Abstract
Cardiovascular disease is the leading cause of death in United States and worldwide. Failure to properly repair or regenerate damaged cardiac tissues after myocardial infarction is a major cause of heart failure. In contrast to humans and other mammals, zebrafish hearts regenerate after substantial injury or tissue damage. Here, we review recent progress in studying zebrafish heart regeneration, addressing the molecular and cellular responses in the three tissue layers of the heart: myocardium, epicardium, and endocardium. We also compare different injury models utilized to study zebrafish heart regeneration, and discuss the differences in responses to injury between mammalian and zebrafish hearts. By learning how zebrafish hearts regenerate naturally, we can better design therapeutic strategies for repairing human hearts after myocardial infarction.
Keywords: zebrafish heart regeneration, myocardium, epicardium, endocardium, cryoinjury
INTRODUCTION
Most tissues and organs in the human body have very limited regenerative capacity after injury. Many degenerative diseases, including heart disease, are thus a result of tissue damage without regeneration. Consequently, scar tissues replace the lost cell population and damaged extracellular matrix and this leads to impaired organ function. Inducing the regenerative capacity of damaged tissues or organs, therefore, has become one of the major goals of modern medicine.
Myocardial infarction (MI) is among the leading causes of mortality in the United States and worldwide (1, 2). It is often a result of coronary heart disease and leads to irreversible loss of cardiomyocytes by necrosis and apoptosis in the heart after ischemia (3). Most mammalian cardiomyocytes are terminally differentiated and unable to divide; the hearts respond to injury by scarring, which results in decreased cardiac function (4, 5). To compensate for decreased cardiac function, the post-infarct hearts develop hypertrophy or dilation, which eventually leads to heart failure.
Several different strategies have been attempted to repair damaged hearts and they include cell replacement, cytokine and growth factor therapy, and tissue engineering. Stem cells and cardiac progenitor cells are promising cell sources for cell replacement therapy. Autologous skeletal myoblasts and bone marrow stem cells have been utilized for randomized, double blinded clinic trials for cardiac repair. However, transplanting these cells has so far failed to significantly improve cardiac functions (measured as left ventricular ejection fraction), and any slight improvement in the test group was attributed to possible paracrine effects of the implanted cells (6, 7). Cytokine and growth factor therapy is a promising non-invasive treatment for heart diseases and has been utilized to stimulate angiogenesis, to enhance cardiomyocyte proliferation and survival, and to recruit stem and progenitor cells to the damaged hearts (7, 8). Fibroblast growth factor 4 (FGF4) and vascular endothelial growth factor (VEGF) were among the first to be tested in clinical trials for revascularization. Their effects, however, were statistically insignificant (8). Furthermore, several cytokines have been shown to mobilize bone marrow stem cells in animal myocardial infarction models. Although initial clinical trials with granulocyte colony-stimulating factor (G-CSF) treatment were promising, subsequent double-blinded trials did not show enhanced left ventricle ejection fraction or reduced infarct size (7–9). Tissue engineering approaches aim to create functional cardiac tissue implants with various combinations of cells and matrix scaffolds to replace damaged cardiac tissues. However, there are still many technical challenges in this approach; for instance, one must decide which human cell types to use, what cell matrix to use as a scaffold, and how the engineered grafts can be vascularized such that they could reach the desired size (7, 10). These considerations may also apply to stem cell or cytokine based approaches. Therefore, despite the recent progress on stem cells, cytokine and growth factor therapy (7, 11, 12), and tissue engineering (10, 13, 14), strategies for repairing diseased or injured hearts are still far from being able to regenerate a perfect heart. A more thorough understanding of the functional structure and the repair mechanism of cardiac tissues is required to improve current approaches in repairing and functional restoration of the injured heart.
Zebrafish as a model for human heart development and diseases
In contrast to the four-chambered human hearts, zebrafish hearts are two-chambered with one ventricle and one atrium (Fig. 1). Nonetheless, there are many striking similarities between zebrafish and mammals in terms of heart structure and embryonic morphogenesis. As in mammals, the zebrafish heart is composed of three layers of tissue called the myocardium, epicardium and endocardium. The myocardium can be divided into the compact layer and the trabecular layer (15) (Fig. 1). Embryonic studies and forward genetic screens using zebrafish contributed greatly to the understanding of both human cardiac development and disease, e.g. dextrocardia (a congenital defect in which the heart is situated on the right side of the body), aortic valve stenosis defect, dilated cardiomyopathy, mitral valve malformation, transposition of the great arteries, double-outlet-right-ventricle and hypertrophic cardiomyopathy (16). Moreover, adult zebrafish hearts exhibit an amazing capacity to regenerate after substantial injury and have been used as a model for studies that might lead to approaches in regenerative repair of human heart injuries.
