Abstract
Carbon nanotube (CNT) modification of microelectrodes can result in increased sensitivity without compromising time response. However, dip coating CNTs is not very reproducible and the CNTs tend to lay flat on the electrode surface which limits access to the electroactive sites on the ends. In this study, aligned CNT forests were formed using a chemical self-assembly method, which resulted in more exposed CNT ends to the analyte. Shortened, carboxylic acid functionalized single-walled CNTs were assembled from a DMF suspension onto a carbon-fiber disk microelectrode modified with a thin iron hydroxide-decorated Nafion film. The modified electrodes were highly sensitive, with 36-fold higher oxidation currents for dopamine using fast-scan cyclic voltammetry than bare electrodes and 34-fold more current than electrodes dipped in CNTs. The limit of detection for dopamine was 17 ± 3 nM at a 10 Hz repetition rate and 65 ± 7 nM at 90 Hz. The LOD at 90 Hz was the same as a bare electrode at 10 Hz, allowing a 9-fold increase in temporal resolution without a decrease in sensitivity. Similar increases were observed for other cationic catecholamine neurotransmitters and the increases in current were greater than for anionic interferents such as ascorbic acid and 3,4-dihydroxyphenylacetic acid (DOPAC). The CNT forest electrodes had high sensitivity at 90 Hz repetition rate when stimulated dopamine release was measured in Drosophila. The sensitivity, temporal resolution, and spatial resolution of these CNT forest modified disk electrodes facilitate enhanced electrochemical measurements of neurotransmitters release in vivo.
Introduction
Carbon-fiber microelectrodes have been extensively used to probe electroactive species in the brain with high spatial resolution and minimal tissue damage.1-5In vivo measurements are challenging because the amount of neurotransmitter is low, as evidenced by the nanomolar to micromolar binding affinities of many neurotransmitter receptors,6,7 and many electroacive endogenous intereferents are present in the extracellular fluid.3,5,8 Fast-scan cyclic voltammetry (FSCV) is the most popular electrochemical technique for measuring changes in electroactive neurotransmitters and neuromodulator concentrations because it has fast temporal resolution and provides a cyclic voltammogram fingerprint of the species identified.1,3,9 Electrochemical detection of many cationic neurotransmitters, such as dopamine, is highly dependent on adsorption to the carbon electrode.8,10,11 With FSCV, scans are normally repeated at 10 Hz, as a trade-off between fast measurements and sensitive detection of adsorbed species.12 To improve sensitivity and selectivity, many treatments have been developed to change the surface chemistry including polymer coatings13,14 and overoxidation of the electrode surface.15,16 While these treatments increase the sensitivity of the electrodes, they also slow the time response, because polymer coatings restrict diffusion to the electrode surface and electrode overoxidation adds oxide groups and promotes adsorption. Thus, electrode treatments that increase sensitivity while maintaining high temporal resolution still need to be developed.
Recently, many researchers have demonstrated that carbon nanotubes (CNTs) have good electrochemical properties and can increase the sensitivity and electron transfer of traditional electrodes, such as glassy carbon electrodes.17-20 CNT-modified electrodes exhibit faster electron transfer kinetics, reduced electrode fouling, and increased sensitivity and selectivity for adsorption-controlled species.21-23 CNT-modified microelectrodes show increased sensitivity without compromising the temporal response,22,23 an advantage over polymer-coated and electrochemically treated electrodes. The most popular method to deposit CNT films on a microelectrode surface is to dip carbon-fiber microelectrodes into either a CNT suspension or a CNT/polymer solution.21-25 Two main drawbacks limit the optimization of this method. First, nanotubes are randomly distributed throughout the CNT films and thus most of the area exposed to the analyte solution is the sidewall of the CNTs. However, the ends of the CNTs are likely to be the best sites for electron transfer because they have similar properties to graphitic edge planes and are oxide functionalized during CNT purification process.26-31 Second, a dense and uniform layer of single CNTs is hard to deposit on the electrode surface and large CNT agglomerations are easily formed, causing high noise and low reproducibility.23 Therefore, to fully benefit from the CNTs, an electrode modification strategy must be developed to deposit CNTs on the microelectrode surface with controlled orientation and density.
