Abstract
The fundamental observation that the temporal spacing of learning episodes plays a critical role in the efficiency of memory encoding has had little effect on either research on long-term potentiation (LTP) or efforts to develop cognitive enhancers. Here we review recent findings describing a spaced trials phenomenon for LTP that appears to be related to recent evidence that plasticity thresholds differ between synapses in the adult hippocampus. Results of tests with one memory enhancing drug suggest that the compound potently facilitates LTP via effects on high threshold synapses and thus alters the temporally extended timing rules. Possible implications of these results for our understanding of LTP substrates, neurobiological contributors to the distributed practice effect, and the consequences of memory enhancement are discussed.
Keywords: hippocampus, actin, spaced trials, integrin, cofilin, cytoskeleton, ampakine, brefeldin, glutamate uncaging
Introduction
It is well established that short bursts of afferent stimulation are more effective at inducing LTP when separated by the period of the theta rhythm (~200 msec) than when delivered at other intervals (Larson et al., 1986). This observation, in addition to providing a physiologically relevant and stereotyped means for generating synaptic modifications in adult forebrain, linked LTP to activity patterns occurring during learning (Otto et al., 1991; Buzsaki, 2005) and thus indirectly to memory encoding (Vertes, 2005; Axmacher et al., 2006). Subsequent work identified the mechanisms responsible for the peculiar efficiency of theta burst stimulation (TBS) and showed that the pattern is also highly effective in studies using transcranial magnetic stimulation of human cortex (Teo et al., 2011). Given these points, it is surprising that the types of parametric studies used to develop the TBS paradigm have not been repeated using periodic delivery of theta patterns across much longer time frames. This is all the more so in light of the improved memory encoding obtained by spacing learning trials (Wickelgren, 1974; Braun and Rubin, 1998; Cepeda et al., 2006; Benjamin and Tullis, 2010), that are each likely associated with cue-initiated theta activity, by hours or days. By itself, the ubiquitous spaced trials effect found in behavioral studies raises the expectation that widely separated theta trains will affect LTP in ways not found with more closely spaced applications.
The present paper surveys recent studies that confirmed the above prediction and describe candidate mechanisms that would allow theta trains separated by long intervals to greatly enhance the magnitude of LTP. Specifically, the LTP version of the spaced trials effect appears to involve recruitment into the potentiated state of synapses with initially very high plasticity thresholds. These results, along with analyses using single spine glutamate uncaging, show that the majority of synaptic contacts in adult hippocampus are not modified by a single train of theta burst stimulation but are in some manner primed by the theta train so as to become responsive to a second bout of theta delivered after a long delay. Possible explanations for this unexpected temporal requirement for capturing synapses ‘missed’ by a single TBS episode will be discussed in some detail. We will also summarize the first experiments asking if a memory enhancing compound interacts with the newly identified LTP timing rules. The results raise new questions about the meaning of cognitive enhancement.
Rules for producing maximal LTP
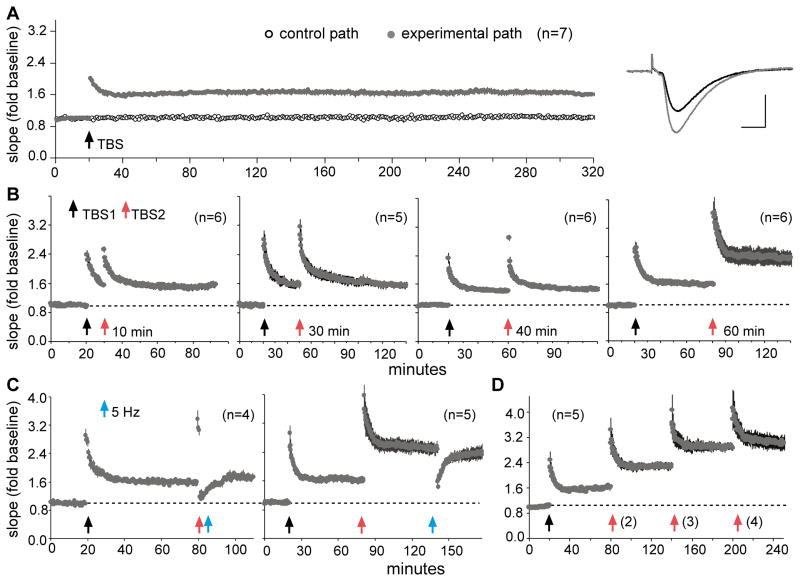
Early work relating theta to LTP suggested that a single 10 burst TBS train produced a near maximal degree of potentiation. Shortening the train reduced the percent LTP while extending it yielded no further enhancement of synaptic responses (Larson et al., 1986). Moreover, the potentiation induced by TBS was found to be remarkably stable, showing no evidence of delayed changes over extended periods of recording. Chronic recording studies first established this point (Staubli and Lynch, 1987; Abraham et al., 2002; Abraham, 2003) and it also holds for hippocampal slices (Fig 1A). However, evidence gradually emerged suggesting that one episode of TBS in fact produces a partial LTP effect. First, drugs that enhance excitatory transmission greatly amplify the magnitude of LTP above that found with TBS delivered under baseline conditions (Staubli et al., 1994; Arai and Kessler, 2007) thereby implying that the conventional single TBS paradigm fails to fully engage the LTP process. Second, work by Frey and colleagues (1995) showed that a second set of high frequency trains delivered after a delay of four hours, and at reduced stimulation current, enhances the EPSPs elicited by single (reduced intensity) pulses. This result suggests that already potentiated synapses can exhibit further facilitation when long inter-stimulation periods are used.
Figure 1. The timing of successive theta trains determines the capacity for augmenting LTP.
Theta burst stimulation (TBS) was applied to the Schaffer-commissural projections and field EPSPs (fEPSPs) were recorded from CA1b stratum radiatum in adult rat hippocampal slices. (A) Plot of fEPSP slopes shows that one TBS train (10 bursts of 4 pulses at 100 Hz, separated by 200 ms) increases synaptic responses by about 50% and that the effect is stable throughout a 5 hour recording session. Traces show individual responses collected before (black) and 300 min after (gray) TBS (calibration: 1mV, 5ms). (B) Application of a second TBS train (TBS2) has no effect on the magnitude of potentiation if applied 10, 30, or 40 min after the first, but doubled the level of potentiation if applied after a delay of 60 min. (C) A train of 5 Hz pulses reverses LTP2 if applied 60 sec (left plot) but not 60 min (right plot) after TBS2, thereby demonstrating that the augmented potentiation exhibits the same reversal characteristics as previously described for TBS1. (D) With 60 min inter-train intervals, the level of potentiation is augmented by a second and third, but not a fourth, train of TBS (Portions of this figure are modified from Kramár et al., 2012).
Recent parametric studies exploring the effects of multiple bouts of theta stimulation produced striking results (Kramár et al., 2012). A conventional TBS train (TBS1) was followed by an identical second train (TBS2) at intervals ranging from 10 to 90 minutes; TBS2 caused no evident changes to the LTP elicited by TBS1 when delayed by 10 or 30 minutes but nearly doubled the level of potentiation when delivered after a 60-minute delay. We have recently established that TBS2 is ineffective when delayed by 40 minutes (Fig 1B), while preliminary results suggest that it produces an LTP2 effect at 50 minutes (not shown). TBS2 delayed by 90 minutes did not produce a greater degree of EPSP facilitation than TBS2 separated from TBS1 by 60 minutes (p>0.5). Collectively, these observations point to the conclusion that the machinery for producing LTP becomes refractory for 40–50 minutes after its initial engagement. The added potentiation elicited by the second theta train showed no evidence of decaying over one hour of recording and with time was resistant to reversal by low frequency stimulation. Specifically, one minute trains of 5 Hz stimulation (no bursts) are known to reverse LTP when applied in the first few minutes after TBS but then become progressively less effective (Lynch et al., 2008); similar to this, 5Hz pulses reversed LTP2, but not LTP1, when delivered one minute after TBS2 but were ineffective 60 minutes later (Fig 1C). It thus appears that the new phase of potentiation (LTP2) undergoes the same stabilization process found with the initial, conventionally studied LTP.
The discovery of LTP2 raised the question of how many widely spaced theta trains are needed to fully potentiate synapses innervated by a given small population of afferents. Tests of this showed that TBS3, delayed by one hour after TBS2, produced a further increment in net potentiation while TBS4 did not (Fig 1D)(Kramár et al., 2012). The final level of LTP after three trains was about 150% above baseline, a value almost three times greater than that typically described for LTP studies using field potentials in area CA1.