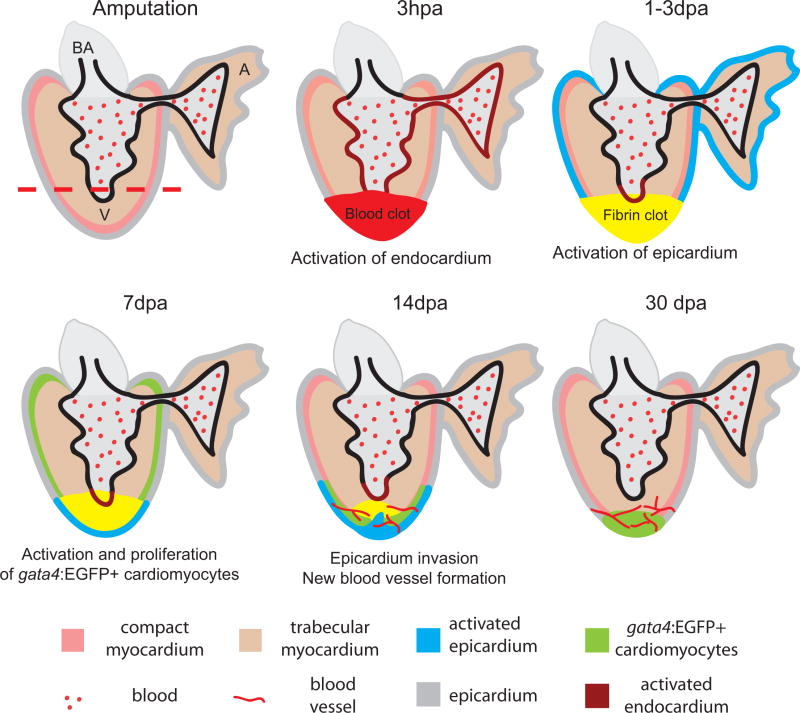
Figure 1. Summary of events during zebrafish heart regeneration.
The process begins at amputation; time points of 3 hours post amputation (hpa) and 1–3, 7, 14 and 30 days post amputation (dpa) are illustrated. The amputation plane is marked by a red dashed line. Right after amputation, a blood clot (red) forms. Within hours, the endocardium (brown) is activated and shows morphological and gene expression changes. At 1–3 dpa, the blood clot becomes a fibrin clot (yellow). The activated raldh2 expression in endocardium becomes localized to the injury site (brown). At the same time, the epicardium (blue) is activated and expresses embryonic markers. At 7 dpa, the epicardium encloses the apex and starts to invade the fibrin clot while a population of gata4:EGFP positive cardiomyocytes appears at the sub-epicardium and begins to proliferate. At 14 dpa, the gata4:EGFP positive cardiomyocytes localize to the apex and newly formed blood vessels vascularize the newly formed myocardium. By 30 dpa, the myocardium is almost fully regenerated. The new blood vessels vascularize the new myocardium. A, atrium; V, ventricle; BA, bulbus arteriosus.
Adult zebrafish hearts regenerate after ventricular resection
In contrast to humans, many vertebrates can regenerate lost tissues and organs. One well-known example is the newt. Newts can fully regenerate their limbs after amputation (17). Zebrafish also have remarkable regenerative abilities and can regenerate many organs and tissues, such as fin, optic nerve, liver, retina, spinal cord, and heart (18–29). Zebrafish fully regenerate their myocardium after 20% ventricular resection in 1–2 months (18, 20). After amputation, a blood clot forms at the site of injury to seal the ventricle and stop bleeding (Fig. 1). With the disappearance of red blood cells in the clot and an enrichment of the clot protein fibrin, the blood clot is turned into a fibrin clot in 2–3 days post amputation (dpa) (18) (Fig. 1). Gene expression profiling analysis suggests that the early stages of heart regeneration are similar to a wound healing response (30).
In contrast to the human response after MI, three major regeneration events occur naturally in the zebrafish hearts. Cardiomyocytes start to proliferate to replace lost myocardium. The epicardium is activated by re-expressing embryonic markers and can contribute to coronary vessel formation that vascularize the regenerated tissues. The endocardium is also activated and starts retinoic acid synthesis, which supports cardiomyocyte proliferation (31). Recent advances in our understanding of cardiomyocyte proliferation, epicardial activation and neovascularization, and endocardial activation during zebrafish heart regeneration are reviewed in the following sections, along with some approaches used in the studies. The ventricular resection model for heart injury is used in these studies. Other more recently developed injury models are discussed and compared with the resection model towards the end of this review.
Zebrafish myocardial regeneration is mainly achieved by existing cardiomyocyte proliferation
Replacing the damaged or lost cardiomyocytes is the most important step in repairing injured cardiac muscles of mammalian hearts. Studies have shown that during zebrafish heart regeneration, cardiomyocytes initiate DNA synthesis and cell proliferation at 7 dpa. The proliferative activity peaks at 14 dpa (Fig. 2) (18, 20). New cardiomyocytes replace most of the lost ventricular tissue, and the structure of the heart is fully restored by 30 dpa (18). Understanding the origin of these proliferating cardiomyocytes is of significant interest not only because it will provide information on the underlying mechanism of zebrafish heart regeneration, but also because it will impact the strategies and approaches that will be taken for regenerative repair of the injured human heart. Two research groups have used the technique of genetic tracing to clarify whether these cells are from resident or distant stem/progenitor cells, or if they are from existing cardiomyocytes via de-differentiation and expansion. This was carried out in one study by permanently labeling the existing cardiomyocyte population (i.e., cmlc2 positive) in zebrafish embryos using a GFP reporter gene with a floxed stop cassette. A modified Cre recombinase (CreERt2) that can be induced with the estrogen analogue 4-hydroxy-tamoxifen (4-OHT) was expressed in cmlc2 positive cardiomyocytes (32). After transient exposure to 4-OHT, only cmlc2 positive cardiomyocytes expressed GFP (Fig. 3). These results showed that the cardiomyocytes of the regenerated adult fish heart are GFP positive and therefore derived from preexisting cardiomyocytes (32). Nonetheless, these experiments do not address whether cardiomyocytes are a heterogeneous population of cells and new cardiomyocytes may arise from a less-differentiated subpopulation and contribute to regeneration.
