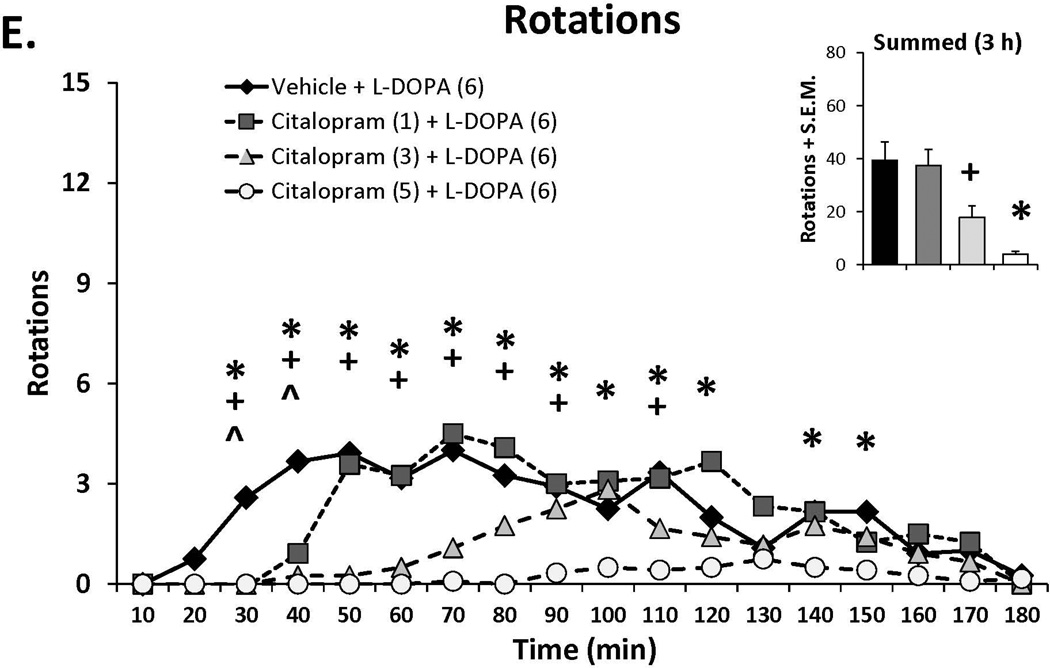
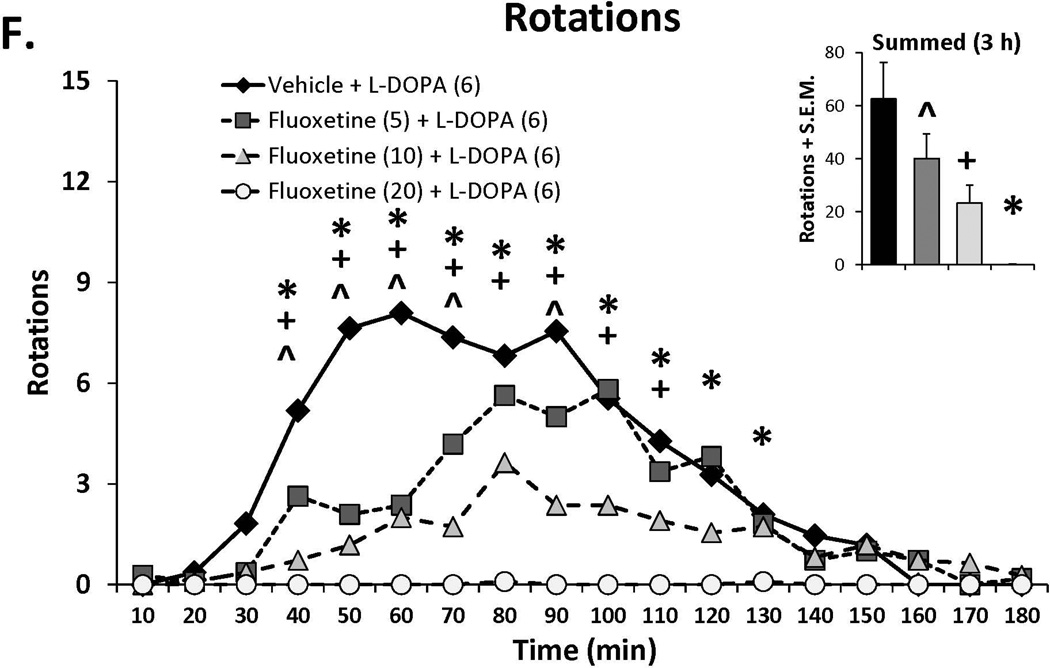
Three doses of each SSRI were tested in L-DOPA-primed rats to determine their effects on L-DOPA-induced AIMs and rotations. Statistical analyses of dose (bar graph insets) and dose at each time point (time courses), depicted in Figure 1A–C, revealed that all SSRIs dose- and time-dependently reduced ALO AIMs. (all χ2, df 3 >9.8, all p<0.05). At each dose tested, paroxetine pre-treatment attenuated LID with increasing doses enhancing and prolonging the anti-dyskinetic effects. Citalopram also reduced ALO AIMs in a dose- and time-dependent manner though the lowest dose was only mildly effective. The effects of fluoxetine pretreatment were similar to paroxetine and citalopram, with the reduction in LID being most pronounced with the highest dose. Paroxetine, citalopram and fluoxetine (Figure 1D–F) also modified L-DOPA-induced rotations with analyses demonstrating dose × time interactions for each (F(51,459)=3.20, p<0.01; F(51,561)=3.60, p<0.01; F(51,510)=4.06, p<0.01, respectively). The pattern of these effects was, by in large, a reflection of those seen for ALO AIMs, though fluoxetine produced more pronounced effects on rotations.
Figure 1.
Effects of the serotonin reuptake inhibitors (SSRIs) paroxetine (A,D), citalopram (B,E) and fluoxetine (C,F) on L-DOPA-induced abnormal involuntary movements (AIMs; A–C) and rotations (D–F). Rats were treated with vehicle, paroxetine (0.5 or 1.25 mg/kg, i.p.), citalopram (3 or 5 mg/kg, i.p.) or fluoxetine (10 or 20 mg/kg, i.p.) 30 minutes prior to vehicle or L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.). Summed axial, limb and orolingual (ALO) AIMs and rotations were evaluated every 10 minutes for 3 hours after L-DOPA. Values are expressed as medians (AIMs+median absolute difference) or means (+standard error of the mean). Significant differences were determined by non-parametric Friedman ANOVAs with Wilcoxon post-hocs (AIMs) or 2-way repeated measures ANOVAs (rotations) and Fischer LSD post-hocs. *p<0.05 vehicle + L-DOPA vs. paroxetine (1.25), citalopram (5) and fluoxetine (20) + L-DOPA, +p<0.05 vs. vehicle + L-DOPA vs. paroxetine (0.5), citalopram (3) and fluoxetine (10) + L-DOPA, ^p<0.05 vs. vehicle + L-DOPA vs. paroxetine (0.3), citalopram (1) and fluoxetine (5) + L-DOPA.