Fabrication of vertically aligned CNTs has also received much attention recently and two main strategies have been developed.32-34 One strategy is to directly grow CNTs in an aligned manner through chemical vapor deposition with a solid-phase catalyst deposited on the substrate surface.35 This strategy can be robust but requires specialized equipment and is less amenable to mass production. Another strategy is to chemically self-assemble vertically aligned CNTs on a substrate with a solution deposition method.36-38 The immobilization is based on strong interactions between functional groups at the end of CNTs and the modified electrode surface, and alignment is driven by hydrophobic interactions between the sidewalls of CNTs. Chemical self-assembly does not require specialized equipment so it should be possible to fabricate an ordered, dense coating of CNTs on a microelectrode surface to preferentially expose the ends of the CNTs to the solution.
In this study, we used a chemical self-assembly mechanism to deposit shortened, carboxylic acid functionalized single-walled CNTs onto a disk carbon-fiber microelectrode modified with a thin iron hydroxide-decorated Nafion film.37,39 These shortened assembled CNT layers are referred to as CNT forests because of their needlelike domains.37 The CNT forest modified microelectrodes showed greater than 30-fold increases in current for cations such as dopamine. The time response of the electrodes did not change after coating and the large increases in sensitivity facilitated measurements using faster repetition rates with FSCV. Finally, these CNT forest electrodes were used to detect endogenous dopamine changes in the ventral nerve cord of Drosophila melanogaster and were able to maintain both high sensitivity and rapid measurements in vivo.
Methods
Electrochemistry
Carbon-fiber microelectrode fabrication was previously described22 and details about fabrication, the electrochemistry set-up, and solutions can be found in the supplemental information. For all neurochemical measurements, the electrode was scanned with a triangular waveform from –0.4 V to 1.0 V vs. a Ag/AgCl reference electrode at a scan rate of 400 V/s. The repetition rate is 10 Hz unless otherwise noted in the text.
The functionalization of single-walled carbon nanotubes (SWCNTs) and the assembly process were adapted from Chattopadhyay et al.37,39 SWCNTs (HiPCO, Carbon Nanotechnologies, Inc. Houston, TX) were sonicated in a 3:1 mixture of HNO3/H2SO4 for 4 hours at 70 °C and then were filtered with 0.22 μm pore filter membrane (Durapore Membrane; Millipore) and washed repeatedly with deionized water until pH was neutral. During the filtration, the short (below 0.22 μm) nanotubes passed through the membrane pores initially, but the pores were quickly blocked with longer nanotubes. The shortened CNTs were dried in vacuum overnight and then suspended in DMF by sonication for 15 hours at a concentration of 0.02 mg/ml. CNT suspensions were stable for several months. TEM imaging confirmed that there were some short nanotubes, tens of nm long, in the CNT suspensions (Fig. S1). Disk carbon-fiber microelectrodes were sequentially dipped in 0.1 % Nafion (5 wt % stock solution from Liquion-1105-MeOH, Ion Power, New Castle, DE, diluted with 9:1 v/v MeOH:H2O) for 15 minutes and freshly made aqueous FeCl3 (0.5 wt %, pH=2.2) for 15 minutes. The electrodes were washed with basic DMF (pH~12, adjusted with 1% NaOH), immersed in the CNT suspension (pH~8, adjusted with ammonium hydroxide) for 5 minutes, and washed immediately with isopropanol to remove loose nanotubes. Electrodes were dried in vacuum for at least 24 hours before use.
Drosophila experiments
Ventral nerve cord (VNC) preparation and data collection were performed as described previously.40 Homozygous 3-day-old larvae with a th-GAL4; UAS-H134R-ChR2 genotype were fed all-trans retinal for 2-3 days prior to the dissection and were shielded from light. The central nervous system of a wandering 3rd instar larva (5 day-old) was removed in modified Schneider's buffer and the optic lobes cut to yield an isolated VNC, which was adhered to the bottom of a plastic Petri dish containing 3 mL of buffer. An electrode was implanted into the VNC 4-6 segments away from the cut edge and the VNC was allowed to equilibrate for 5 minutes before data collection. 30 seconds of baseline electrochemistry data were collected before a pulse train (4 ms, 60 Hz, 500 pulses) of a 473 nm diode laser was applied (IkeCool Corporation, Los Angeles, CA). A FFT Filter smoothing (Origin-Lab OriginPro 7.5) with a cutoff frequency of 1 Hz was used for the in vivo data to remove noise. All experiments were performed in a dark room. Each individual electrode was tested in a separate VNC.