LTP2 involves potentiation of synapses ‘missed’ by TBS1
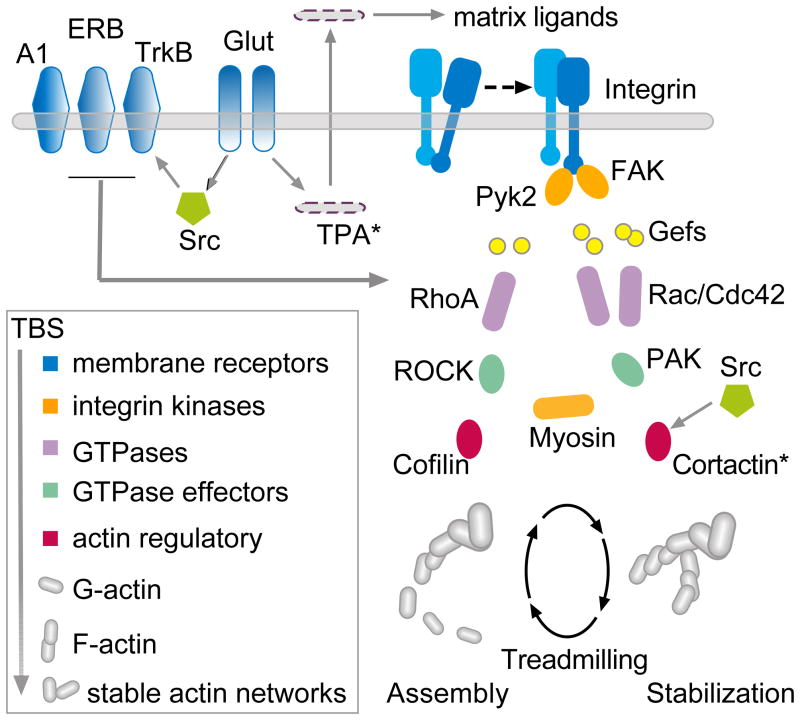
The existence of LTP2 could indicate that individual synapses have multiple potentiation steps or that TBS1 fails to modify all of the contacts formed by the axons stimulated by TBS1. The literature is unclear with regard to these ideas. However, recent advances in the analysis of the cell biological underpinnings of LTP describe methods that could in principle be used to test if TBS2 triggers events associated with enduring potentiation at synapses other than those engaged by TBS1. Those studies show that theta bursts and induction of LTP activate a complex collection of serial and parallel signaling pathways leading to the assembly and subsequent stabilization of actin filaments in the sub-synaptic cytoskeleton (Fig 2) (Fukazawa et al., 2003; Chen et al., 2007; Kramár et al., 2009b; Rex et al., 2009; Chen et al., 2010a; Rex et al., 2010; Murakoshi and Yasuda, 2012; Seese et al., 2012). Disrupting any of several steps in this machinery blocks LTP stabilization and the consolidation of long term memory (Lamprecht et al., 2006; Rex et al., 2009; Rex et al., 2010; Gavin et al., 2012). As described below, tests of whether TBS2 initiates actin filament assembly (polymerization) at synapses in addition to those at which the effect occurs after TBS1 proved positive.
Figure 2. Proposed actin signaling pathways for TBS1.
Studies using adult hippocampal slices have shown that a single round of theta burst stimulation (TBS) elicits actin signaling through two cascades with either the RhoA or Rac/Cdc42 GTPases as upstream elements. It is known that integrin adhesion proteins signal through both GTPase streams and modulate the assembly and stabilization of F-actin; β1 integrin function is required for TBS-induced increases in spine F-actin and for stable LTP (tissue plasminogen activator (TPA) and metalloproteinases generate extracellular matrix ligands for the newly activated integrins (Wang et al., 2008)). Both the estrogen receptor beta (ERB) and the BDNF receptor TrkB facilitate signaling through the RhoA path with the latter being required for TBS initiated actin filament assembly within spines. These effects are opposed by actions of the adenosine A1 receptor that blocks RhoA-to-cofilin signaling. Blocking signaling through ROCK or myosin IIb motors prevents increases in spine F-actin and initial stabilization of LTP whereas Rac antagonists prolong the vulnerability of LTP to reversal by other manipulations (e.g., low frequency stimulation). For details see primary publications (Chen et al., 2007; Kramár et al., 2009b; Kramár et al., 2009a; Rex et al., 2009; Chen et al., 2010a; Rex et al., 2010).
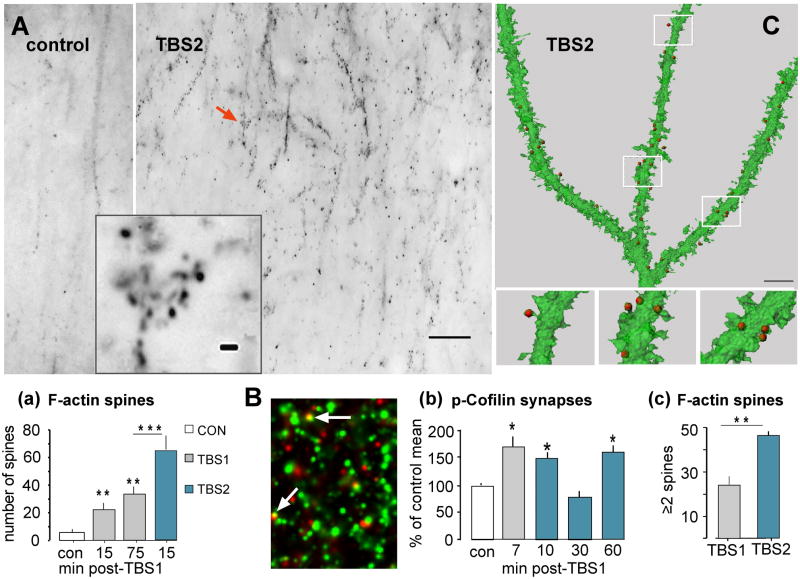
Increases in filamentous (F-) actin within dendritic spines can be assessed by topically applying fluorescence tagged variants of the mushroom toxin phalloidin to recording sites at the conclusion of an experimental session (Fig 3A)(Lin et al., 2005a; Kramár et al., 2006). Past work showed that TBS-induced increases in phalloidin-tagged spines are long lasting (Rex et al., 2010) and recent studies confirmed this: the number of such spines is increased to the same level above baseline at 15 and 75 minutes after TBS1 (Fig. 3a). The experimental question then became one of whether TBS2 delayed by one hour increases the number of F-actin dense spines above that obtained with TBS1 alone. The results were clear: TBS2 nearly doubled the incidence of densely phalloidin-positive cases relative to the effects produced by a single theta train (Fig 3a)(Kramár et al., 2012). We later confirmed that TBS2 fails to produce an essential step in actin polymerization -- phosphorylation (inactivation) of the actin severing protein cofilin (see Fig 2) -- at synapses when applied at 10 or 30 minutes after TBS1 but triggers the effect after a 60 minute delay (Fig 3B,b). Close examination of phalloidin labeling images suggests that after TBS2 the F-actin enriched spines occur in small clusters (Fig 3A, inset). Analyses using confocal imaging of in situ phalloidin labeling in conjunction with 3D reconstruction of GFP-labeled dendrites reinforced this conclusion by showing that labeled spines more frequently formed small groups (i.e., two or more labeled spines on a single 5μm dendritic segment) after TBS2 than was found after TBS1 alone (Fig 3C,c). This strongly suggests that synapses differentially affected by the two theta trains occur in close proximity and may be innervated by the same axon or by tightly coupled afferents that terminate on neighboring spines (Kleindienst et al., 2011; Magee, 2011).
Figure 3. TBS2 causes a marked increase in F-actin positive (+) spines over values found after TBS1.