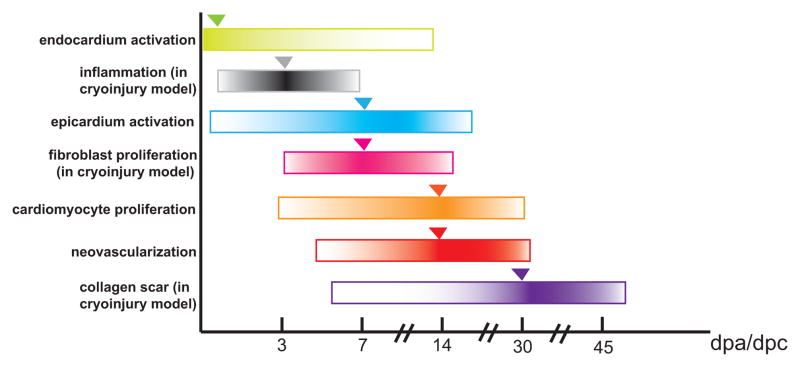
Figure 2. Summary of the timing of events during zebrafish heart regeneration.
dpa, days post amputation; dpc, days post cryoinjury. Time course of events are marked with horizontal bars. The relative level of activity is shown as a gradient; darker colors indicate stronger activity. The peak of activity is marked with triangles.
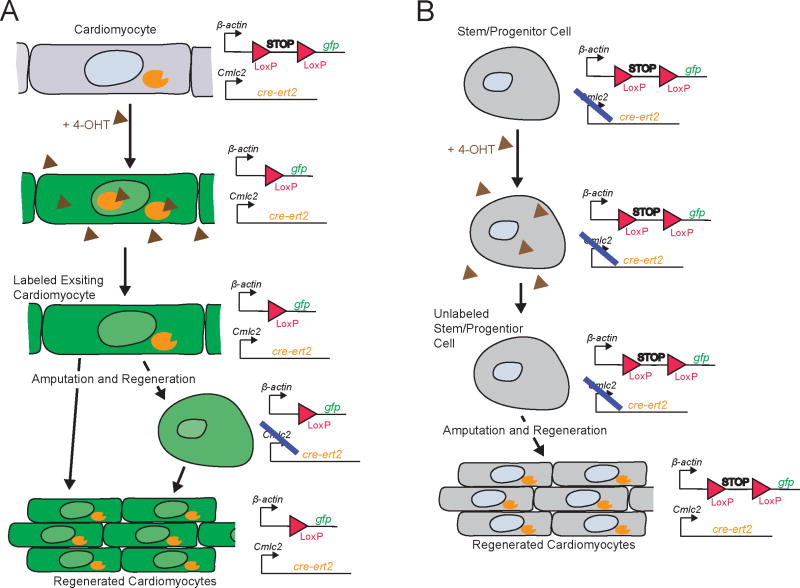
Figure 3. Strategy for Cre-lox based lineage tracing of the source of newly formed cardiomyocytes during zebrafish heart regeneration.
Cre recombinase coding sequence is fused to the estrogen receptor coding sequence (cre-ert2) to make an inducible Cre protein (orange), which is then induced using the estrogen agonist 4-OHT (brown) prior to amputation; this fusion protein is specifically expressed in pre-existing cardiomyocytes using the cardiacmyosin light chain 2 (cmlc2) promoter. Expression of a gfp reporter (green) is driven by the constitutive β-actin promoter, but expression is blocked by a stop sequence (STOP) flanked byloxP sites. A. Expected result if newly regenerated cardiomyocytes are derived from existing cardiomyocytes. The stop sequence is specifically excised via Cre-mediated site-specific recombination in the cardiomyocyte lineage when 4-OHT is added and gfp is therefore expressed in newly formed cardiomyocytes (this is the result reported in ref (32, 33)), even if the cardiomyocytes transiently dedifferentiate into a non-cmlc expressing cell. B. Theoretical result if newly regenerated cardiomyocytes were derived from stem/progenitor cells. Since these cells would not express cmlc2-driven cre-ert-2 prior to amputation, 4-OHT would have no effect on the stop sequence, which would therefore remain intact and continue to block gfp expression in this lineage, even after differentiation to form new cardiomyocytes. The blue line indicates that there is no expression from the promoter.