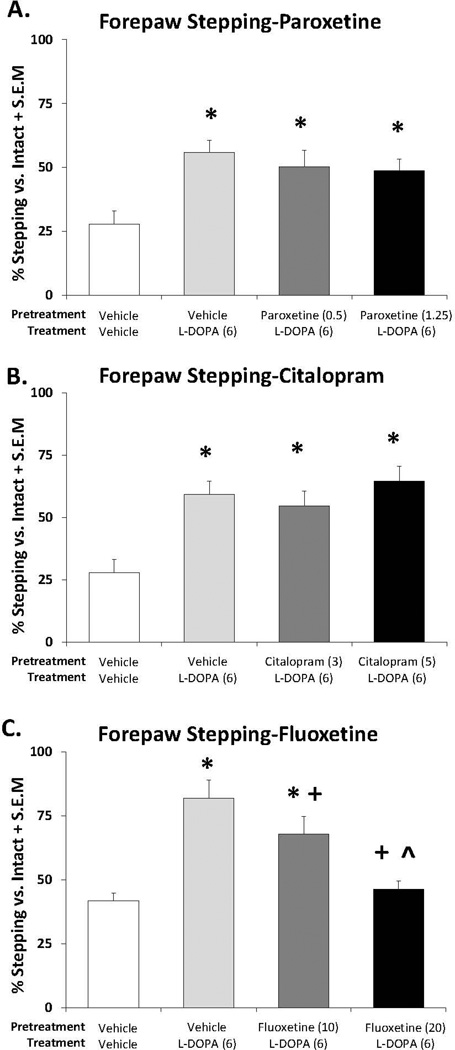
L-DOPA efficacy is not altered by SSRI administration
In order to determine whether the anti-parkinsonian efficacy was altered by SSRI pretreatment, rats were pretreated with anti-dyskinetic doses of each compound followed by L-DOPA. Motor performance using the FAS was measured 60 min later. Analyses of the results shown in Figure 2A–C demonstrated main effects of treatment for paroxetine (F(3,33)=11.24, p<0.001), citalopram (F(3,33)=8.36, p<0.001) and fluoxetine (F(3,30)=14.84, p<0.001). Further post-hoc analyses revealed that L-DOPA significantly reversed lesion-induced stepping deficits (p<0.05) and that both doses of paroxetine and citalopram, when combined with L-DOPA, maintained this anti-parkinsonian effect (p<0.05 vs. vehicle treatments). Interestingly, while the lower dose of fluoxetine did maintain L-DOPA’s efficacy above vehicle treatment (p<0.05), the higher dose squelched anti-parkinsonian effects.
Figure 2.
Effects of the serotonin reuptake inhibitors (SSRIs) paroxetine, citalopram and fluoxetine on L-DOPA reversal of motor deficit on the forepaw adjusting steps test. Rats were treated with vehicle, paroxetine (0.5 or 1.25 mg/kg, i.p.), citalopram (3 or 5 mg/kg, i.p.) or fluoxetine (10 or 20 mg/kg, i.p.) 30 minutes prior to vehicle or L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.). Forepaw adjusting steps were evaluated 60 minutes after final treatments. Values (as means ± S.E.M.) are expressed as percent stepping with the lesioned forepaw vs. intact forepaw. Significant differences were determined by 1-way ANOVAs for each SSRI. When appropriate, treatment differences were analyzed with Fischer LSD post-hocs. *p<0.05 vs. vehicle + vehicle, +p<0.05 vs. vehicle + L-DOPA ^p<0.05 vs. fluoxetine (10) + L-DOPA.
DA lesions, L-DOPA and SSRIs alter striatal and raphe monoamines
HPLC analysis of striatal and raphe monoamines was used to examine the possible effects of lesion, L-DOPA and SSRI administration in unilateral 6-OHDA lesioned, L-DOPA-primed rats (n=7–8/group). Subjects were killed 60 min after receiving: vehicle treatments, vehicle and L-DOPA or citalopram (5 mg/kg, i.p.) and L-DOPA (6 mg/kg, s.c.). Results are shown in Table 1.
Table 1.
Effects of unilateral 6-hydroxydopamine (6-OHDA) lesions and systemic drug treatments on concentrations of dopamine (DA), 3,4-Dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT) and 5-Hydroxyindoleacetic acid (5-HIAA) and their turnover in the striatum or dorsal raphe of rats (n=7–8/group). Rats were treated with vehicle or the serotonin reuptake inhibitor citalopram (5 mg/kg, i.p.) 30 minutes prior to vehicle or L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.). Rats were killed 60 minutes after final treatments. Values (as means ± S.E.M.), determined by high performance liquid chromatography were expressed as picograms monoamine or metabolite per milligram wet weight tissue, % intact (in parentheses) or metabolite/monoamine for estimates of turnover. Differences between sides (striata contralateral vs. ipsilateral to lesion), treatments and side × treatment interactions were determined by 1 and 2-way ANOVAs. Individual cell differences were analyzed with Fischer LSD post-hocs.