A) Theta burst stimulation was applied to Schaffer-commissural afferents of field CA1b and Alexafluor-tagged phalloidin was subsequently infused for in situ labeling of F-actin. (A) Reverse contrast epifluorescent images of the proximal stratum radiatum in field CA1 (the cell body layer is just beyond the top of the micrograph) in slices that received control baseline stimulation (left) or two rounds of TBS show that theta stimulation increased the incidence of densely F-actin+ puncta. Higher magnification images (inset) of one subfield (arrow) indicate that the labeled spines commonly occur in clusters (calibration bars = 200 μm and 5μm for main panels and inset, respectively). (Aa) Quantification of F-actin rich puncta in the CA1 stratum radiatum sample field in slices collected 15 or 75 min after TBS1, or 15 min after TBS2 delayed by one hour after TBS1. The first theta train increased numbers of labeled spines to comparable levels at the 15 or 75 min time points while TBS2 approximately doubled the number of F-actin+ spines over TBS1-alone values. (B) Slices were harvested at 7 min after TBS and processed for immunofluorescent localization of p-Cofilin (red) and PSD95 (green) followed by restorative deconvolution. Synapses were counted as double-labeled when puncta labeled for PSD95 and pCofilin overlapped (arrows). (Bb) Plot shows quantification of pCofilin+ PSDs in the CA1b sample field. Numbers of synapses associated with pCofilin were increased 7 min after TBS1 alone; TBS2 applied 10 min later did not induce further increases above control (levels at 10 min post-TBS2 likely include the increase produced by TBS1 which in prior work required more than 15 min to decay (Chen et al., 2007). TBS2 applied 30 min after TBS1 had no effect on pCofilin puncta whereas stimulation after a 60 min delay elicited a full pCofilin response. (C) Three dimensional rendering (reconstructed from a multi-photon image z-stack) of an GFP-labeled dendrite showing that phalloidin-labeled F-actin aggregates (red dots) are localized within spines in a slice harvested after two trains of TBS. (Cc) Percent of 5μm dendritic segments, with an F-actin+ spine, that contained one or more additional labeled spines in 3D builds (as in panel C). This value was twice as great following TBS2 than after TBS1, suggesting that newly labeled spines after a second round of theta stimulation are clustered near those responding to TBS1. Statistical comparisons: *p<0.05; **p<0.01; ***p<0.001 vs. con or comparison indicated. Panels ‘a’ and ‘c’ are modified from Kramar et al., 2012.
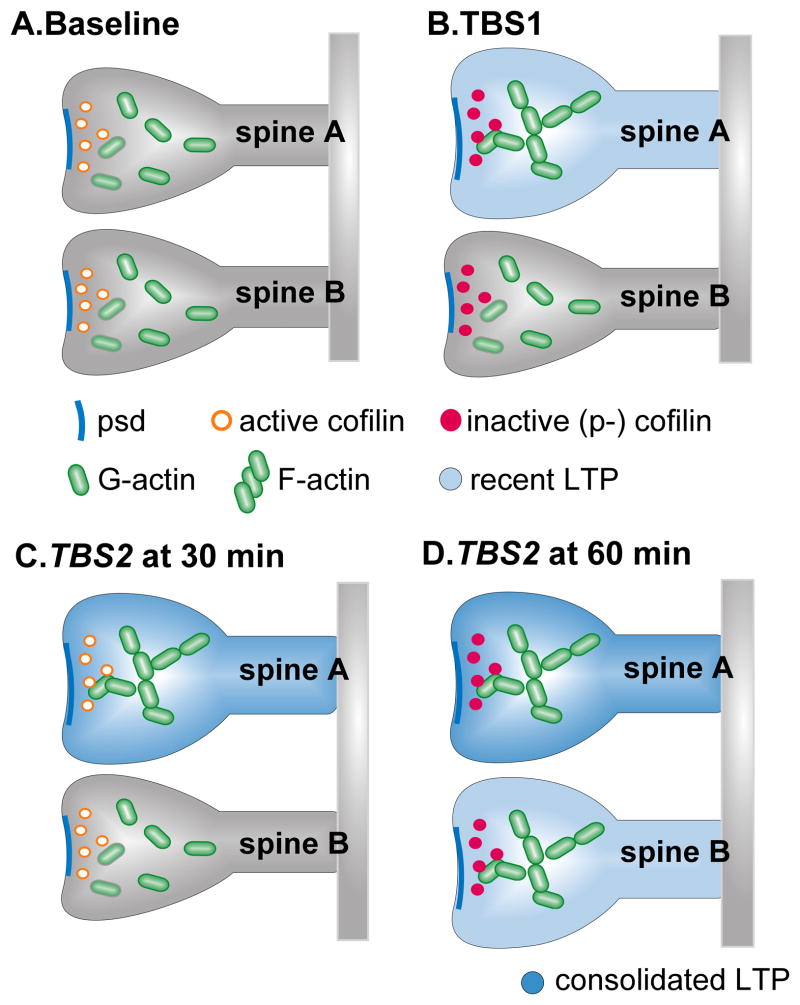
This collection of results leads to the conclusion that TBS2 stimulates LTP consolidation processes at synapses that failed to respond (with potentiation) to TBS1. More specifically, we propose that TBS1 i) initiates signaling leading to LTP at all synapses innervated by the stimulated afferents but fails to engage consolidation mechanisms in the majority of these, and ii) triggers some type of refractory period for signaling, again throughout the entire population of activated contacts. In this scenario TBS2 activates potentiation in the synapses that did not exhibit enduring plasticity after TBS1 -- hence the evident short-term potentiation it produces at 10 and 30 minutes post-TBS1 (see Fig 1) -- but is only able to induce stable LTP when applied after the refractory period has dissipated (Fig 4). This hypothesis makes the necessary prediction that adult hippocampal synapses have different thresholds for induction of long lasting modifications by brief periods of intense stimulation. The following section describes a recent attempt to test this point.
Figure 4. Refractoriness in high and low threshold spines.
Schematic illustration of mechanisms proposed to underlie recruitment of high threshold spines by TBS2. (A) Each panel shows neighboring spines with low levels of F-actin and normal sized PSDs. (B) TBS1 initiates actin signaling through the cofilin phosphorylation stage in both spines but this goes to completion (formation of stable actin filaments; persistent PSD enlargement) only in the low threshold case (‘spine A’). (C) TBS2 applied at 30 min after TBS1 fails to elicit signaling in either spine although morphological and cytoskeletal changes induced by TBS1 in spine ‘A’ endure. (D) TBS2 applied 60 min after TBS1 increases p-cofilin in both spines, as did the initial round of stimulation, and triggers actin polymerization in the spine in which this response did not occur after TBS1 (‘spine B’); this, in effect, doubles both the number of F-actin rich spines and the magnitude of stable LTP relative to measures obtained after TBS1 alone.
A test of the hypothesis that adult hippocampus contains spines with different plasticity thresholds
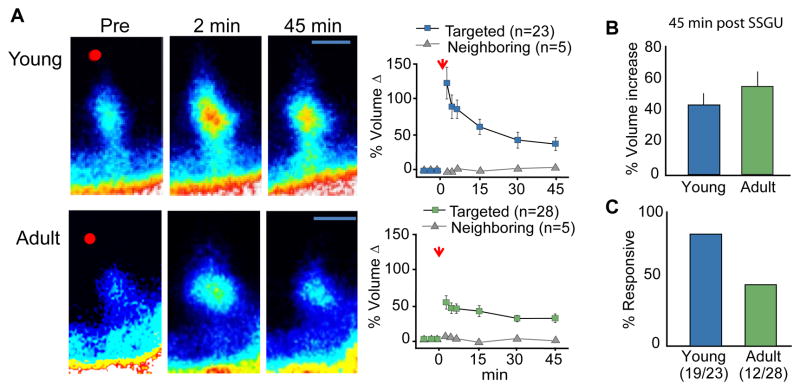
A now sizable body of studies first using electron microscopy and then live imaging or immunostaining indicates that LTP is associated with substantial changes to spine and synapse morphology (Chen et al., 2007; Fortin et al., 2010; Gu et al., 2010; Wang and Zhou, 2010; Bosch and Hayashi, 2011). Previous studies have also shown that local single spine glutamate uncaging (SSGU) can elicit coordinated and enduring increases in spine head volume and increases in synaptic function (Matsuzaki et al., 2004; Harvey and Svoboda, 2007). This approach made it possible for us to test both the proposition that individual spines have different thresholds for synaptic plasticity and if individual spines respond to repeated, spaced stimulation. In accord with prior work using immature tissue, we found that uncaging adjacent to single spines caused a marked increase in spine head volume, in both young (2–3 wk old) and adult (4–8 wk old) mouse hippocampal slices, that persisted throughout the 45 minute testing session (Fig. 5A,B); the absence of a response in neighboring spines indicated that activation was synapse specific (Fig. 5A). However, although the same morphological type spines were activated at the two ages, only 43% of the spines tested in adult slices exhibited this effect, in contrast with the over >80% of spines responding in immature (2–3 week old) tissue (Fig 5C). This result suggests that maturation engages stability mechanisms that decrease the proportion of spines primed for activity-induced morphological plasticity. A second application of glutamate, at previously responding spines, did not produce further head enlargement even when that pulse was delayed by one hour after the first (Kramár et al., 2012). This last finding accords with our hypothesis that TBS2 augments fEPSP responses by eliciting plasticity in spines that were activated but did not potentiate with the first round of stimulation (and not by augmenting the level of potentiation at spine synapses that exhibited LTP after TBS1). Regarding this point, it is noteworthy that the percentage of spines resistant to enlargement with one round of SSGU corresponds with that expected from the LTP and phalloidin labeling experiments described in figures 1 and 3. We conclude from these studies that spines in adult hippocampus have different plasticity thresholds and that delayed events set in motion by TBS1 shift the high threshold cases into a more responsive state.