Kikuchi et al. addressed the origin of proliferating cardiomyocytes using a similar approach, but induced cmlc2:CreERT2 recombination in adults. They also genetically labeled a subpopulation of sub-epicardial compact cardiomyocytes using the promoter of gata4, a gene important for heart development (33). gata4:EGFP and gata4:ERCreER labeled cells in the amputated heart were found to be both proliferative and to have entered the wound site by 14 dpa. This study further defined the contribution of these gata4:EGFP labeled cardiomyocytes in regenerating myocardium, and also suggests that this specific subpopulation of cardiomyocytes may play a major role for myocardium regeneration in adult zebrafish (33).
Cardiomyocytes in the regenerating myocardium appear to lose some of their differentiated cellular features. For instance, the sarcomeric structure in these cardiomyocytes, which is normally required to generate contractile force, is altered (32). This also indicates that dedifferentiation of cardiomyocytes must have occurred in order for the cells to reenter the cell cycle. Exactly how far the cardiomyocytes have to dedifferentiate down the cardiac linage to reenter the cell cycle is still unclear. Nonetheless, the electrical coupling of the injured heart is restored upon regeneration (33). The expression of developmental markers such as hand2 and nkx2.5 in the regenerative process shown in an earlier study points to progenitor population involvement in the regenerative process (34). However, new evidence (32, 33) brings into question the role of stem /progenitor cell involvement in the regenerative process.
Activation of the epicardium and neovascularization of the regenerating myocardium
Within 1–2 days after amputation, the epicardium of regenerating heart starts to express the embryonic epicardial markers retinaldehyde dehydrogenase 2 (raldh2) (which encodes the rate-limiting enzyme for retinoic acid (RA) synthesis) and tbx18 (which encodes a T-box transcription factor) (34). It indicates a re-activation of embryonic morphogenesis in the injured epicardium. The injury-induced activation of the epicardium starts throughout the entire ventricle and gradually becomes localized to the apex (34), although the mechanism for this is unknown. Proliferation is detected from 3 to 7 dpa in cells of the activated epicardium (34). Similar to mice and chick heart development where epicardial cells undergo epithelial-to-mesenchymal transition (EMT) to form coronary vessels, markers of EMT (i.e., snail and twist genes) were detected in regenerating zebrafish hearts (35). The epicardial EMT process gives rise to fibroblasts and pericyte-like perivascular mural cells of the coronary vessels. Indeed, we found that expression of mural cell markers sm22αb/transgelin (tagln), α smooth muscle actin (αSMA)/acta2 (36, 37) and pdgfrβ is increased at the wound site (35).
To study the functions of epicardial cells, we established an in vitro primary culture model of adult zebrafish epicardial cells on a fibrin gel to mimic the in vivo environment (35, 38). We showed that epicardial cells from sham-operated hearts have strong nuclear staining of the epicardial marker tcf21 and exhibit a typical epithelial phenotype of close cell-cell contact with subcortical actin bundles and expression of the tight junction marker ZO1 (35). Furthermore, PDGF-BB induces epicardial cells to reorganize their subcortical actin into stress fibers, to lose cell-cell contacts in the cultured epicardial cells, and to form mesenchymal-like cell types (35).
Previous lineage tracing experiments in mice using the epicardial genes Wt1 and Tbx18 suggested that epicardial cells can give rise to coronary vascular smooth muscle and cardiomyocytes during mammalian development (39, 40). This in vivo myogenic property was not reported in earlier fate mapping experiments using avian and other vertebrate models, wherein it was suggested that epicardium was the primary source of perivascular and smooth muscle populations in the heart (41–46). In zebrafish it appears that the expression of both wt1 and tbx18 is not restricted to the epicardium, but is also expressed in intra-myocardial cells and cardiac muscle (47). This is also the case in mouse, wherein developmental studies suggested that Tbx18 is also expressed in a subset of cardiomyocytes; this indicates that Tbx18 may not be ideal for epicardial cell lineage tracing (48). To determine the fate of epicardium-derived progenitor cells (EPDCs) in vivo during zebrafish heart regeneration, Kikuchi et al. (2011) carried out genetic lineage tracing of tcf21-positive cells. tcf21 is expressed in the outer epithelial epicardial layer and in EPDCs of the subepicardium (47). Following amputation, tcf21-positive cells give rise to EPDC and perivascular mesenchymal cells. The authors carefully analyzed if any of the tcf21-labeled population gave rise to cardiomyocytes during zebrafish development and regeneration and conclusively found that in both cases, cardiomyocytes are not derived from epicardial cells. This suggests that epicardial cells may not be a major potential source of cardiomyocytes and do not readily acquire a myocardial phenotype.
Interestingly, quiescent mammalian epicardium can also be activated after MI, similar to zebrafish heart after myocardium resection. Embryonic epicardial markers such as Tbx18, Raldh2 and Wt1 are reexpressed (49–51). However, unlike adult zebrafish and fetal mammalian epicardium, EPDC of adult mouse hearts after MI largely remain a thickened layer at the surface of the heart and fail to migrate into the myocardium. These cells mainly contribute to post MI response by releasing paracrine factors that stimulate angiogenesis (51). Nonetheless, epicardial cells may still provide an attractive target for therapeutically inducing cardiac muscle regeneration in situ after acute myocardial infarction. Smart et al. (2011) demonstrated that epicardial Wt1-expressing cells do have the potential to adopt a cardiomyocyte fate in vivo in a mouse myocardial infarct model (52). However, it appears that only a small sub-population of EPDCs may have such potential, and furthermore this population needs to be directed to adapt the cardiomyocyte fate with Thymosin β4 induction (52). It remains to be determined as to how effective or efficient such an intervention can be therapeutically.