| STRIATUM | Treatment | DA | DOPAC | DA turnover | 5-HT | 5-HIAA | 5-HT turnover |
|---|---|---|---|---|---|---|---|
| Lesion Side | |||||||
| Contralateral (Control) | Vehicle + Vehicle | 14428±707 | 3336±292 | 0.24±0.03 | 616±66 | 633±32 | 1.09±0.11 |
| Vehicle + L-DOPA | 17228±1130 | 4345±599 | 0.25±0.03 | 506±27 | 673±46 | 1.33±0.05 | |
| Cit (5) + L-DOPA | 17888±1156 | 4054±198 | 0.23±0.01 | 683±57 | 564±40 | 0.84±0.03 | |
| Significant Main effects | Side, Treatment | Side | Side | Side, Treatment | Side, Treatment | Side, Treatment | |
| Ipsilateral (Lesion) | Vehicle + Vehicle | 85±23 (0.6%) | 52±14 (1.6%) | 0.77±0.19 (321%) | 472±57 (77%) | 871±70 (138%) | 2.14±0.47 (196%) |
| Vehicle + L-DOPA | 357±133 (2.1%) | 261±54 (6.0%) | 1.10±0.24 (440%) | 459±34 (91%) | 926±51 (138%) | 2.11±0.26 (157%) | |
| Cit (5) + L-DOPA | 299±35 (1.7%) | 183±32 (4.5%) | 0.66±0.12 (287%) | 653±75 (96%) | 712±49 (126%) | 1.17±0.15 (139%) | |
| DORSAL RAPHE | Treatment | DA | DOPAC | DA turnover | 5-HT | 5-HIAA | 5-HT turnover |
| Lesion Side | |||||||
| Midline Structure | Vehicle + Vehicle | 581±48 | 139±13 | 0.24±0.03 | 2286±120 | 2247±220 | 0.99±0.09 |
| Vehicle + L-DOPA | 802±32 | 373±54 * | 0.46±0.06* | 2252±198 | 2809±164 | 1.31±0.17* | |
| Cit (5) + L-DOPA | 1219±202 *+ | 405±51 * | 0.38±0.07 | 3773±625 *+ | 2067±272 | 0.60±0.07*+ | |
p<0.05 vs. vehicle + vehicle,
p<0.05 vs. vehicle + L-DOPA.
Within the striatum, analyses of DA revealed main effects of lesion side (F(1,20)=763.77, p<0.001) and treatment (F(2,20)=3.93, p<0.05) indicating DA levels were reduced by lesion and that L-DOPA treatment increased DA regardless of citalopram. Striatal DOPAC was also reduced by lesion (F(1,20)=310.63, p<0.001), though no significant effects of treatment were observed. A main effect of side (F(1,20)=35.79, p<0.001), demonstrated that DA turnover was increased preferentially in the DA lesioned hemisphere (p<0.05), Striatal 5-HT tissue content was altered significantly by lesion (F(1,20)=4.35, p<0.05) and treatment (F(2,20)=3.79, p<0.05). Post hoc analyses showed lesion reduced overall 5-HT levels, while citalopram increased 5-HT regardless of hemisphere, in corroboration of its putative pharmacological actions. Striatal 5-HIAA was also altered by lesion and