Statistics
All values are given as mean ± standard error of the mean (SEM) for n number of electrodes and all error bars are given as SEM. Unpaired t-tests were performed to compare properties between two groups including differences between bare and SWCNT forest electrodes and the time response of SWCNT forest electrodes at two different frequencies in Drosophila experiment. A one-way ANOVA with Bonferonni post-tests was used to compare effects among multiple groups. All statistics were performed in GraphPad Prism (GraphPad Software,Inc., La Jolla, CA).
Results and Discussion
Fast-scan cyclic voltammetry of dopamine at SWCNT forest electrodes
Previous studies have modified larger substrates, such as pyrolytic graphite electrodes (PGEs) with self-assembled CNT forests.37,39 Here, we coated disk carbon-fiber microelectrodes because they have a flat surface that is easier for CNT self-assembly than cylindrical electrodes. With a high concentration of CNTs (0.1 mg/ml) and long assembly time (30 min) similar to that used with PGEs, large oxidation currents (29 ± 7 nA for 1 μM dopamine) were observed but the response was slow and did not return to baseline (Fig. S2). CFMEs have an area five orders of magnitude smaller than PGEs so those conditions caused a thick film to be deposited that trapped and accumulated dopamine, leading to high currents. Restricted diffusion in and out of the thick film caused the slow temporal response.
To optimize the response, shorter coating times and lower CNT concentrations were used. A representative cyclic voltammogram from the optimized procedure is shown in Fig. 1A, where CNTs were assembled from a 0.02 mg/ml suspension for 5 min. The SWCNT forest electrode has a 15-fold larger background current than a bare electrode, indicative of about a 15-fold increase in electroactive surface area. The oxidation current of 1 μM dopamine is approximately 30-fold greater than the bare electrode in the background subtracted cyclic voltammograms in Fig. 1B. Thus, the increase in oxidation current is greater than the increase in background current. The shape of the current vs time curve at the SWCNT forest electrode (Fig. 1C) is similar to the bare electrode so the sensitivity is increased without compromising time response.
Fig.1.
Comparison of a bare (solid line) and a SWCNT forest modified (dotted line) disk carbon-fiber microelectrode for the detection of 1 μM dopamine. A. The modified electrode exhibits a 15-fold higher background current than the bare electrode. The inset is the background CV for the bare electrode. B. The oxidation current of 1 μM dopamine is 30-fold higher at the SWCNT forest electrode. The inset shows the dopamine CV for the bare electrode. C. Current vs. time responses for a flow injection analysis experiment. The responses were averaged from 10 electrodes each (error bars are not shown for clarity). The inset shows traces normalized to the same peak height for better comparison in peak shapes.
Carbon nanotube coating could increase electrode sensitivity by increasing electroactive surface area or adding more adsorption sites for adsorption controlled species.22,23 The ends of the CNT are proposed to have similar electrochemical activity to edge plane graphite30 and are functionalized with carboxyl groups during the shortening process.26 Here, it is highly likely that the functionalization is more responsible for the greatly improved sensitivity because electrochemical detection of dopamine is inner sphere, surface dependent, and kinetically dominated by adsorption processes at the electrode surface.10,12 The self-assembly mechanism results in highly organized CNTs on the electrode surface and would preferentially expose the ends of the CNTs. If these ends preferentially adsorb dopamine, this would increase Faradaic current compared to the background current, which scales with area.