Figure 5. Spines have different thresholds for glutamate-induced, LTP-related morphological changes.
(A) Images of representative GFP labeled spines in slices from young (2–3 wk old; left) and adult (4–8 wk old, right) mouse hippocampal slices before and after glutamate uncaging demonstrate that the local uncaging event (at red dot) increases spine head volume at both ages. Plots of spine volume change for groups of SSGU-responsive spines (blue symbols) and their untargeted neighbors (gray symbols): as shown for the targeted spines, although the initial volume changes are greatest for young spines, similar sized morphological changes endure over 45 min at both ages. (B) Plot shows that the % volume increase is the same for responsive spines in young and adult slices as assessed at 45 min after stimulation. (C) The proportion of spines that respond to SSGU declines with age: for young slices 19 of 23 targeted spines enlarged with stimulation whereas in adult cases only 12 of 28 spines (43%) were responsive. Panel A modified from Kramár et al., 2012.
Mechanisms underlying the delayed reduction of plasticity thresholds
The most straightforward interpretation of the results obtained with the TBS1/TBS2 paradigm is that a single train of theta bursts i) induces LTP in low threshold synapses and ii) ‘primes’ high threshold connections to respond to the delayed arrival of a second theta train. What type of mechanism might account for the latter effect? Uncaging studies using immature neurons have uncovered evidence for spine crosstalk involving the diffusion of material from stimulated spines to neighbors located up to five microns away on the same dendritic branch (Harvey et al., 2008; Murakoshi et al., 2011). This is particularly intriguing in the present context because certain of the transferred proteins are known to be critical for the production of LTP. The results summarized in figure 3c, suggesting that TBS2 triggers actin polymerization in spines adjacent to those affected by TBS1, accord well with the idea that local crosstalk participates in the priming process. Another mechanistic possibility for priming is that TBS activates long lasting, LTP-related enzymatic changes in previously high threshold spines that serve to facilitate subsequent synaptic modifications. Of interest in this regard are reports that high frequency stimulation causes a long lasting activation of CaMKII (Barria et al., 1997; Lisman et al., 2012), a kinase that has also been implicated in the genesis of LTP. If this effect were to occur at all synapses formed by the stimulated input, then it could be factor in the lowering of thresholds in the initially non-responsive cases. Indeed, Ras-GTP, which spreads from stimulated spines to its neighbors (Harvey et al., 2008), relays the potentiating effects of CaMKII signaling in response to synaptic activation (Zhu et al., 2002). Thus, the active-Ras spreading phenomenon could lower the threshold for CAMKII-induced potentiation in previously activated (by TBS1), but unpotentiated, synapses. This process could convert previously unresponsive synapses into carriers for LTP2.
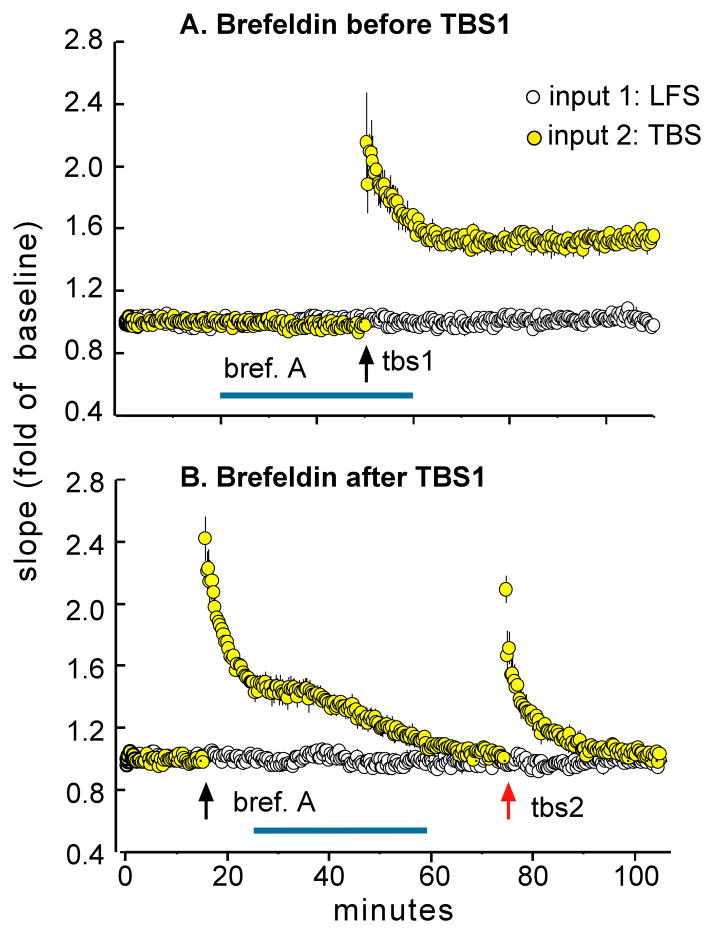
The spine crosstalk and prolonged enzyme activation hypotheses do not address the question of why TBS2 becomes effective only after long delays. One possibility is that TBS1 inactivates a step required for the production of stable potentiation and that 40–60 minutes are required for recovery to occur. While there is little evidence for this suggestion, it is known that theta bursts and NMDA receptor stimulation activate the calcium-dependent protease calpain (Vanderklish et al., 1995; Vanderklish et al., 2000), resulting in the breakdown of key cytoskeletal elements at synapses. Calpain-mediated proteolysis has been studied only in the context of LTP formation but, if present at high threshold synapses where potentiation failed, it could contribute to refractoriness. Recovery, in this scenario, would involve assembling replacement copies for cleaved proteins followed by transport and insertion into the synaptic membrane (Fig 6). This argument makes the explicit prediction that blocking protein movement to the membrane will prevent the formation of LTP2. The fungal toxin brefeldin A disrupts vesicle formation and transport to the membrane (Klausner et al., 1992; Nebenfuhr et al., 2002) and is known to prevent the insertion of LTP-related proteins into membranes (Lin et al., 2005b). Initial tests of whether brefeldin interferes with LTP2 produced positive results (Kramár et al., 2012). A thirty-minute infusion of the toxin had no detectable influence on baseline synaptic responses or on the induction of LTP1 (Fig 7A); however, treatments begun after TBS1 and prior to TBS2 eliminated LTP2 (Fig 7B). We interpret these results as indicating that a brief interruption of vesicular transport to the synapse has no effect on LTP under baseline conditions, when the machinery for induction and consolidation is already in place, but prevents the delivery of materials required for the stabilization of LTP2. It thus sets the delay that defines the extended LTP timing rule.