treatment (F(1,20)=33.94, p<0.01; F(2,20)=4.77, p<0.05, respectively). Post hoc analysis of these main effects demonstrated that lesion increased while, citalopram reduced 5-HIAA. Analysis of striatal 5-HT turnover demonstrated both side (F(1,20)=18.73, p<0.001) and treatment (F(2,20)=5.49, p<0.05) main effects. Further post hocs indicated that lesion increased turnover and citalopram treatment suppressed it (all p<0.05).
In order to better understand neurochemical alterations at 5-HT cell bodies, monoamine tissue content was also examined in the dorsal raphe of these same rats. Main effects of treatment were found for DA (F(2,20)=6.83, p<0.01), DOPAC (F(2,20)=11.94, p<0.001) and DA turnover (F(2,20)=4.12, p<0.05). 5-HT (F(2,20)=4.84, p<0.05) and 5-HT turnover (F(2,20)=11.79, p<0.001) main effects were also observed. Raphe DA levels were found to be significantly increased by combined citalopram + L-DOPA treatment, DOPAC levels were increased in raphe regardless of citalopram (all, p<0.05) and L-DOPA treatment increased DA turnover. Similar to the striatum, citalopram, when combined with L-DOPA, augmented 5-HT levels in raphe tissue (p<0.05). 5-HT turnover was increased by L-DOPA and significantly reduced by citalopram co-administration (p<0.05), again supporting the actions of this SSRI on 5-HT neurotransmission.
Experiment 2
Apomorphine dose-dependently induces AIMs
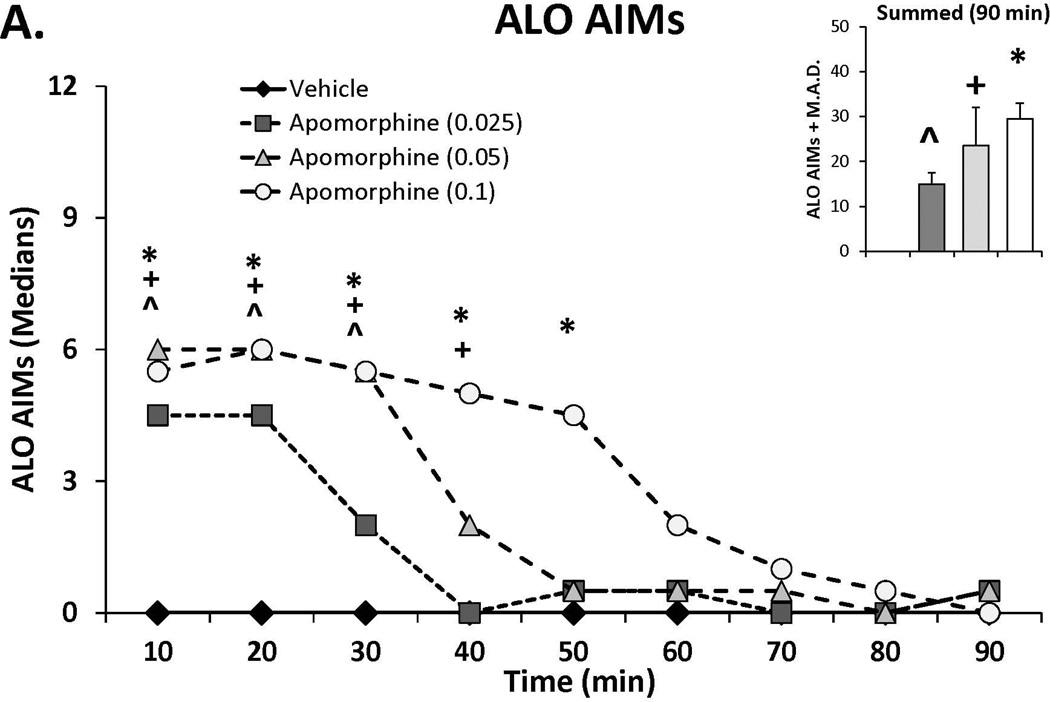
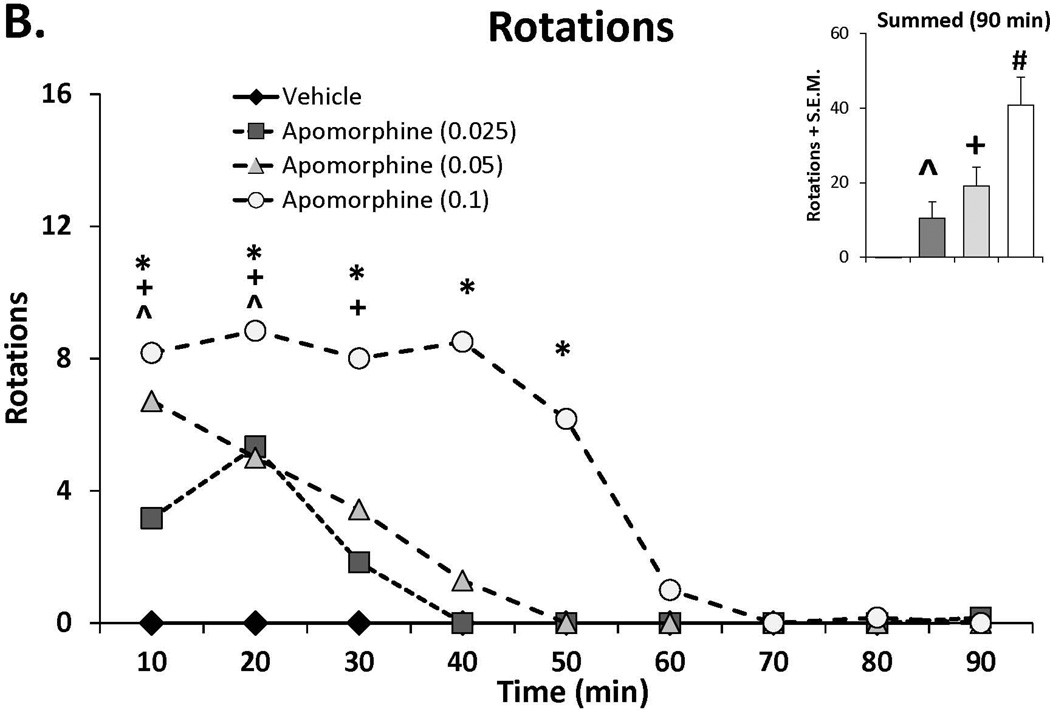
As a way to determine whether the anti-dyskinetic effects of SSRIs occur via post-synaptic influences, a dose-response for AIMs and rotations induced by the non-selective DA agonist apomorphine was first established. As shown in Figure 3A and 3B, apomorphine dose- and time-dependently induced ALO AIMs and rotations as indicated by main effects of treatment for ALO AIMs (χ2, df 3 = 14.60; p<0.01) and rotations (treatment effect: F(3,15)=26.33, p<0.001), as well as treatment by time effects from 10–50 min post-injection (AIMs: χ2, df 3 >12.0; all p<0.05; rotations: F(24,120)=3.94, all post hocs, p<0.001). Based on this dose response, a test dose of 0.05 mg/kg (s.c.) apomorphine was chosen for the final experiment.
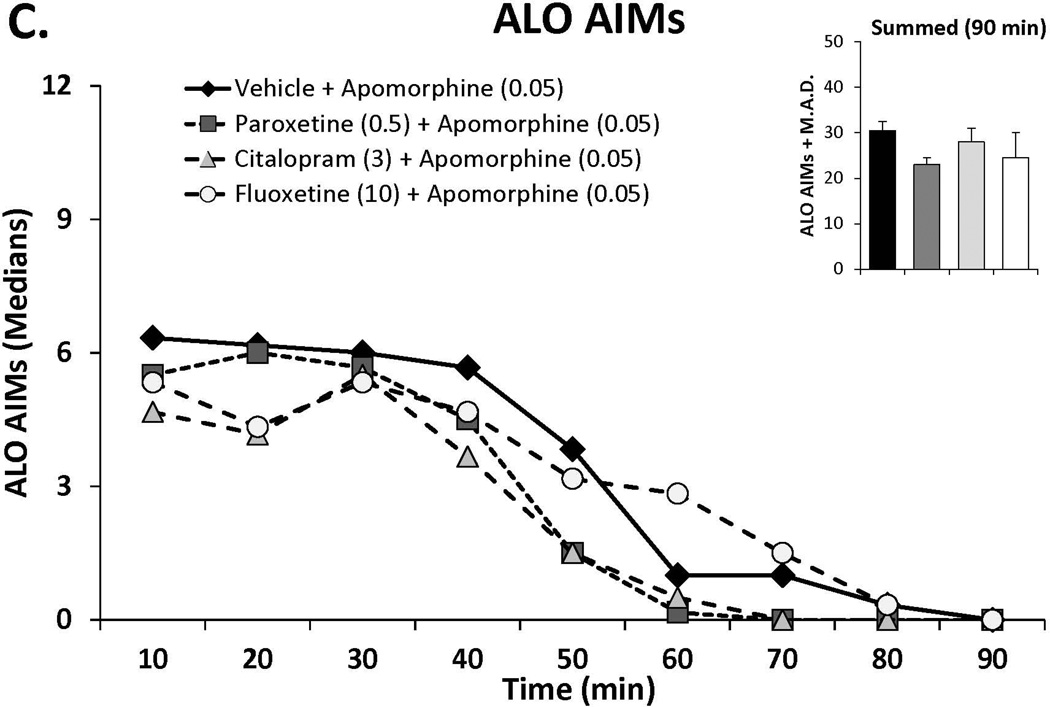
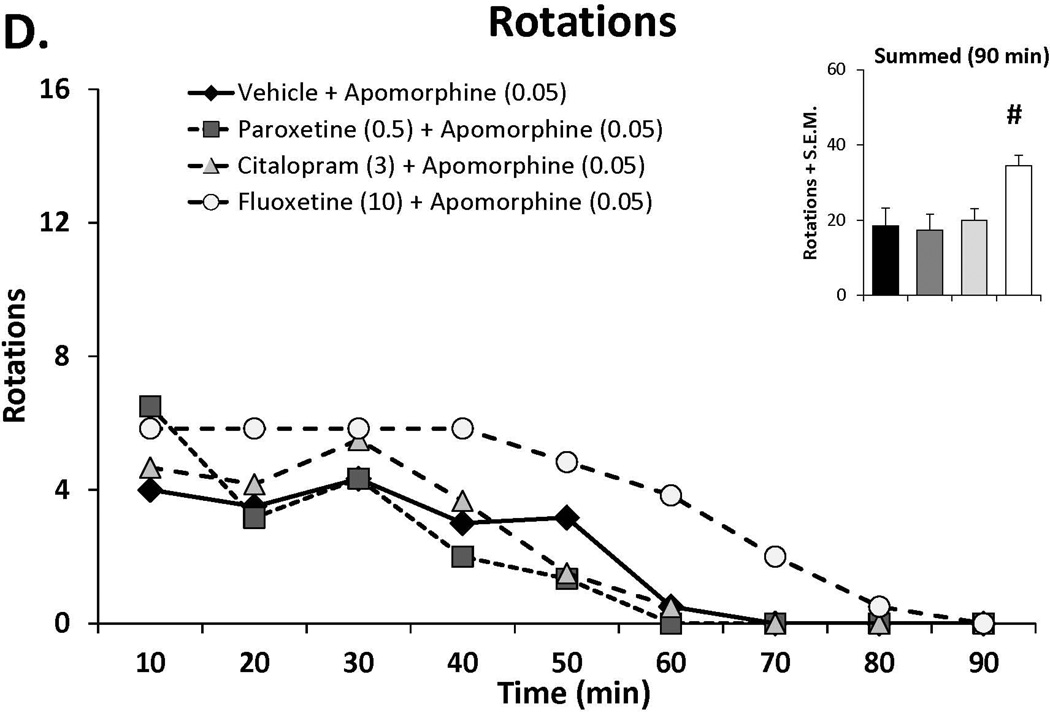
Figure 3.