Table 1 shows the average oxidation and reduction currents for bare and SWCNT forest electrodes and the ratio of the two currents. The modified electrodes had both increased oxidation and reduction currents. The increase in the reduction current was larger than for the oxidation current so the ratio of the reduction to oxidation current was significantly higher at the SWCNT forest electrodes than bare electrodes (p < 0.05). This higher ratio indicates stronger adsorption and slower desorption kinetics of dopamine-o-quinone at the nanotubes, causing more dopamine-o-quinone to be recycled back to dopamine on the electrode surface where it may be oxidized again. This recycling may partly account for the high oxidation current at the modified electrodes. Table 1 also lists ΔEp values and the ΔEp values were not significantly different between bare and modified electrodes (p = 0.314). This result is consistent with a previous study that showed increased electron transfer kinetics at SWCNT dip-coated electrodes were not observed at traditional FSCV waveforms.22 The rise time from 10% to 90% of peak was calculated to quantitate the time response and the rise times were not significantly different between bare and SWCNT forest electrodes (p = 0.404).
Table 1.
Anodic and cathodic peak currents, peak separation and rise time values for 1 μM dopamine.
| ip,a (nA) | ip,c (nA) | ip,c/ip,a | ΔEp (V) | rise time (s) | |
|---|---|---|---|---|---|
| Bare disk electrodes, n=18 | 0.43 ± 0.03 | 0.22± 0.02 | 0.49 ± 0.03 | 0.65 ± 0.01 | 0.51 ± 0.07 |
| SWCNT forest electrodes, n=46 | 14 ± 2*** | 8.0 ± 0.9*** | 0.61 ± 0.04* | 0.67 ± 0.01 | 0.58 ± 0.04 |
Data are mean ± SEM. Significant differences between bare and modified electrodes were determined by unpaired t-test
p < 0.001
p < 0.05.
For in vivo measurements, cylindrical electrodes are preferred because they have larger surface area and thus higher sensitivity.41 However, a highly sensitive disk electrode would be advantageous for spatially resolved measurements. The average oxidation current of 1 μM dopamine at SWCNT forest electrodes was 14 ± 1 nA, compared to 0.38 ± 0.03 nA at bare disk electrodes. The currents at SWCNT forest modified disk electrodes were higher than 30 μm long, bare cylindrical electrodes which averaged 4.3 ± 0.3 nA. To correct for differences in current due to surface area, the apparent geometric area of each electrode was determined from the electrode dimensions and then the current was divided by that area. The apparent current density for the SWCNT forest modified disk electrodes was 184 ± 19 pA/μm2, larger than the 5.0 ± 0.4 pA/μm2 for bare disk electrodes and 6.1 ± 0.5 pA/μm2 for bare cylindrical electrodes (p < 0.001). The 30-fold larger current density is likely due to enhanced surface roughness and increased adsorption sites for dopamine.
Effects of different coating methods
To confirm the effect of the self-assembly of SWCNT forests, three control experiments were performed. First, electrodes were coated only in the dilute Nafion solution and second, by iron hydroxide-decorated Nafion. Third, bare electrodes (without Nafion/FeO(OH) bilayer) were dipped in the same CNT-DMF suspension used for self-assembly. Fig. 2 shows that the oxidation currents for all three control groups are not significantly different from the bare electrodes, while the SWCNT forest electrodes show a significant difference from all groups (p < 0.001). While Nafion is known to increase the sensitivity of electrodes, the concentration used here was 25-fold more dilute than the typical Nafion coating procedure,42 so a complete film may not form in 5 min. Elemental analysis of the electrode surface by energy dispersive X-ray spectroscopy (EDS) comparing the Nafion and Nafion/FeO(OH) groups revealed the presence of iron hydroxide on the Nafion/FeO(OH) electrode (Fig. S3). The small currents for electrodes dip coated with SWCNTs might be due to the low concentration of CNTs compared to previous studies.22,23,25 The large increases for the SWCNT forest group compared to the dip coated group demonstrate an effect of the self-assembly procedure, not just CNTs.
Fig. 2.
Comparison of different coatings on the oxidation current of 1μM dopamine. The Nafion only electrodes were dipped in 0.1% Nafion for 15 min. The Nafion-FeO(OH) electrodes were dipped sequentially in 0.1% Nafion for 15 min and 0.5% FeCl3 for 15 min and washed with high pH DMF. For the SWCNT dip coated group, bare electrodes were dipped in 0.02 mg/ml SWCNT DMF suspension for 5 min. The SWCNT forest electrodes were modified with Nafion-FeO(OH) and dipped in SWCNT for 5 min. Error bars are standard error of the mean and the number of replicates is the number in the bars. The oxidation current of the SWCNT forest group is significantly different from all the control groups (*** p < 0.001, one-way ANOVA with Bonferroni post-test).