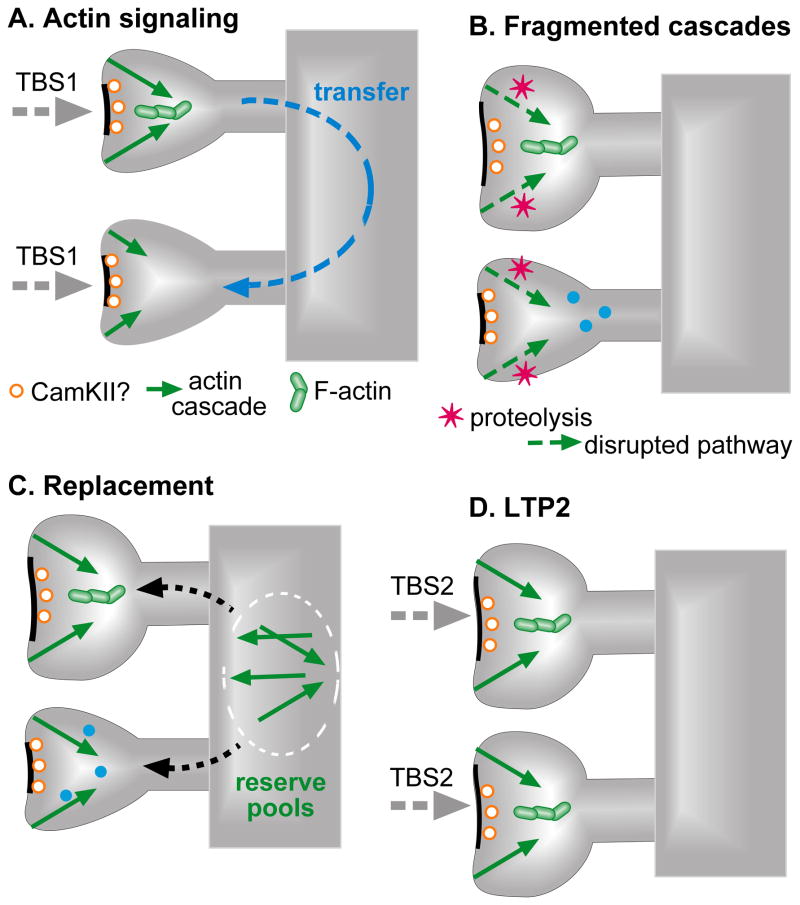
Figure 6. Hypothesized mechanisms responsible for spine ‘priming’ and the LTP refractory period.
Panels represent successive TBS-induced processes that mediate the post-TBS1 refractory period, and priming of adjacent spines for potentiation with TBS2. (A) TBS1 initiates actin signaling at all innervated synapses but the effects go to completion only in low threshold cases, resulting in morphological transformation of the spine and its synapse. These effects are rapid but the theta bursts also activate long lasting enzymatic events (e.g., CaMKII activation) that facilitate, or ‘prime’, the stabilization of later LTP. Moreover, TBS1 causes the diffusion of LTP promoting materials from low to high threshold spines (Harvey et al., 2008; Murakoshi and Yasuda, 2012); this adds to the priming process. (B) TBS1 induces proteolysis in all innervated spines and thereby eliminates essential elements in the actin signaling cascades. Thus, the primed high threshold spines are, for a period, unable to generate LTP in response to a TBS2. (C) Signaling proteins cleaved by TBS1 are gradually replaced over ~50 min either by synthesis or drawdown from extant pools. (D) Primed, previously high threshold, spines are now able to respond to TBS2 with cytoskeletal reorganization and the production a stable LTP2 effect.
Figure 7. A toxin that blocks transport of proteins to the membrane eliminates LTP2.
(A) Infusion of brefeldin A (horizontal blue line) had no effect on LTP if initiated prior to one train of TBS; plot shows fEPSPs for the control pathway (receiving 3 pulse per minute low frequency stimulation [LFS] only) and the experimental path that received one 10 burst train of TBS (at arrow). (B) Brefeldin infusion initiated 20 min after TBS1 caused a delayed decay in LTP1 and totally prevented the expression of LTP2. Brefeldin did not influence responses in the control pathway in either experiment.
As shown in figure 7B, brefeldin produced an entirely unexpected effect in addition to the predicted disruption of LTP2: it caused LTP1 to rapidly decay back to baseline (Kramár et al., 2012). This result suggests that the delayed arrival of vesicular proteins serves two LTP-related functions: i) to enable primed, high threshold synapses to respond to TBS2, and ii) to stabilize LTP1 in the low threshold cases modified by TBS1. The two results can be integrated by assuming that TBS1 causes the loss of proteins in all synapses formed by the stimulated inputs, with delayed replenishment serving to anchor already present potentiation in the low threshold contacts, and providing materials needed for stabilizing LTP2 in high threshold connections.
A memory enhancing drug modifies LTP timing rules
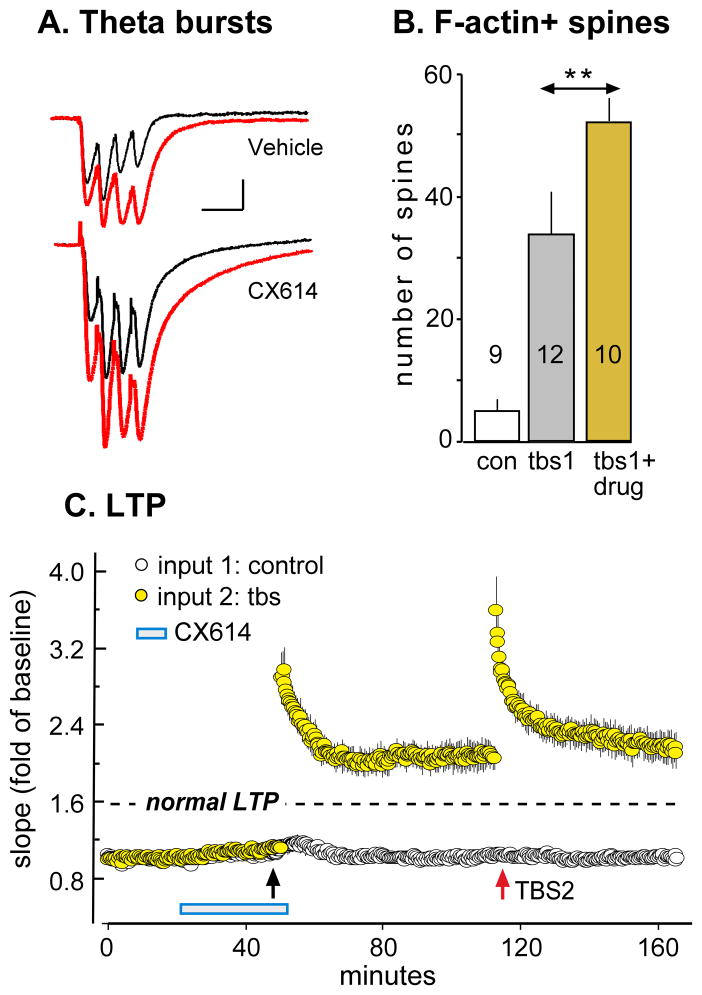
As noted, drugs that positively modulate AMPA receptors, and thereby increase the size of fast EPSCs (‘ampakines’), both lower the threshold and raise the ceiling for LTP (Staubli et al., 1994; Arai and Kessler, 2007). Ampakines also improve retention scores in diverse learning paradigms using rodents, rabbits, and primates (e.g., Staubli et al., 1994a; Shors et al., 1995; Porrino et al., 2005; Hampson et al., 2009). A likely explanation for these effects is that the compounds markedly increase the magnitude of the depolarization produced by theta bursts (Fig 8A) and thereby increase the voltage-dependent NMDA receptor responses occurring during a burst response. These results raise the possibility that augmenting the triggering events for LTP induction serves to overcome the barriers to actin polymerization, and thus to LTP consolidation, in high threshold synapses. It follows from this argument that TBS delivered in the presence of an ampakine should increase the number of spines with high concentrations of F-actin to a much greater degree than is found under control conditions. This prediction has been confirmed: a conventional ten theta burst train causes an approximately 70% greater than normal increase in phalloidin-positive spines (Fig 8B) and an equivalent enhancement of LTP (Fig 8C) (Kramár et al., 2012). If, as suggested by these observations, ampakines allow TBS1 to initiate the full sequence of steps leading to stable potentiation in high threshold synapses, then the effects of TBS2 should be correspondingly reduced. This appears to be the case because, as shown in figure 8C, a second train of appropriately delayed theta bursts failed to induce additional potentiation when applied against a background of an ampakine-enhanced LTP1.
Figure 8. Positive allosteric modulation of AMPA receptors enhances LTP1 at the expense of LTP2.