The effects the direct dopamine receptor agonist apomorphine (A,B) and their modulation by serotonin reuptake inhibitors (SSRIs) paroxetine, citalopram and fluoxetine (C,D) on abnormal involuntary movements (AIMs; A,C) and rotations (B,D). In experiment 1, rats (n=6) were treated with vehicle or apomorphine (0.025, 0.05 or 0.1 mg/kg, s.c.). In experiment 2 rats were pretreated with vehicle, paroxetine (0.5 mg/kg, i.p.), citalopram (3 mg/kg, i.p.) or fluoxetine (10 mg/kg, i.p.) 30 minutes prior to apomorphine (0.05 mg/kg, s.c.). Summed axial, limb and orolingual (ALO) AIMs and rotations were evaluated every 10 minutes for 90 minutes after apomorphine. Values are expressed as medians (AIMs+median absolute difference) or means (+standard error of the mean). Significant differences were determined by non-parametric Friedman ANOVAs with Wilcoxon post-hocs (AIMs) or 2-way repeated measures ANOVAs (rotations) and Fischer LSD post-hocs. *p<0.05 vehicle vs. apomorphine (0.1) +p<0.05 vehicle vs. apomorphine (0.05), ^p<0.05 vehicle vs. apomorphine (0.025) #p<0.05 vs. all other treatments.
SSRIs do not alter apomorphine-induced AIMs
Doses of paroxetine (0.5 mg/kg), citalopram (3 mg/kg) and fluoxetine (10 mg/kg), shown to reduce L-DOPA-induced AIMs were administered 30 prior to apomorphine (0.05 mg/kg), after which ALO AIMs and rotations were examined. As shown in Figure 3C, SSRI pretreatment did not alter AID at doses shown to convey anti-dyskinetic effects against L-DOPA. Surprisingly, examination of SSRI treatment on apomorphine-induced rotations (Figure 3D) revealed a main effect of treatment (F(3,15)=7.72, p<0.01), which post-hocs indicated was a result of enhanced rotations in fluoxetine-treated subjects compared to all other conditions (all p<0.05).
DISCUSSION
In the present study, several findings support further exploration of SERT inhibition as a potential adjunctive approach to L-DOPA pharmacotherapy in PD and shed light upon possible mechanism(s) of action. First, acute administration of the SSRIs citalopram, paroxetine and fluoxetine all conveyed dose-dependent anti-dyskinetic effects in L-DOPA-primed rats. Second, at anti-dyskinetic doses, the anti-parkinsonian efficacy of L-DOPA was not modified. Finally, SERT blockade did not alter dyskinesia induced by the direct DA agonist apomorphine, suggesting that SSRIs’ putative therapeutic effects likely occur by alterations in pre-synaptic processes.
Accumulating evidence over the past several years has implicated a more primary role for the 5-HT system in the development and expression of LID. 5-HT neurons can transport exogenously administered L-DOPA into the cytosol, convert it to DA via amino acid decarboxylase, package L-DOPA-derived DA into vesicles via vesicular monoamine transporters and release it as a “false neurotransmitter” (Ng et al., 1970; Arai et al., 1995; Kannari et al., 2000; Carta et al., 2007). While the contribution of 5-HT neurons to the pool of L-DOPA-derived DA is likely limited to advanced stages of PD when the majority of DA neurons have degenerated, this is also when patients are most likely to experience LID (Nutt and Nygaard, 2001; Troiano et al., 2009). Thus, in late stage PD, 5-HT neurons, which lack the autoregulatory DA transporters and DA D2 receptors, may be releasing L-DOPA-derived DA in a pulsatile and unregulated manner that leads to LID (Nutt et al., 2000; de la Fuente-Fernández et al., 2004). Therefore, anti-dyskinetic strategies have focused on ways to temper extraphysiological DA release from 5-HT neurons by targeting the inhibitory 5-HT1 agonists. Such a strategy has proven somewhat beneficial (Bonifati et al., 1994; Bara-Jiminez et al., 2005; Goetz et al., 2007), though translation of these compounds to the clinic remains an unmet goal.
Another less studied target with potential to modulate L-DOPA-derived DA neurotransmission is SERT. Striatal SERT is both upregulated in dyskinetic rats, monkeys and humans and has been shown to be accompanied by an increased density of striatal 5-HT innervation (Rylander et al., 2011). While such neuroplasticity does not establish a causal link between SERT-expressing terminals and LID, removal of 5-HT terminals from raphestriatal projections has been demonstrated to squelch LID (Carta et al., 2007; Eskow et al., 2009). Therefore, in order to better examine the potential role of SERT in LID and its treatment, the current study set out to systematically address the effects of SERT blockade on the expression of AIMs in a rodent model of dyskinesia.