Endocardial activation is required for cardiomyocyte proliferation
Within 1–3 hours post amputation (hpa) of zebrafish hearts, the endocardium reacts to the heart injury by undergoing organ-wide morphological changes (Fig. 2). The endocardial cells appear rounded and are detached from the myocardium (31). At the same time, the endothelial cells start to express raldh2. Strong raldh2 expression persists in both the atrium and ventricle until 6 hpa but becomes localized to the injury site by 1 dpa. This endocardial expression of raldh2 appears earlier than its expression in the epicardium and is maintained until 14 dpa. Interestingly, similar organ-wide raldh2 expression can be induced by lipopolysaccharide (LPS), which can cause systemic inflammation and increased permeability of endothelial cells. It has been speculated that cardiac injury causes inflammation and increased permeability of endothelial cells, which triggers raldh2 expression, and leads to RA synthesis as a response to the injury (31). Although retinoic acid has been implicated in mammalian heart development, Raldh2 expression is confined to the epicardium (53, 54) and no such response has been observed in injured mammalian hearts. These results suggest a potential therapeutic intervention strategy to increase the regenerative capacity of human hearts by modulating nonmyocardial sources of retinoic acid production.
When RA signaling is inhibited in zebrafish expressing a dominant-negative retinoid acid receptor alpha (RARα) or the RA degrading enzyme Cyp26, cardiomyocyte proliferation is severely impaired (31). However, RA or a synthetic retinoid agonist is not sufficient to induce cardiomyocyte proliferation in adult zebrafish (31). RA signaling has been well studied in heart development and it is known that RA signaling is important for cardiomyocyte proliferation and myocardial growth during mid-gestation of embryonic development (55, 56). However, RXRα does not function cell-autonomously for myocardial cardiomyocyte proliferation (44, 57, 58). Not until recently was RA action during development demonstrated to function through the hepatic erythropoietin (EPO) signaling pathway that activates the expression of IGF2, which in turn activates cardiomyocyte proliferation through the cardiac IGF receptor (59, 60). It will be interesting to determine whether a similar mechanism is reactivated during zebrafish heart regeneration.
Different injury models to study zebrafish heart regeneration
Amputation of the ventricular apex, in which 20% of the ventricle is removed by a clean cut, has been used to induce injury in the zebrafish heart for almost a decade. All the findings described above were done using the ventricular resection model. Recently, cryoinjury has been established as an alternative injury model by three groups in Europe (61–63). Although the methods used by these groups are different, in general the heart cells are all damaged by freezing and thawing after coming into contact with dry ice or a probe pre-chilled in liquid nitrogen. However, depending on whether a cryoprobe or dry ice is used and how severe the cryoinjury is, zebrafish hearts may form either a collagen scar (61, 62) or a fibrin (63) filled wound (also our unpublished observations) in the early stages of regeneration. The size of the wound can vary from to 15–25% of the ventricular volume (Table 1). Furthermore, the time required to complete regeneration can vary from 60 (62, 63) to 120 days (61). These results suggest that it takes more time to regenerate after more severe injuries even for zebrafish. Despite these inconsistencies, the major difference between cryoinjury and amputation is that dead cells and tissue remain in the heart after cryoinjury, mimicking the pathogenesis after myocardial infarction. Compared to ventricular amputation, cryoinjury is probably more clinically relevant and involves tissue and cell death through apoptosis and necrosis. Many of the post cryoinjury responses are similar to regeneration after ventricular amputation, including cardiomyocyte proliferation and reactivation of epicardium (Table 1).
Table 1.
Comparison between different injury models for studying zebrafish heart regeneration
| Type of injury | Ventricular amputation (18, 20) | Cryoinjury (61, 62), (63) | Genetic Ablation (65) |
|---|---|---|---|
| Method of damage | Removal of cells | Cell damage caused by freezing and thawing | Cardiomyocyte specific cell death caused by expression of diptheria toxin A chain (DTA) |
| Size of injury | 20% of the ventricle | 15–25% of the ventricle | >60% of the myocardium |
| Mortality after the procedure | 10% | 5% | Dose dependent |
| Cell death | Some apoptosis and necrosis along the amputation plane | Apoptosis and necrosis throughout the heart | Apoptosis throughout the ventricle uniformly at 3 and 5 dpi |
| Inflammation | Yes ((30) and unpublished) | Yes | Yes |
| Cardiomyocyte proliferation | Yes | Yes | Yes |
| Epicardial activation | Yes | Yes | Yes |
| Initial collagen deposition | No | Yes (61, 62) No (63) |
No |
Cardiomyocyte death after cryoinjury first elicits an inflammatory response in zebrafish hearts. The wound area is infiltrated with leukocytes with characteristics of neutrophil granulocytes at one days post cryoinjury (dpc) (63) (Fig. 2). At 3 and 7 dpc, both neutrophil and eosinophil granulocytes are found in the wound (63). The inflammatory response induced after cryoinjury likely triggers the fibrosis that leads to collagen deposition, akin to what has been described in mammalian wound healing. During regeneration after ventricular resection, there is little or no collagen scar formation throughout the entire process. By contrast, a large collagen scar can form after cryoinjury is induced with a probe pre-chilled in liquid nitrogen (61, 62) (Table 1). During the infarct healing of mammalian hearts (ischemia-reperfusion model), inflammation occurs during the first 72 hrs when leukocytes infiltrate the infracted myocardium. Between 72 hrs and 7 days, granulation tissues form in response to cytokines and growth factors released by macrophages, and fibroblasts and endothelial cells start to proliferate. Activated myofibroblasts will start to produce extracellular matrix proteins. A mature collagen-based scar forms during 7–14 days post MI and (reviewed in (64)). Comparing the time courses of inflammation and fibrosis after zebrafish heart cryoinjury and mammalian heart after MI suggests that inflammation occurs during a similar time frame (Fig. 2). The scar tissue in zebrafish heart does not appear as mature as in mammalian hearts (unpublished data). Interestingly, the scar is later absorbed or resolved and the zebrafish heart still fully regenerates after cryoinjury (61, 62). However, it can take almost twice the amount of the time to regenerate after severe cryoinjury compared to amputation (63). One possibility is that extra time is needed to resolve the scar. The mechanisms underlying collagen scar resolution are currently unknown.