Adult hippocampal slices received TBS of Schaffer-commissural afferents to field CA1 in normal aCSF (Vehicle) or in the presence of the positive AMPA receptor modulator (ampakine) CX614 (20 μM). (A) Traces show fEPSPs elicited by two successive theta bursts (red for second burst) in the presence or absence of the ampakine: the compound increased the amplitude and duration of both first and second burst responses. (B) In situ phalloidin labeling was used to determine the effects of the experimental treatments on spine F-actin (as in Figure 3). Bar graph shows that TBS1 increased the number of F-actin enriched spines in normal aCSF (gray bar) but had a much larger effect when delivered in the presence of the ampakine drug CX614 (gold bar; p<0.001, con vs TBS1; **p<0.01, tbs1 vs tbs1+drug). (C) Plot of fEPSP slopes shows that TBS1 (black arrow) elicited a much greater LTP1 effect in ampakine treated slices than is found in drug free aCSF (indicated by dashed line). TBS2 (red arrow) in the same slices did not induce further potentiation indicating that the initial supranormal response to TBS1 occluded further potentiation by a second round of theta stimulation (modified from Kramár et al, 2012).
The ampakine results could indicate that high threshold contacts have reduced machinery for engaging NMDA receptor signaling, as would occur if the synapses had relatively sparse populations of AMPA or NMDA receptors. Boosting AMPA receptor gated currents would then provide depolarization of sufficient magnitude and duration to generate a robust NMDA receptor response. However, the glutamate uncaging studies described earlier used spines of similar volume and it is known from electron microscopic work that synapse diameter and receptor numbers correlate with spine size. An alternative, and perhaps more likely, explanation for the drug effects involves potential differences between spines in the magnitude of the NMDA receptor-mediated response needed to initiate actin signaling. In this argument the locus of the high threshold would be found in the downstream events leading to the formation and stabilization of cytoskeletal networks. This idea can be tested using single spine glutamate uncaging studies paired with varying induction intensities and pharmacological agents that either enhance or suppress the generation of polymerized spine actin linked to stable LTP (Kramár et al., 2009b; Rex et al., 2009; Rex et al., 2010). We propose that the ‘state’ of elements in the polymerization machinery downstream from the NMDA receptors (Fig. 2) will have profound influences on the probability of plasticity induction at individual spines, and thus on spine plasticity thresholds.
Whatever their origins, the ampakine results describe an instance in which a drug obviates a basic timing rule for maximizing LTP. The occurrence of these effects in learning studies would lead to new questions about the relative advantages of various enhancement strategies. Specifically, one explanation of the distributed practice effect is that temporal spacing focuses learning on elements in the environment that are relatively constant across time, thereby avoiding the acquisition of ‘noise’. Combining the encoding strength produced by multiple trials into a single session could thus reduce the fidelity of the memory trace with regard to relevance of the information it contains. These points emphasize the potential utility of tests for enhancement under conditions in which the advantages of distributed practice are prominent.
Discussion
Three lines of evidence described here lead to the conclusion that the threshold for inducing stable LTP differs between synapses in the adult hippocampus. First, TBS2 increases the number of spines containing high concentrations of polymerized actin to values that are substantially greater than those found after stimulation of the same fibers with TBS1. Second, live spine imaging has shown that while many spines in adult hippocampus undergo LTP-related morphological changes in response to intense glutamatergic stimulation, the majority do not. Third, a drug (ampakine) that markedly enhances depolarization and NMDA receptor channel opening during TBS triggers actin polymerization in a much broader than normal population of spines, markedly enhances the magnitude of the corresponding LTP, and does so at the expense of LTP2. The argument that most spines in hippocampus have elevated thresholds for TBS-induced actin filament formation and/or stabilization, and thus for consolidating newly generated LTP, accounts for this collection of observations.
The origin of the differences in plasticity thresholds is unclear although the experimental results provide several interesting clues. It is unlikely that the variations reflect a failure, in the non-responsive cases, of TBS1 to engage the initial steps in the sequences leading to induction of LTP. Specifically, TBS2 delayed by 60 minutes did not induce cofilin phosphorylation at a greater number of synapses than did TBS1. These data indicate that TBS2 either i) triggers these events in a different, but equal sized, population of contacts than did TBS1, or ii) affects all of the same synapses that were activated by TBS1 including those in which signaling events (in addition to cofilin phosphorylation) were not sufficient to generate new, stable actin filaments. Preliminary analyses indicate that TBS2 produces the same sized theta burst responses when applied at 30 (no LTP2) and at 60 (LTP2 induced) minutes after TBS1 (p>0.05, 30 vs 60 min delay). This result strongly suggests that theta stimulation activates the same pool of synapses at the two time points. Since theta burst responses contain a large NMDA receptor-mediated component (Larson and Lynch, 1988), it also provides evidence that the initial triggering event for LTP occurs at all contacts in that pool. Finally, the absence of any evidence for theta-induced cofilin phosphorylation at 30 minutes after TBS1 indicates that the refractoriness generated by the first theta train occurs at all connections (potentiated or not) formed by the stimulated axons. Based on these arguments, we propose that TBS1 triggers many of the events needed for eliciting stable LTP at all innervated contacts but that an additional factor (or factors) downstream from NMDA receptors has an elevated triggering point in a large subpopulation of these.
If correct, the above conclusion significantly narrows the field of candidate explanations for why so many spines do not transition into the LTP state after TBS1. If cofilin phosphorylation responses are, as proposed here, present at all synapses stimulated by TBS1, then the high threshold feature would involve additional elements needed for the production or stabilization of actin networks. Cofilin constitutively severs dynamic actin filaments and its inactivation via phosphorylation is highly correlated with polymerization (Sarmiere and Bamburg, 2004; Hotulainen et al., 2005; Wiggan et al., 2012). However, elaboration and maintenance of actin filament networks requires a battery of additional proteins including cortactin, the WAVE-ARP2/3 complex, cross-linking elements, and more (Burridge and Wennerberg, 2004; Penzes and Cahill, 2012). Recent work indicates that TBS activates multiple, small GTPase-initiated signaling pathways, at least one of which is linked to the observed phosphorylation of synaptic cofilin (Fig 2)(Rex et al., 2009; Chen et al., 2010b). It is known from the cell biological literature that stimulation of other Rho and Ras family GTPases leads to the further steps required for stable reorganization of the submembrane cytoskeleton (Fig 2)(Daly, 2004; Szczepanowska, 2009; Murakoshi et al., 2011; Penzes and Cahill, 2012). Possibly then, the elements that do not respond to a normal sized, first TBS train are to be found in GTPase-initiated pathways other than those associated with cofilin. Indeed, we have recently shown that myosin II activation in dendritic spines is also required for the polymerization of F-actin that supports stable LTP (Rex et al., 2010) and memory consolidation. We concluded from these and earlier studies (Rex et al., 2009; Chen et al., 2010b) that multiple, parallel signaling pathways are simultaneously activated to initiate and then stabilize spine F-actin, which then leads to stable LTP. Stochastic events within individual spines (i.e. the phosphorylation state of Myosin Light Chain or the ratio of F/G actin) may prevent any one of these required pathways from being “online” at the time of TBS1. However, the first theta train may serve to minimize such stochastic processes, thereby enabling the polymerization capability of the spine and increasing the chances that TBS2 induces stable potentiation.
We suggested two means whereby the first application of TBS could adjust the threshold for actin polymerization in nearby spines: transfer of materials from spines that potentiated with TBS1 and/or prolonged activation of the kinase CaMKII. There is good evidence for both effects (Harvey et al., 2008; Lisman et al., 2012; Murakoshi and Yasuda, 2012) and each is logically related to the construction of persistent actin networks. It remains now to test the prediction that suppressing the activity of proteins known to diffuse into neighboring spines, or of CaMKII, will block LTP2 without disturbing baseline physiology or the induction of LTP1. Studies summarized here provided evidence that the additional spines containing high levels of F-actin after TBS2 occur in close proximity to those labeled after TBS1, as expected from the crosstalk hypothesis. Since the large majority of dendritic branches did not have labeled spines, the clustering effect presumably reflects multiple innervation of short segments by individual, or functionally linked, axons. It is interesting to note that the combination of diffusion and CaMKII activation could result in a single input variation on the ‘synaptic tagging’ theory advanced by Frey and Morris (Frey and Morris, 1998). That is, diffusion from newly potentiated spines to their neighbors would provide materials that offset elevated thresholds but only in those in which synapse specific activation of CaMKII had occurred (‘synaptic capture’ in the tagging theory).