In the first experiment we found that each SSRI dose- and time-dependently reduced the expression of ALO AIMs. The highest doses of each SSRI produced a >75% reduction in AIMs, however the magnitude and timing of these effects was dependent on the compound, likely reflecting differential pharmacological properties for SERT affinity (paroxetine > citalopram > fluoxetine) and SERT selectivity over DA and norepinephrine transporters (citalopram > fluoxetine > paroxetine) (Owens et al., 1997; Tatsumi et al., 1997; Bymaster et al., 2002). Interestingly, rotations were also markedly reduced by SSRIs. While the utility of measuring rotational activity remains debatable (Cenci et al., 2002), these findings may have reflected both anti-dyskinetic effects as well as alterations in L-DOPA efficacy, as discussed below. While the present work is the first systematic study of SERT inhibition on LID, several other studies have demonstrated comparable effects. For example, Dekundy et al. (2007) reported that a lower dose of fluoxetine (5 mg/kg) produced a mild, though non-significant reduction in AIMs in L-DOPA-primed rats. Kuan and colleagues (2008) found that chronic administration of citalopram, at a high dose (40 mg/kg), attenuated L-DOPA-induced AIMs and rotational activity in 6-OHDA-lesioned rats. Potential anti-dyskinetic effects of SERT blockade for LID treatment have also been reported in MPTP-treated primates (Johnston et al., 2011; Huot et al., 2012). It remains to be determined whether the pronounced anti-dyskinetic effects of SSRIs in our rat model will translate to the PD patient. To this point, the few studies that have addressed this question varied in method. Durif and colleagues (1995) reported that fluoxetine reduced AID in L-DOPA-responsive PD patients, while Chung et al. (2005), using intravenous L-DOPA, reported no significant change to LID in patients co-administered paroxetine. Further work is required to discern potential anti-dyskinetic benefit in the clinical population.
One controversial concern regarding the use of SSRI treatment in PD is the potential for deterioration of L-DOPA efficacy (Leo, 1996; Rampello et al., 2002). In the present study, this possibility was highlighted by the dose-dependent reduction in rotations observed with each SSRI. Therefore, in order to determine whether SSRI treatment also altered L-DOPA’s anti-parkinsonian properties, rats were subject to the FAS test (Schallert et al., 1979; Olsson et al., 1995; Chang et al. 1999; Eskow et al. 2007). In the present study, the pronounced motor performance deficits produced by DA lesions were significantly reversed by L-DOPA treatment. More importantly, anti-dyskinetic doses of the SSRIs maintained L-DOPA’s anti-parkinsonian efficacy. The one exception to this effect was the highest dose of fluoxetine (20 mg/kg), which appeared to attenuate L-DOPA’s beneficial effects on motor performance. This is interesting given clinical reports that fluoxetine can exacerbate PD symptoms in a small subset of patients (Caley et al., 1992; Steur, 1993; Montastruc et al., 1995). That said, most studies report either no deleterious motor consequence or improvement in motor symptoms with SSRI treatment to PD patients (Ceravolo et al., 2000; Rampello et al., 2002; Chung et al., 2005). Moreover, though qualitative in assessment, we did not observe a drug-induced suppression of motor activity in SSRI-treated rats. In fact, in the first 30 min of testing, these rats often reared, touching the sides of the cylinder with both forepaws. However across testing days, rats tended to acclimate to the environment confounding quantitative assessments. These observations corroborate previous work showing that similar SSRI doses did not alter locomotor activity in intact rats (Lightowler et al., 1994; Griebel et al., 1997; Szewczyk et al., 2009).
Though the aforementioned behavioral findings strongly support the targeting of SERT inhibition as an anti-dyskinetic strategy, the mechanisms by which these compounds convey their effects remain largely unexplored. In order to better understand this, we examined the effects of DA lesion, L-DOPA treatment and citalopram treatment (5 mg/kg) on monoamine function in the striatum and dorsal raphe. As expected, 6-OHDA injections produced severe loss of striatal DA and its metabolite DOPAC, while DA turnover was augmented by lesion, likely reflecting a compensatory response to DA loss (Zigmond et al., 1984; Dupre et al., 2007). It is notable that DA lesions also reduced 5-HT, but increased 5-HIAA and 5-HT turnover. Interestingly, citalopram corrected these imbalances in the lesioned striatum, increasing 5-HT and reducing 5-HT turnover, commensurate with SSRI’s putative pharmacological and antidepressant actions in the parkinsonian brain (Perry and Fuller, 1992; Honig et al., 2009). Lesion and intact side comparisons within the midline dorsal raphe were not possible, as cell bodies and ascending 5-HT fibers are intermixed (Bjorklund et al., 1971), however treatments affected several neurochemical indices. One of the more intriguing effects occurred following combined L-DOPA and citalopram treatment where SERT inhibition led to increased local DA levels. L-DOPA and citalopram also significantly increased raphe 5-HT levels and reduced 5-HT turnover, suggesting that increased 5-HT tone proximal to dorsal raphe neurons may lead to stimulation of local 5-HT1A autoreceptors responsible for the effects of SSRIs on LID (Kriess and Lucki, 1998; Yamato et al., 2000; Eskow et al., 2007). This is also in concert with previous work demonstrating that the anti-dyskinetic effects of the SERT-acting compounds 3,4-methylenedioxymethamphetamine and fenfluramine are conveyed in part via indirect stimulation of 5-HT1A receptors (Iravani et al., 2003; Bishop et al., 2006).
As an additional way to examine the mechanism(s) by which SERT inhibition reduces LID, we tested whether SSRIs modified dyskinesia evoked by direct DA receptor stimulation by apomorphine. This question originated from a rich literature describing the integral role of post-synaptic signaling in the development and expression of LID (for examples see Feyder et al., 2011; Gerfen and Surmeier, 2011). Under these circumstances, SSRIs did not alter AID. In fact, fluoxetine actually augmented apomorphine-induced rotations. This discrepancy between the anti-dyskinetic effects of SERT blockade for LID vs. AID along with the neurochemical results implicates presynaptic modulation of monoaminergic function as a mechanism for SSRI’s effects since direct DA receptor stimulation bypasses L-DOPA uptake, conversion and release.
Despite the clear preclinical benefits, it remains to be determined whether the pronounced anti-dyskinetic effects of SSRIs in our rat model will translate to patients. SSRI are ubiquitously employed for treatment of depression and anxiety in PD (Richard et al., 1997; Weintrab et al., 2003). Yet, despite their utility for the treatment of mood dysfunction, the few studies that have addressed SSRI effects on LID have varied in question and method. Durif and colleagues (1995) reported that fluoxetine reduced AID in L-DOPA-responsive PD patients, while Chung et al. (2005), using intravenous L-DOPA, reported no significant change to LID in patients co-administered paroxetine. Further work is clearly required to discern the potential anti-dyskinetic benefits of SERT inhibition in the clinical population.