In addition to mechanical injury such as ventricular amputation and cryoinjury, genetic ablation has also been used to determine if zebrafish hearts can regenerate after killing cardiomyocytes specifically (65). There are some caveats to amputation or cryoinjury as an injury model. For instance, the damage is limited to the apex area; one cannot test whether internally isolated areas can effectively regenerate. Furthermore, amputation and cryoinjury removes and/or damages all tissue layers; it is not possible to address how zebrafish respond to cardiomyocyte-specific damage without tissue disruption and blood clotting (65). Cardiomyocyte-specific genetic ablation is achieved by expressing the diptheria toxin A chain (DTA) using a Cre-lox based strategy similar to the one described in the previous section. This strategy causes 60% of cardiomyocytes to be ablated and results in a physiological condition similar to heart failure (65). Interestingly, this severe injury can cause a robust regenerative response of adult zebrafish hearts with 42% of cardiomyocytes undergoing proliferation ((65) and Table 1). Furthermore, this cardiomyocyte-specific death is sufficient to induce a strong inflammatory response involving macrophage and/or neutrophil recruitment independent of blood/fibrin clot formation. This model also eliminates the need for doing microsurgery, which is challenging to inexperienced researchers. Similar strategies could be utilized to ablate different cell populations in the hearts to determine their roles in heart regeneration.
Future directions
An immediate question raised by the recent report by Kikuchi et al (2010) (33) is whether the gata4:EGFP positive sub-epicardial cardiomyocyte population is present in mammalian hearts and whether these cells can be utilized for cardiac repair. If an analogous population exists in the mammalian heart then they could provide an excellent target for therapeutic intervention, particularly if we can determine what growth factors and signaling pathways can stimulate the proliferation of these gata4:EGFP positive cells. As these cells are located in the sub-epicardium/compact myocardium, are these growth factors stimulating the proliferation of the gata4:EGFP cells from epicardium? We did not detect gata4 transcript expression by section in situ hybridization (unpublished observation). It is not clear whether these sub-epicardial cells really express gata4 or if the gata4 promoter utilized to drive the GFP expression is an enhancer fragment that happens to mark this population. It is also possible that the gata4 mRNA is rapidly degraded in these cells while the GFP protein is much more stable. It will be interesting to determine whether Gata4 protein is expressed in these sub-epicardial cells.
Neovascularization is required for continued regeneration after the creation of regenerated muscle. It remains unclear whether the new endothelial cells of the coronary vessels derive from wound angiogenesis or de novo vasculogenesis during zebrafish regeneration. Epicardial cells appear to contribute to the majority of perivascular cells during this process (47). Proepicardial cells had been thought to be the source of endothelial cells of the coronary vasculature during development, but more recent observations in mice suggest that the sinus venosus may be the source of such cells during development (66). Consistent with these more recent developmental studies in mouse, it appears the epicardium is not the source of such cells during zebrafish heart regeneration. Mammalian hearts also reactivate a fetal-like state after MI that involves induction of embryonic epicardial marker expression, proliferation, and EMT (51). The activated epicardium can secrete multiple proangiogenic factors (51). Conditional medium from cultured mammalian EPDC injected into the heart at the time of MI was shown to promote angiogenesis, which may then promote myocardial survival (51). However, unlike zebrafish, growth factors and cytokines secreted from mammalian EPDC are not sufficient for the mammalian heart to fully regenerate itself naturally. As these approaches are being investigated, the zebrafish will provide guidance as to how regenerative therapies for mammalian hearts can be developed.