SEM images revealed that only random, small bundles of SWCNTs were present on the dip-coated electrode surface (Fig. S4A). A previous study using phase contrast AFM imaging observed a more patchy appearance after SWCNT forests with 20-30 nm thickness assembled on a mica/Nafion/FeO(OH) substrate.43 Here the SEM images show that the microstructure of FeO(OH) precipitate (Figure S4B) were filled in by aligned CNTs after the assembly of SWCNT forests (Fig. S4C). The electrochemistry and SEM results prove that self-assembly deposits CNTs on the electrode surface in a controllable way which facilitates a highly increased current.
The SWCNT forest electrodes also showed a significant 1.6-fold increase in S/N ratios compared to bare electrodes and a significant 1.8-fold increase compared to SWCNT dip-coated electrodes (p < 0.01). Dip-coated electrodes can have large agglomerations on the surface that cause large amounts of noise and decreased S/N ratios.22,23,25 With self assembly, the CNT forest layer maximizes the accessible electroactive surface area.
Enhanced electrochemical detection for other neurochemicals
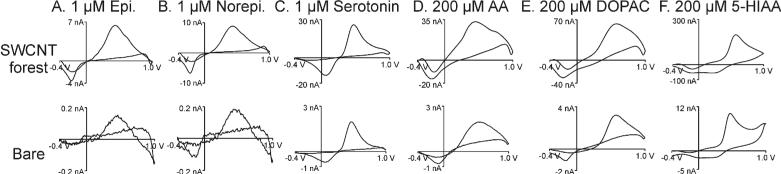
Fig. 3 and Table 2 compare the response for other electroactive neurochemicals using SWCNT forest electrodes. Norepinephrine and epinephrine are catecholamine neurotransmitters and have similar electrochemical properties to dopamine, while serotonin is an indolamine neurotransmitter with a similar oxidation potential to dopamine.8 These compounds are cationic at physiological pH. Norepinephrine and epinephrine had similar trends as dopamine, with higher increases in oxidation current than background current. However, for serotonin, the oxidation current increase was similar to the background current increase. Serotonin has different adsorption properties than dopamine and CFMEs are highly sensitive to serotonin.8,44 Thus, the CNT ends might not preferentially adsorb serotonin as they do catecholamines. Future studies with further chemical modification of the ends of the CNT forests could test the preference for serotonin adsorbing to specific functional groups. Ascorbic acid and 3,4-dihydroxyphenylacetic acid (DOPAC) are anionic compounds, present in high concentrations in the brain, that can interfere with dopamine detection.3 5-hydroxyindoleacetic acid (5-HIAA) is an anionic metabolite of serotonin that interferes with its detection.8 The increases in oxidation current for these anionic species were smaller than that for dopamine and similar to increase in background current. Because the current for catecholamines increased more than for the anions, the SWCNT forest electrodes have better selectivity than bare electrodes.
Fig. 3.
Detection of different neurochemicals at SWCNT forest electrodes. Top: CVs at SWCNT forest electrodes. Bottom: CVs at bare electrodes. The anionic species show smaller increases in oxidation current than the cationic species. Serotonin has a smaller increase than other cationic species. See table 2 for average values.
Table 2.
Average oxidation currents for different neurochemicals at bare and SWCNT forest electrodes.
| Analytes | Bare electrodes (n=13) | SWCNT forest electrodes (n=12) | Ratio (Modified/Bare) |
|---|---|---|---|
| Dopamine (1 μM) | 0.40 ± 0.04 | 11 ± 3 | 28 |
| Epinephrine (1 μM) | 0.14 ± 0.01 | 5 ± 1 | 36 |
| Norepinephrine (1 μM) | 0.21 ± 0.03 | 7 ± 1 | 33 |
| Serotonin (1 μM) | 1.8 ± 0.1 | 25 ± 4 | 14 |
| Ascorbic acid (200 μM) | 2.0 ± 0.1 | 33 ± 5 | 17 |
| DOPAC (200 μM) | 3.1 ± 0.1 | 61 ± 12 | 20 |
| 5-HIAA (200 μM) | 10.0 ± 0.5 | 203 ± 45 | 20 |
Values are mean ± SEM. The ratios (modified/bare) are calculated from the mean values.