In conclusion, this present preclinical work demonstrated that common, FDA-approved SSRIs were able to attenuate the expression of LID in hemi-parkinsonian rats. Several important questions remain regarding translation of these findings to the clinic, the mechanism by which these compounds work, and the long-term efficacy of the purported anti-dyskinetic effects. Finally, the current study complements the accumulating literature supporting the 5-HT system as a target for the improved treatment of PD patients.
ACKNOWLEDGEMENTS
This work was supported by NIH NS059600, the Michael J Fox Foundation and the Center for Development and Behavioral Neuroscience at Binghamton University. The authors would especially like to thank Nirmal Bhide, Kristin B. Dupre, David Lindenbach and Corinne Ostock for their help in the design and management of the current work.
REFERENCES
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Geffard M, Nagatsu I. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett. 1995;195(3):195–198. doi: 10.1016/0304-3940(95)11817-g. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, Chase TN. Effects of serotonin 5-HT1A agonist in advanced Parkinson's disease. Mov Disord. 2005;20(8):932–936. doi: 10.1002/mds.20370. [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin Wochenschr. 1961;73:787–788. [PubMed] [Google Scholar]
- Bishop C, Krolewski DM, Eskow KL, Barnum CJ, Dupre KB, Deak T, Walker PD. Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. J Neurosci Res. 2009;87(7):1645–1958. doi: 10.1002/jnr.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD. MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci. 2006;23(10):2669–2676. doi: 10.1111/j.1460-9568.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- Björklund A, Falck B, Stenevi U. Classification of monoamine neurones in the rat mesencephalon: distribution of a new monoamine neurone system. Brain Res. 1971;32(2):269–285. doi: 10.1016/0006-8993(71)90324-6. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. Buspirone in levodopa-induced dyskinesia. Clin Neuropharmcol. 1994;17(1):73–82. doi: 10.1097/00002826-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160(4):353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Caley CF, Friedman JH. Does fluoxetine exacerbate Parkinson’s disease? J Clin Psychiatry. 1992;53:278–282. [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat. Nat Rev Neurosci. 2002;3(7):574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Nuti A, Piccinni A, Dell'Agnello G, Bellini G, Gambaccini G, Dell'Osso L, Murri L, Bonuccelli U. Paroxetine in Parkinson's disease: effects on motor and depressive symptoms. Neurology. 2000;55(8):1216–1218. doi: 10.1212/wnl.55.8.1216. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88(2):617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Chung KA, Carlson NE, Nutt JG. Short-term paroxetine treatment does not alter the motor response to levodopa in PD. Neurology. 2005;64(10):1797–1798. doi: 10.1212/01.WNL.0000161841.41885.80. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Gessa GL. Pharmacology and neurochemistry of apomorphine. Adv Pharmacol Chemother. 1978;15:87–160. doi: 10.1016/s1054-3589(08)60482-2. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias. Brain. 2004;127(12):2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179(1):76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Negron G, Bishop C. The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Res. 2007;1158:135–143. doi: 10.1016/j.brainres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Durif F, Vidailhet M, Bonnet AM, Blin J, Agid Y. Levodopa-induced dyskinesias are improved by fluoxetine. Neurology. 1995;45(10):1855–1858. doi: 10.1212/wnl.45.10.1855. [DOI] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. The role of the dorsal raphe nucleus in the development, expression, and treatment of L-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse. 2009;63(7):610–620. doi: 10.1002/syn.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow K, Gupta V, Alam S, Park J, Bishop C. The partial 5-HT1A agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Feyder M, Bonito-Oliva A, Fisone G. L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front Behav Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Henry B, Hill MP, Peggs G, Crossman AP, Brotchie JM. Neural mechanisms underlying peak-dose dyskinesia induced by levodopa and apomorphine are distinct: evidence from the effects of the alpha(2) adrenergic adrenoreceptor antagonist idazoxan. Mov Disord. 2001;16(4):642–650. doi: 10.1002/mds.1148. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Damier P, Hicking C, Laska E, Müller T, Olanow CW, Rascol O, Russ H. Sarizotan as a treatment for dyskinesias in Parkinson's disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22(2):179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus maze. Pharmacol Biochem Behav. 1997;57:817–827. doi: 10.1016/s0091-3057(96)00402-9. [DOI] [PubMed] [Google Scholar]
- Honig G, Jongsma ME, van der Hart MC, Tecott LH. Chronic citalopram administration causes a sustained suppression of serotonin synthesis in the mouse forebrain. PLoS One. 2009;4(8):e6797. doi: 10.1371/journal.pone.0006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS. Abnormal bidirectional plasticity-like effects in Parkinson's disease. Brain. 2011;134(8):2312–2320. doi: 10.1093/brain/awr158. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM. Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of ON-time. J Neurosci. 2011;31(19):7190–7198. doi: 10.1523/JNEUROSCI.1171-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Jackson MJ, Kuoppamäki M, Smith LA, Jenner P. 3,4-methylenedioxymethamphetamine (ecstasy) inhibits dyskinesia expression and normalizes motor activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci. 23(27):9107–9115. doi: 10.1523/JNEUROSCI.23-27-09107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with increased motor disability. J Pharmacol Exp Ther. 2006;319(3):1225–1234. doi: 10.1124/jpet.106.110429. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Millar Z, Huot P, Wagg K, Thiele S, Salomonczyk D, Yong-Kee CJ, Gandy MN, McIldowie M, Lewis KD, Gomez-Ramirez J, Lee J, Fox SH, Martin-Iverson M, Nash JE, Piggott MJ, Brotchie JM. A novel MDMA analogue, UWA-101, that lacks psychoactivity and cytotoxicity, enhances l-DOPA benefit in parkinsonian primates. FASEB J. 2012 doi: 10.1096/fj.11-195016. [Epub]. [DOI] [PubMed] [Google Scholar]
- Kannari K, Shen H, Arai A, Tomiyama M, Baba M. Reuptake of L-DOPA-derived extracellular dopamine in the striatum with dopaminergic denervation via serotonin transporters. Neurosci Lett. 2006;402(1–2):62–65. doi: 10.1016/j.neulet.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Kannari K, Tanaka K, Maeda T, Tomiyama M, Suda T, Matsunaga M. Reserpine treatment prevents increases in extracellular striatal dopamine following L-DOPA administration in ras with nigrostriatal denervation. J Neurochem. 2000;74:263–269. doi: 10.1046/j.1471-4159.2000.0740263.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46(6):1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Differential regulation of serotonin (5-HT) release in the striatum and hippocampus by 5-HT1A autoreceptors of the dorsal and median raphe nuclei. J Pharmacol Exp Ther. 1994;269(3):1268–1279. [PubMed] [Google Scholar]