The inflammation response is closely intertwined with the process of injury repair. It is fundamentally a protective response to remove damaged tissues or infection that are consequences of the injury. Acute inflammation lasts for a relatively short duration and is participated principally by neutrophils. However, in the case of severe injury, inflammation may last longer and can result in tissue or organ fibrosis. Interestingly, skin injury at early stages of fetal development heals in a regenerative fashion without scar formation in many mammals. It may be attributed to a very limited inflammatory response in fetal mammals, because skin begins to heal with significant inflammation and concurrent scarring as it becomes more mature (67). Although inflammation does occur in zebrafish heart after cryoinjury, it is still not clear how severe the inflammatory response is comparing to the mammalian heart after MI.
Recently, neonatal mouse hearts were shown to regenerate within 21 days after resection of the apex (68). The regenerative response is highly similar to what occurs in adult zebrafish hearts. However, this regenerative capacity is lost by postnatal (P) day 7. One obvious question is whether the regenerative capacity in mouse or mammalian hearts can be reactivated in P7 or even in adult hearts. Interestingly, the time point at which decreased regenerative capacity of neonatal hearts begins correlates with the time point at which binucleation and hypertrophy of cardiomyocytes occurs (68). Furthermore, the majority of cardiomyocytes in zebrafish hearts are mononucleated, although the size and shape of cardiomyocytes vary significantly (23, 30, 69). It is possible that the mononucleated cardiomyocytes are less differentiated than bi-or multinucleated cardiomyocytes and reenter cell cycle more readily to contribute to myocardial regeneration. However, the difference in contractile force between zebrafish and neonatal and adult mouse hearts might also greatly affect the regenerative capacity. There is still a small population of mononucleated cardiomyocytes present in adult mouse hearts that can be stimulated to proliferate with neuregulin-1 which acts through epidermal growth factor receptor family members ErbB2 and ErbB4 (70). This population of mononucleated cardiomyocytes can be a potential target of therapeutic approaches. However, the localization, differentiation and migration potential of the mononucleated cardiomyocytes in the mammalian heart is unknown and it is not clear if their number decreases with age. The studies in zebrafish hearts provide many interesting candidate genes and factors that might be important for reactivation of the regenerative capacity in neonatal mice older than P7 or for proliferation and differentiation of mononucleated cardiomyocytes in adults. These studies will continue to provide valuable insights and a blueprint for strategies to regenerate human hearts using approaches such as stem cell replacement, cytokine and growth factor therapy, or tissue engineering.
Acknowledgments
We thank Drs. V. Kaartinen, H. Sucov, and M. Chao for critical reading of the manuscript. This work was supported by the American Heart Association (0730214N, C.-L. L.); the National Heart, Lung and Blood Institute (R01HL096121, C.-L. L.); the Wright Foundation (C.-L. L.); a CAFA (Chinese American Faculty Association) Faculty Development Grant (C.-L. L.), a Research Career Development Award from the Saban Research Institute (C.-L. L.); the National Institute of General Medical Sciences (R01GM055081 T.-L. T.); and a California Institute of Regenerative Medicine Postgraduate Training Grant (M.H).
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeve JL, Duffy AM, O’Brien T, Samali A. Don’t lose heart--therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med. 2005;9(3):609–22. doi: 10.1111/j.1582-4934.2005.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolli P, Chaudhry HW. Molecular physiology of cardiac regeneration. Ann N Y Acad Sci. 2010;1211:113–26. doi: 10.1111/j.1749-6632.2010.05814.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 6.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5(4):364–77. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Krane M, Wernet O, Wu SM. Promises and pitfalls in cell replacement therapy for heart failure. Drug Discov Today Dis Mech. 2010;7(2):e109–e15. doi: 10.1016/j.ddmec.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beohar N, Rapp J, Pandya S, Losordo DW. Rebuilding the damaged heart: the potential of cytokines and growth factors in the treatment of ischemic heart disease. J Am Coll Cardiol. 2010;56(16):1287–97. doi: 10.1016/j.jacc.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohlnhofer D, Dibra A, Koppara T, de Waha A, Ripa RS, Kastrup J, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51(15):1429–37. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 10.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16(2):169–87. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segers VF, Lee RT. Protein therapeutics for cardiac regeneration after myocardial infarction. J Cardiovasc Transl Res. 2010;3(5):469–77. doi: 10.1007/s12265-010-9207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivas G, Anversa P, Frishman WH. Cytokines and myocardial regeneration: a novel treatment option for acute myocardial infarction. Cardiol Rev. 2009;17(1):1–9. doi: 10.1097/CRD.0b013e31817bd7ab. [DOI] [PubMed] [Google Scholar]
- 13.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyagawa S, Roth M, Saito A, Sawa Y, Kostin S. Tissue-engineered cardiac constructs for cardiac repair. Ann Thorac Surg. 2011;91(1):320–9. doi: 10.1016/j.athoracsur.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 15.Hu N, Yost HJ, Clark EB. Cardiac morphology and blood pressure in the adult zebrafish. The Anatomical record. 2001;264(1):1–12. doi: 10.1002/ar.1111. [DOI] [PubMed] [Google Scholar]
- 16.Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovascular research. 2011;91(2):279–88. doi: 10.1093/cvr/cvr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockes JP, Kumar A, Velloso CP. Regeneration as an evolutionary variable. J Anat. 2001;199(Pt 1–2):3–11. doi: 10.1046/j.1469-7580.2001.19910003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science (New York, NY) 2002;298(5601):2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 19.Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dyn. 2003;226(2):202–10. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- 20.Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, et al. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2003;100 (Suppl 1):11889–95. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kan NG, Junghans D, Izpisua Belmonte JC. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J. 2009;23(10):3516–25. doi: 10.1096/fj.09-131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadler KC, Krahn KN, Gaur NA, Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(5):1570–5. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poss KD. Getting to the heart of regeneration in zebrafish. Seminars in cell & developmental biology. 2007;18(1):36–45. doi: 10.1016/j.semcdb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. The Journal of comparative neurology. 1997;377(4):577–95. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Bernhardt RR, Tongiorgi E, Anzini P, Schachner M. Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. The Journal of comparative neurology. 1996;376(2):253–64. doi: 10.1002/(SICI)1096-9861(19961209)376:2<253::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci. 2000;17(5):789–97. doi: 10.1017/s0952523800175121. [DOI] [PubMed] [Google Scholar]