Improved temporal resolution at highly sensitive microelectrodes
The high sensitivity of the SWCNT forest electrodes allows them to be used at higher repetition rates, improving temporal resolution. With traditional FSCV, the entire scan of the triangle waveform takes less than 10 ms, but the scans are only repeated every 100 ms to allow time for dopamine to adsorb.12 As shown in Fig. 4A, the oxidation current of 1 μM dopamine decreases for both SWCNT forest and bare electrodes at higher repetition frequencies. However, the modified electrodes still maintain a large current at 90 Hz, which is higher than the bare electrode at 10 Hz (Fig. 4B). The increased sensitivity of the modified electrodes facilitates faster measurements and will allow more accurate determinations of the kinetics of neurotransmitter release events under physiological conditions.12,45
Fig. 4.
Comparison of bare and SWCNT forest modified electrodes at higher repetition frequencies. A. Modified electrodes and bare electrodes both exhibit decreases in current at higher frequencies, but the modified electrodes still maintain a high current at 90 Hz. The inset shows graph for the bare electrodes. B. A bare electrode at 10 Hz and a SWCNT forest modified electrode at 90 Hz for the detection of 1 μM dopamine. The modified electrode has a 10-fold higher current with 9-fold better temporal resolution.
Different concentrations of dopamine were tested with bare and SWCNT forest electrodes at 10 Hz and 90 Hz, respectively (Fig. S5). At 10 Hz, bare disk electrodes exhibited a limit of detection (LOD, S/N = 3) of 63 ± 10 nM while the SWCNT forest electrodes had a LOD of 17 ± 3 nM. At 90 Hz, the LOD was 190 ± 35 nM for bare electrodes and 65 ± 7 nM for the SWCNT forest electrodes. Thus, the SWCNT forest electrodes not only exhibit a 3-fold better LOD than the bare electrodes at 10 Hz, but notably can be used at 9-times faster repetition rate while maintaining the same LOD as traditional bare electrodes. The linear range for the SWCNT forest electrodes was comparable to the bare electrodes, with linear response up to 25 μM dopamine.
Measurements in Drosophila
To validate that the SWCNT forest electrodes maintained the high sensitivity and improved temporal resolution in vivo, these electrodes were used to measure endogenous dopamine changes in the ventral nerve cord (VNC) of the fruit fly, Drosophila melanogaster. The flies were genetically modified to express Channelrhodopsin-2, a blue light sensitive cation channel, in only dopaminergic neurons. Dopamine release was stimulated by a pulse train (60 Hz, 500 pulses) of a 473 nm laser.
Fig. 5A shows a representative color plot of stimulated dopamine release recorded at 10 Hz with a bare disk microelectrode. The current is small, only 0.1 nA, because the disk microelectrode has a small surface area and no current was detected when the repetition rate was increased to 90 Hz. Fig. 5B shows a representative color plot recorded with a SWCNT forest electrode at 90 Hz. The currents are larger than the bare electrode at 10 Hz. Fig. 5C shows the SWCNT forest electrodes maintain significantly higher currents than the bare electrodes at both 10 Hz and 90 Hz. The SWCNT forest electrodes in Drosophila also showed a slight decrease in current at higher frequencies, but the decrease was not as great as in vitro.
Fig. 5.
Comparison of bare and SWCNT forest modified electrodes for dopamine detection in Drosophila. A pulse train (4ms, 60Hz, 500 pulses, denoted by line under figure) of a 473 nm laser was applied to stimulate dopamine release A. detected with a bare electrode at 10 Hz and B. with a SWCNT forest electrode at 90 Hz. The electrodes were placed in different samples. The color plots show all data and dopamine oxidation is the green feature in the middle of the plot. C. The average current detected with bare electrodes at 10 Hz was significantly lower than with SWCNT forest electrodes at 10 Hz and 90 Hz (* p<0.05, ** p<0.01, 1-way ANOVA with Bonferroni post-test, n=6 for bare and n=5 CNT-modified electrodes). D. Representative concentration traces in the same nerve cord recorded with a SWCNT forest electrode at 10 Hz and 90 Hz.