As noted, the above suggestions about how the LTP threshold is adjusted do not of themselves help explain why an approximately 50 min long delay is needed before the modified spines can respond to TBS. Diffusion and CaMKII activation, as found in experimental studies, occur too rapidly to account for this time frame (Lee and Yasuda, 2009). We proposed instead that this period reflects the time needed to transfer proteins, from extant pools or via de novo synthesis, to replace synaptic elements targeted by the NMDA receptor-dependent proteolytic activity associated with TBS1. At present the balance of evidence supports the protein transfer hypothesis. Ongoing studies indicate that recovery from the refractory period, at least regarding signaling to actin, is not blocked by the translation inhibitor anisomycin (Babayan, Kramár and Lynch, personal communication). In contrast, infusion of brefeldin A, a toxin that selectively blocks vesicular transport to the membrane, prevented the development of LTP2 when applied prior to TBS2. Brefeldin had no effect on the induction of LTP1 when delivered before the first theta train suggesting that all ingredients needed for potentiation in low threshold spines were present. It is noteworthy that Broutman and Baudry (2001) found that prolonged treatment with brefeldin, encompassing both pre- and post-stimulation intervals, disrupted NMDA-induced LTP in hippocampus; although the stimulation paradigms and degrees of depolarization were markedly different in this work and our own, the results are consistent with the idea that protein transfer in the post-induction period is critical for enduring stabilization of LTP.
Our hypothesis raises the further question of what influence the delayed arrival of replacement proteins might have at the synapses in which LTP was generated by TBS1. Tests of this point produced a striking effect: brefeldin administered after the rapid phase of consolidation caused a complete reversal of LTP1. A simplifying assumption here would be that the same transported material serves to anchor LTP in low threshold synapses and to promote the transition of high threshold spines into an LTP-ready state (i.e., to enable them for later potentiation). Synaptic integrins are an intriguing candidate for this role because they are i) cleaved by proteases activated by TBS (Huttenlocher et al., 1997; Vanderklish et al., 2000), ii) transported to hippocampal membranes by glutamate receptor activation (Lin et al., 2005b), and iii) essential for LTP consolidation (Staubli et al., 1998; Chun et al., 2001; Kramár et al., 2006; Wang et al., 2008).
Collectively, the new set of synaptic conditions to be satisfied for maximizing LTP described above is suggestive of a system in which maximal encoding during behavior is a far from normal occurrence. This point has been made previously with regard to the initial stages of LTP consolidation where recently induced potentiation is readily erased by neuronal firing patterns generated by commonplace activities (Xu et al., 1998; Zhou and Poo, 2004). Plasticity under these conditions requires not only the production of a sufficient number of theta bursts, each with an appropriate number of spikes, but also the avoidance for several minutes of behaviors that cause erasure by initiating low frequency afferent activity at the potentiated contacts. Functionally, these neurobiological rules could ensure that hippocampal networks do not form representations of spurious signals or, in computational terms, have properties that extract regularities from the environment. The LTP refractory period discussed here adds a new dimension to this argument in that it implies that strong encoding can only be achieved by repetitively sampling the external world with long interposed delays. Behavioral research into the importance of distributed practice sessions confirms this point with regard to the strength of memory (Fanselow and Tighe, 1988; Cepeda et al., 2006; Kornell and Bjork, 2008). Psychologists have theorized that separating learning episodes ensures that only constant, and thus presumably task relevant, features are incorporated into memory. Other researchers in this field emphasize the possibility that temporal spacing is related to the time needed to consolidate the memory formed by earlier learning (Wickelgren, 1974), an idea consonant with the demonstration that preparation for LTP2 is accompanied by stabilization of LTP1. It should be emphasized that these potential relationships between LTP and the spaced trials effect relate only to one segment of the much broader time period over which the latter phenomenon operates.
The surprising effect of maturation on the percentage of spines that undergo stable morphological changes in response to single synapse stimulation merits comment. The results could be interpreted as evidence that high threshold spines either appear later in development than their more readily modified counterparts or reflect conversion of a subpopulation of the latter into the former. It will be of interest to test if LTP2, or enhancement of LTP1 by ampakines, is present in the immature hippocampus. Such studies could add a new set of constraints on hypotheses about the cellular origins of threshold differences. Similarly, work is needed on the generality of the TBS1/TBS2 effects discussed here, particularly in light of multiple reports showing that the magnitude of LTP is substantially greater in the basal than apical dendrites of field CA1 (Kaibara and Leung, 1993; Arai et al., 1994; Roth and Leung, 1995). Available evidence suggests that this difference reflects enhanced theta burst responses in the basal arborizations (Arai et al., 1994), an effect that resembles the results produced by ampakines in the apical dendrites. If so, then it is conceivable that the significance of threshold differences is minimized in some synaptic fields because induction conditions in those zones are sufficiently robust to modify all stimulated connections. These arguments lead to the further question of whether developmental processes responsible for the emergence of spines resistant to glutamate uncaging, as found in apical dendrites, also occur basally. There is evidence that basal synapses do not fully transition from the immature condition into adult state with regard to LTP consolidation (Kramár and Lynch, 2003), an observation that in the present context raises the question of whether they also fail to develop other features including high threshold spines. These issues relate directly to the substrates and significance of the new LTP rules surveyed here but could also be of importance to current ideas about how the timing of learning sessions impacts on the nature of the material encoded into long term memory.
What are the implications of these findings for the effects of cognitive enhancers, such as the ampakines, on memory encoding and content? First, the LTP results suggest that ampakine enhancement of theta burst responses may diminish the advantage of spaced versus massed training or, minimally, influence the spacing over which subsequent trials can enhance encoding. As noted, spaced trials serve to minimize the encoding of noise into stable memory; ampakines, and other drugs that facilitate LTP, may therefore be most effective under conditions in which the material to be learned has been filtered. Second, by reducing the need for spaced reinforcement of the memory trace, enhancers would minimize the number of opportunities for erasure of recent memories by behaviors that elicit low frequency activity. This point again emphasizes the possibility that memory enhancement is costly with regard to mechanisms likely used to ensure that the newly formed memories do not include irrelevant information. The same arguments could have very different implications for circumstances in which LTP and memory consolidation are impaired. For example, TBS elicits normal LTP, and increases in spine F-actin, in the standard mouse model of Fragile X mental retardation syndrome, but both changes remain vulnerable to disruption by low frequency stimulation for abnormally long periods (Chen et al., 2010b). It remains to be seen if enhancement of LTP1 in such models, if indeed it occurs, results in full encoding that is resistant to erasure by activity patterns associated with commonplace behaviors (Zhou and Poo, 2004).
Finally, evidence that maximal LTP arises from optimally spaced bursts of afferent activity raises the question of whether conditions associated with severe learning disabilities and cognitive dysfunction are rooted in the inability of networks to recruit high threshold synapses and cluster plasticity within dendritic segments. It has been suggested that clustered plasticity serves to bind features of a common experience to a single target neuron (Govindarajan et al., 2011). From this viewpoint, impairments in lateral influences of the first-potentiated spines might lead to impairments in organizing information into associated, or hierarchical, clusters. It is possible that among the various cognitive enhancers that have been proposed, and discussed in this volume, some may be effective in such ‘cross-linking’ disorders but completely ineffective in other forms of cognitive impairment. Findings of this nature would be informative, as they would provide unique insight into the substrates within neural structures that contribute to memory and cognition. These results would also guide future development of cognitive enhancers that target intellectual/cognitive disability based on the neurobiological underpinnings of the disorder instead of a general clinical feature, such as low IQ or poor memory performance.
Highlights.