- 27.Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82(4):558–75. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Morgan TH. Regeneration in teleosts. Arch Entwickslungsmech Org. 1901;10:120–31. [Google Scholar]
- 29.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes & development. 2007;21(11):1292–315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 30.Lien CL, Schebesta M, Makino S, Weber GJ, Keating MT. Gene expression analysis of zebrafish heart regeneration. PLoS biology. 2006;4(8):e260. doi: 10.1371/journal.pbio.0040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, et al. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Developmental cell. 2011;20(3):397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–9. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–5. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127(3):607–19. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Wu Q, Zhang Y, Wiens KM, Huang Y, Rubin N, et al. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(40):17206–10. doi: 10.1073/pnas.0915016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoro MM, Pesce G, Stainier DY. Characterization of vascular mural cells during zebrafish development. Mechanisms of development. 2009;126(8–9):638–49. doi: 10.1016/j.mod.2009.06.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgijevic S, Subramanian Y, Rollins EL, Starovic-Subota O, Tang AC, Childs SJ. Spatiotemporal expression of smooth muscle markers in developing zebrafish gut. Dev Dyn. 2007;236(6):1623–32. doi: 10.1002/dvdy.21165. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Rubin N, Huang Y, Tuan TL, Lien CL. In vitro culture of epicardial cells from adult zebrafish heart on a fibrin matrix. Nature protocols. 2012;7(2):247–55. doi: 10.1038/nprot.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental biology. 1998;193(2):169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 42.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation research. 1998;82(10):1043–52. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 43.Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. The Anatomical record. 1999;255(2):212–26. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18455–60. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with in growth of the epicardial organ. Developmental biology. 1996;174(2):221–32. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 46.Lavine KJ, Ornitz DM. Shared circuitry: developmental signaling cascades regulate both embryonic and adult coronary vasculature. Circulation research. 2009;104(2):159–69. doi: 10.1161/CIRCRESAHA.108.191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, et al. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development (Cambridge, England) 2011;138(14):2895–902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458(7240):E8–9. doi: 10.1038/nature07916. discussion E-10. [DOI] [PubMed] [Google Scholar]
- 49.Gittenberger-de Groot AC, Winter EM, Poelmann RE. Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia. J Cell Mol Med. 2010;14(5):1056–60. doi: 10.1111/j.1582-4934.2010.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limana F, Bertolami C, Mangoni A, Di Carlo A, Avitabile D, Mocini D, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. Journal of molecular and cellular cardiology. 2010;48(4):609–18. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121(5):1894–904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474(7353):640–4. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC, et al. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Developmental biology. 1998;199(1):55–71. doi: 10.1006/dbio.1998.8911. [DOI] [PubMed] [Google Scholar]
- 54.von Gise A, Zhou B, Honor LB, Ma Q, Petryk A, Pu WT. WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Developmental biology. 2011;356(2):421–31. doi: 10.1016/j.ydbio.2011.05.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, et al. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78(6):987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 56.Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes & development. 1994;8(9):1007–18. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development (Cambridge, England) 1998;125(10):1943–9. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- 58.Tran CM, Sucov HM. The RXRalpha gene functions in a non-cell-autonomous manner during mouse cardiac morphogenesis. Development (Cambridge, England) 1998;125(10):1951–6. doi: 10.1242/dev.125.10.1951. [DOI] [PubMed] [Google Scholar]
- 59.Brade T, Kumar S, Cunningham TJ, Chatzi C, Zhao X, Cavallero S, et al. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development (Cambridge, England) 2011;138(1):139–48. doi: 10.1242/dev.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li P, Cavallero S, Gu Y, Chen TH, Hughes J, Hassan AB, et al. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development (Cambridge, England) 2011;138(9):1795–805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development (Cambridge, England) 2011;138(9):1663–74. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 62.Chablais F, Veit J, Rainer G, Jazwinska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnabel K, Wu CC, Kurth T, Weidinger G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS ONE. 2011;6(4):e18503. doi: 10.1371/journal.pone.0018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8(11–12):1907–39. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development (Cambridge, England) 2011;138(16):3421–30. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464(7288):549–53. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126(4):1172–80. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science (New York, NY. 2011;331(6020):1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farrell AJD. Fish physiology: the heart. San Diego: Academic Press; 1992. [Google Scholar]
- 70.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]