- Kuan WL, Zhao JW, Barker RA. The role of anxiety in the development of levodopa-induced dyskinesias in an animal model of Parkinson's disease, and the effect of chronic treatment with the selective serotonin reuptake inhibitor citalopram. Psychopharmacology (Berl) 2008;197(2):279–293. doi: 10.1007/s00213-007-1030-6. [DOI] [PubMed] [Google Scholar]
- Larsen MB, Sonders MS, Mortensen OV, Larson GA, Zahniser NR, Amara SG. Dopamine transport by the serotonin transporter: a mechanistically distinct mode of substrate translocation. J Neurosci. 2011;31(17):6605–6615. doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo RJ. Movement disorders associated with the serotonin selective reuptake inhibitors. J Clin Psychiatry. 1996;57:449–454. doi: 10.4088/jcp.v57n1002. [DOI] [PubMed] [Google Scholar]
- Lightowler S, Kennett GA, Williamson IJR, Blackburn TP, Tullock IF. Anxiolytic-like effect of paroxetine in a rat social interaction test. Pharmacol Biochem Behav. 1994;49:281–285. doi: 10.1016/0091-3057(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Lindenbach D, Ostock CY, Eskow Jaunarajs KL, Dupre KB, Barnum CJ, Bhide N, Bishop C. Behavioral and cellular modulation of L-DOPA-induced dyskinesia by β-adrenoceptor receptor blockade in the 6-OHDA lesioned rat. J Pharmacol Exp Ther. 2011;337(3):755–765. doi: 10.1124/jpet.111.179416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson's disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem. 2010;112(6):1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15(1):120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Nagata K, Yoshida Y, Kannari K. Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered l-DOPA. Brain Res. 2005;1046(1–2):230–233. doi: 10.1016/j.brainres.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Montastruc J-L, Fabre N, Blin O, Senard J-M, Rascol O, Rascol A. Does fluoxetine aggravate Parkinson’s disease? A pilot prospective study. Mov Disord. 1995;10:355–357. doi: 10.1002/mds.870100325. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Li Q, Gardoni F, Marcello E, Qin C, Carlsson T, Kirik D, Di Luca M, Björklund A, Bezard E, Carta M. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain. 2008;(12):3380–3394. doi: 10.1093/brain/awn235. [DOI] [PubMed] [Google Scholar]
- Navailles S, Bioulac B, Gross C, De Deurwaerdère P. Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson's disease. Neurobiol Dis. 2010;38(1):136–143. doi: 10.1016/j.nbd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Ng KY, Chase TN, Colburn RW, Kopin IJ. L-DOPA-induced release of cerebral monoamines. Science. 1970;170:76–77. doi: 10.1126/science.170.3953.76. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Nygaard TG. Reponse to levodopa treatment in dopa-responsive dystonia. Arch Neurol. 2011;58:905–910. doi: 10.1001/archneur.58.6.905. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Obeso JA, Stocchi F. Continuous dopamine receptor stimulation in advanced Parkinson’s disease. Trends Neurosci. 2000;23:S109–S115. doi: 10.1016/s1471-1931(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15(5):3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283(3):1305–1322. [PubMed] [Google Scholar]
- Papathanou M, Rose S, McCreary A, Jenner P. Induction and expression of abnormal involuntary movements is related to the duration of dopaminergic stimulation in 6-OHDA-lesioned rats. Eur J Neurosci. 2011;33(12):2247–2254. doi: 10.1111/j.1460-9568.2011.07704.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The Rat Brain in Stereotaxic Coordinates. fourth ed. San Diego: Academic Press; 1998. [Google Scholar]
- Perry KW, Fuller RW. Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci. 1992;50(22):1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- Putterman DB, Munhall AC, Kozell LB, Belknap JK, Johnson SW. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2007;323(1):277–284. doi: 10.1124/jpet.107.126219. [DOI] [PubMed] [Google Scholar]
- Rampello L, Chiechio S, Raffaele R, Vecchio I, Nicoletti F. The SSRI, citalopram, improves bradykinesia in patients with Parkinson's disease treated with L-dopa. Clin Neuropharmacol. 2002;25(1):21–24. doi: 10.1097/00002826-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Richard IH, Kurlan R. A survey of antidepressant drug use in Parkinson’s disease. Parkinson Study Group. Neurology. 1997;49:1168–1170. doi: 10.1212/wnl.49.4.1168. [DOI] [PubMed] [Google Scholar]
- Rylander D, Parent M, O'Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol. 2010;68(5):619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- Schallert T, De Ryck M, Whishaw IQ, Ramirez VD, Teitelbaum P. Excessive bracing reactions and their control by atropine and L-DOPA in an animal analog of Parkinsonism. Exp Neurol. 1979;64(1):33–43. doi: 10.1016/0014-4886(79)90003-7. [DOI] [PubMed] [Google Scholar]
- Smith Y, Wichmann T, Factor SA, DeLong MR. Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37(1):213–246. doi: 10.1038/npp.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steur EN. Increase of Parkinson disability after fluoxetine medication. Neurology. 1993;43(1):211–213. doi: 10.1212/wnl.43.1_part_1.211. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Poleszak E, Wlaz P, Wróbel A, Blicharska E, et al. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):323–329. doi: 10.1016/j.pnpbp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2–3):249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Troiano AR, de la Fuente-Fernandez R, Sossi V, Schulzer M, Mak E, Ruth TJ, Stoessl AJ. PET demonstrates reduced dopamine transporter expression in PD with dyskinesias. Neurology. 2009;72(14):1211–1216. doi: 10.1212/01.wnl.0000338631.73211.56. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Locatelli ER, Bravi D, Mouradian MM, Chase TN. Apomorphine responses in Parkinson’s disease and the pathogenesis of motor complications. Neurology. 1997;48(2):369–372. doi: 10.1212/wnl.48.2.369. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16:178–183. doi: 10.1177/0891988703256053. [DOI] [PubMed] [Google Scholar]
- Yamato H, Kannari K, Shen H, Suda T, Matsunaga M. Fluoxetine reduces L-DOPA-derived extracellular DA in the 6-OHDA-lesioned rat striatum. Neuroreport. 2001;12(6):1123–1126. doi: 10.1097/00001756-200105080-00015. [DOI] [PubMed] [Google Scholar]
- Zhang X, Andren PE, Greengard P, Svenningsson P. Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci U S A. 2008;105(6):2163–2168. doi: 10.1073/pnas.0711839105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Acheson AL, Stachowiak MK, Stricker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch Neurol. 1984;41(8):856–861. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]