New LTP timing rules are identified for adult hippocampus
A post-TBS refractory period for synaptic signaling and LTP induction is identified
There are synapses with high and low threshold for LTP induction in adult hippocampus
We propose spine crosstalk primes high threshold synapses for later potentiation
Ampakine treatment facilitates potentiation of high threshold synapses
Acknowledgments
This research was supported, in part, by NINDS grants NS045260 (G.L., C.M.G.) and NS064079 (G.R.), NIMH grant MH083346 (C.G.), and ONR MURI grant N00014-10-1-0072 (G.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A, Black J, Lynch G. Origins of the variations in long-term potentiation between synapses in the basal versus apical dendrites of hippocampal neurons. Hippocampus. 1994;4:1–9. doi: 10.1002/hipo.450040103. [DOI] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Res Rev. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Benjamin AS, Tullis J. What makes distributed practice effective? Cog Psych. 2010;61:228–247. doi: 10.1016/j.cogpsych.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Rubin DC. The spacing effect depends on an encoding deficit, retrieval, and time in working memory: evidence from once-presented words. Memory. 1998;6:37–65. doi: 10.1080/741941599. [DOI] [PubMed] [Google Scholar]
- Broutman G, Baudry M. Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J Neurosci. 2001;21:27–34. doi: 10.1523/JNEUROSCI.21-01-00027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull. 2006;132:354–380. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Sanaiha Y, Lynch G, Gall CM. Learning induces neurotrophin signaling at hippocampal synapses. Proc Natl Acad Sci U S A. 2010a;107:7030–7035. doi: 10.1073/pnas.0912973107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Babayan AH, Kramár EA, Lynch G, Gall CM, Lauterborn JC. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010b;30:10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D, Gall CM, Bi X, Lynch G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation. Neuroscience. 2001;105:815–829. doi: 10.1016/s0306-4522(01)00173-7. [DOI] [PubMed] [Google Scholar]
- Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro S, Arai A, Kessler M, Bahr BA, Vanderklish P, Rivera S, Lynch G. Stimulation of NMDA receptors activates calpain in cultured hippocampal slices. Neurosci Lett. 1994;167:149–152. doi: 10.1016/0304-3940(94)91049-9. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J Exp Psychol Anim Behav Process. 1988;14:187–199. [PubMed] [Google Scholar]
- Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci. 2010;30:11565–11575. doi: 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Frey U, Schollmeier K, Reymann KG, Seidenbecher T. Asymptotic hippocampal long-term potentiation in rats does not preclude additional potentiation at later phases. Neuroscience. 1995;67:799–807. doi: 10.1016/0306-4522(95)00117-2. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gavin CF, Rubio MD, Young E, Miller C, Rumbaugh G. Myosin II motor activity in the lateral amygdala is required for fear memory consolidation. Learn Mem. 2012;19:9–14. doi: 10.1101/lm.024042.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm Behav. 2011;59:503–511. doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Israely I, Huang SY, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Espana RA, Rogers GA, Porrino LJ, Deadwyler SA. Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology (Berl) 2009;202:355–369. doi: 10.1007/s00213-008-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. J Neurosci. 1999;19:9728–9738. doi: 10.1523/JNEUROSCI.19-22-09728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Kaibara T, Leung LS. Basal versus apical dendritic long-term potentiation of commissural afferents to hippocampal CA1: a current-source density study. J Comp Neurol. 1993;13:2391–2404. doi: 10.1523/JNEUROSCI.13-06-02391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72:1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Knafo S, Venero C, Sanchez-Puelles C, Pereda-Perez I, Franco A, Sandi C, Suarez LM, Solis JM, Alonso-Nanclares L, Martin ED, Merino-Serrais P, Borcel E, Li S, Chen Y, Gonzalez-Soriano J, Berezin V, Bock E, Defelipe J, Esteban JA. Facilitation of AMPA receptor synaptic delivery as a molecular mechanism for cognitive enhancement. PLoS Biol. 2012;10:e1001262. doi: 10.1371/journal.pbio.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornell N, Bjork RA. Learning concepts and categories: is spacing the “enemy of induction”? Psychol Sci. 2008;19:585–592. doi: 10.1111/j.1467-9280.2008.02127.x. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Lynch G. Developmental and regional differences in the consolidation of long-term potentiation. Neuroscience. 2003;118:387–398. doi: 10.1016/s0306-4522(02)00916-8. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci U S A. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Rex CS, Gall CM, Lynch G. Estrogen’s Place in the Family of Synaptic Modulators. Mol Cell Pharmacol. 2009a;1:258–262. [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009b;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, Rumbaugh G, Lynch G. Synaptic evidence for the efficacy of spaced learning. Proc Natl Acad Sci U S A. 2012;109:5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Margulies DS, Farb CR, Hou M, Johnson LR, LeDoux JE. Myosin light chain kinase regulates synaptic plasticity and fear learning in the lateral amygdala. Neuroscience. 2006;139:821–829. doi: 10.1016/j.neuroscience.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Role of N-methyl-D-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 1988;441:111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yasuda R. Spatiotemporal Regulation of Signaling in and out of Dendritic Spines: CaMKII and Ras. Open Neurosci J. 2009;3:117–127. doi: 10.2174/1874082000903020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kramár EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005a;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Lynch G, Gall C. AMPA receptor stimulation increases α5s1 integrin surface expression, adhesive function and signaling. Journal of Neurochemistry. 2005b;94:531–546. doi: 10.1111/j.l471-4159.2005.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Palmer LC, Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 2011;99:116–129. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Observations on clustered synaptic plasticity and highly structured input patterns. Neuron. 2011;72:887–888. doi: 10.1016/j.neuron.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod SJ, Shum AJ, Lee RL, Takei F, Gold MR. The Rap GTPases regulate integrin-mediated adhesion, cell spreading, actin polymerization, and Pyk2 tyrosine phosphorylation in B lymphocytes. J Biol Chem. 2004;279:12009–12019. doi: 10.1074/jbc.M313098200. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 2012;35:135–143. doi: 10.1016/j.tins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuhr A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, Wible CG. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME. Deconstructing signal transduction pathways that regulate the actin cytoskeleton in dendritic spines. Cytoskeleton (Hoboken) 2012 doi: 10.1002/cm.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino L, Daunais J, Rogers G, Hampson R, Deadwyler S. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Gavin CF, Rubio MD, Kramár EA, Chen LY, Jia Y, Huganir RL, Muzyczka N, Gall CM, Miller CA, Lynch G, Rumbaugh G. Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron. 2010;67:603–617. doi: 10.1016/j.neuron.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth LR, Leung LS. Difference in LTP at basal and apical dendrites of CA1 pyramidal neurons in urethane-anesthetized rats. Brain Res. 1995;694:40–48. doi: 10.1016/0006-8993(95)00767-k. [DOI] [PubMed] [Google Scholar]
- Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J Neurobiol. 2004;58:103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- Seese RR, Babayan AH, Katz AM, Cox CD, Lauterborn JC, Lynch G, Gall CM. LTP induction translocates cortactin at distant synapses in wild-type, but not Fmr1-KO mice. J Neurosci. 2012 doi: 10.1523/JNEUROSCI.0968-12.2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ, Thompson RF, Rogers G, Lynch G. Enhanced glutamatergic neurotransmission facilitates classical conditioning in the freely-moving rat. Neurosci Lett. 1995;186:153–156. doi: 10.1016/0304-3940(95)11309-k. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Res. 1987;435:227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- Staubli U, Chun D. Factors regulating the reversibility of long term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994a;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Perez Y, Xu F, Rogers G, Ingvar M, Stone-Elander S, Lynch G. Centrally active modulators of glutamate (AMPA) receptors facilitate the induction of LTP in vivo. Proc Natl Acad Sci USA. 1994;91:11158–11162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanowska J. Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim Pol. 2009;56:225–234. [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Cheeran B, Greenwood RJ, Rothwell JC. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex. 2011;21:1627–1638. doi: 10.1093/cercor/bhq231. [DOI] [PubMed] [Google Scholar]
- Vanderklish P, Saido TC, Gall C, Arai A, Lynch G. Proteolysis of spectrin by calpain accompanies theta-burst stimulation in cultured hippocampal slices. Brain Res Mol Brain Res. 1995;32:25–35. doi: 10.1016/0169-328x(95)00057-y. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Krushel LA, Holst BH, Gally JA, Crossin KL, Edelman GM. Marking synaptic activity in dendritic spines with a calpain substrate exhibiting fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 2000;97:2253–2258. doi: 10.1073/pnas.040565597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Wang XB, Zhou Q. Spine remodeling and synaptic modification. Mol Neurobiol. 2010;41:29–41. doi: 10.1007/s12035-009-8093-9. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. Single-trace fragility theory of memory dynamics. Memory & Cognition. 1974;2:775–780. doi: 10.3758/BF03198154. [DOI] [PubMed] [Google Scholar]
- Wiggan O, Shaw AE, Deluca JG, Bamburg JR. ADF/Cofilin Regulates Actomyosin Assembly through Competitive Inhibition of Myosin II Binding to F-Actin. Dev Cell. 2012;22:530–543. doi: 10.1016/j.devcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]