After use, electrodes were calibrated at the respective frequencies and then the currents in vivo were converted to concentrations. Fig. 5D shows representative concentration traces recorded in the same sample with a SWCNT forest electrode at 10 Hz and 90 Hz. The maximal evoked dopamine concentrations was 0.32 ± 0.04 μM for bare electrodes at 10 Hz, 0.42 ± 0.07 μM for SWCNT forest electrodes at 10 Hz, and 0.46 ± 0.11 μM at 90 Hz. As expected, these concentrations were not significantly different. The time from the start of stimulation to peak concentration and the time from peak to half decay of each trace were also compared at SWCNT forest electrodes at 10 Hz and 90 Hz and the results were not significantly different. In brain slices, Kile et al reported that dopamine evoked by a single pulse electrical stimulation rose slower and took longer to return to baseline with FSCV recorded at 10 Hz compared to 60 Hz.45 With our long stimulations, no differences in rise time or decay time were detected. Future work examining shorter stimulations might reveal more differences. However, the SWCNT forest electrodes showed potential for measuring small amounts of neurotransmitters with a fast repetition rate.
Conclusions
Highly sensitive, disk carbon-fiber microelectrodes were fabricated using a chemical self-assembly method to deposit SWCNTs on the surface. This method allowed deposition of a dense, aligned SWCNT forest on the electrode surface in a controlled fashion. Large increases in current were observed after SWCNT forests formation, likely due to the exposure of the CNT ends to the analyte. The high sensitivity of the modified electrodes facilitated the use of faster repetition rate, improving temporal resolution. The increased sensitivity as well as the improved temporal resolution were maintained after implantation in Drosophila, thus these modified electrodes showed great promise for studying neurotransmitter release events in biological systems.
Supplementary Material
Acknowledgement
This work was funded by grants from the National Science Foundation (grant CHE 0645587522) and the National Institutes of Health (grant R01 MH085159) to B.J.V.
Footnotes
ASSOCIATED CONTENT
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Robinson DL, Hermans A, Seipel AT, Wightman RM. Chem. Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kita JM, Wightman RM. Curr. Opin. Chem. Biol. 2008;12:491–496. doi: 10.1016/j.cbpa.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Clin. Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 4.Troyer KP, Heien ML, Venton BJ, Wightman RM. Curr. Opin. Chem. Biol. 2002;6:696–703. doi: 10.1016/s1367-5931(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 5.Wightman RM. Science. 2006;311:1570–1574. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]
- 6.Richfield EK, Penney JB, Young AB. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- 7.Berke JD, Hyman SE. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 8.Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM. Anal. Chem. 1988;60:1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- 9.Kawagoe KT, Zimmerman JB, Wightman RM. J. Neurosci. Meth. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- 10.Bath BD, Martin HB, Wightman RM, Anderson MR. Langmuir. 2001;17:7032–7039. [Google Scholar]
- 11.Duvall SH, McCreery RL. J. Am. Chem. Soc. 2000;122:6759–6764. [Google Scholar]
- 12.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal. Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 13.Pihel K, Walker QD, Wightman RM. Anal. Chem. 1996;68:2084–2089. doi: 10.1021/ac960153y. [DOI] [PubMed] [Google Scholar]
- 14.Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Anal. Chem. 2009;81:9462–9471. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heien MLAV, Phillips PEM, Stuber GD, Seipel AT, Wightman RM. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 16.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM. Anal. Chem. 2010;82:2020–2028. doi: 10.1021/ac902753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs CB, Peairs MJ, Venton BJ. Anal. Chim. Acta. 2010;662:105–127. doi: 10.1016/j.aca.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Ratinac KR, Ringer SP, Thordarson P, Gooding JJ, Braet F. Angew. Chem. , Int. Ed. 2010;49:2114–2138. doi: 10.1002/anie.200903463. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Yantasee W, Wang J. Front Biosci. 2005;10:492–505. doi: 10.2741/1545. [DOI] [PubMed] [Google Scholar]
- 20.Dumitrescu I, Unwin PR, Macpherson JV. Chem. Commun. 2009:6886–6901. doi: 10.1039/b909734a. [DOI] [PubMed] [Google Scholar]
- 21.Hocevar SB, Wang J, Deo RP, Musameh M, Ogorevc B. Electroanalysis. 2005;17:417–422. [Google Scholar]
- 22.Swamy BEK, Venton BJ. Analyst. 2007;132:876–884. doi: 10.1039/b705552h. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs CB, Vickrey TL, Venton BJ. Analyst. 2011;136:3557–3565. doi: 10.1039/c0an00854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong H, Jeon S. Sensors. 2008;8:6924–6935. doi: 10.3390/s8116924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peairs MJ, Ross AE, Venton BJ. Anal. Chem. 2011;3:2379–2386. [Google Scholar]
- 26.Liu J, Rinzler AG, Dai HJ, Hafner JH, Bradley RK, Boul PJ, Lu A, Iverson T, Shelimov K, Huffman CB, Rodriguez-Macias F, Shon YS, Lee TR, Colbert DT, Smalley RE. Science. 1998;280:1253–1256. doi: 10.1126/science.280.5367.1253. [DOI] [PubMed] [Google Scholar]
- 27.Banks CE, Compton RG. Analyst. 2006;131:15–21. doi: 10.1039/b512688f. [DOI] [PubMed] [Google Scholar]
- 28.Banks CE, Compton RG. Anal. Sci. 2005;21:1263–1268. doi: 10.2116/analsci.21.1263. [DOI] [PubMed] [Google Scholar]
- 29.McCreery RL. Chem. Rev. 2008;108:2646–2687. doi: 10.1021/cr068076m. [DOI] [PubMed] [Google Scholar]
- 30.Banks CE, Davies TJ, Wildgoose GG, Compton RG. Chem. Commun. 2005:829–841. doi: 10.1039/b413177k. [DOI] [PubMed] [Google Scholar]
- 31.Forrest GA, Alexander AJ. J. Am. Chem. Soc. 2007;111:10792–10798. [Google Scholar]
- 32.Diao P, Liu Z. Adv. Mater. 2010;22:1430–1449. doi: 10.1002/adma.200903592. [DOI] [PubMed] [Google Scholar]
- 33.Yan YH, Chan-Park MB, Zhang Q. Small. 2007;3:24–42. doi: 10.1002/smll.200600354. [DOI] [PubMed] [Google Scholar]
- 34.Huang LM, Jia Z, O'Brien S. J. Mater. Chem. 2007;17:3863–3874. [Google Scholar]
- 35.Gao LJ, Peng AP, Wang ZY, Zhang H, Shi ZJ, Gu ZN, Cao GP, Ding BZ. Solid State Commun. 2008;146:380–383. [Google Scholar]
- 36.Diao P, Liu ZF, Wu B, Nan XL, Zhang J, Wei Z. Chemphyschem. 2002;3:898–901. doi: 10.1002/1439-7641(20021018)3:10<898::AID-CPHC898>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Chattopadhyay D, Galeska I, Papadimitrakopoulos F. J. Am. Chem. Soc. 2001;123:9451–9452. doi: 10.1021/ja0160243. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZF, Shen ZY, Zhu T, Hou SF, Ying LZ, Shi ZJ, Gu ZN. Langmuir. 2000;16:3569–3573. [Google Scholar]
- 39.Yu X, Chattopadhyay D, Galeska I, Papadimitrakopoulos F, Rusling JF. Electrochem. Commun. 2003;5:408–411. [Google Scholar]
- 40.Vickrey TL, Condron B, Venton BJ. Anal. Chem. 2009;81:9306–9313. doi: 10.1021/ac901638z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venton BJ, Wightman RM. Anal. Chem. 2003;75:414A–421A. [Google Scholar]
- 42.Jones SR, Garris PA, Wightman RM. J. Pharmacol. Exp. Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- 43.Malhotra R, Papadimtrakopoulos F, Rusling JF. Langmuir. 2010;26:15050–15056. doi: 10.1021/la102306z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson BP, Dietz SM, Wightman RM. Anal. Chem. 1995;67:1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 45.Kile BM, Walsh PL, McElligott ZA, Bucher ES, Guillot TS, Salahpour A, Caron MG, Wightman RM. ACS Chem. Neurosci. 2012;3:285–292. doi: 10.1021/cn200119u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.