Abstract
Paraneoplastic neurological disorders may result from autoimmunity directed against antigens shared by the affected neurons and the associated cancer cells. We have recently reported the case of a woman with breast cancer and paraneoplastic lower motor neuron syndrome whose serum contained autoantibodies directed against axon initial segments and nodes of Ranvier of myelinated axons, including the axons of motoneurons. Here, we show that major targets of the autoantibodies of this patient are βIVΣ1 spectrin and βIV spectrin 140, two isoforms of the novel βIV spectrin gene, as well as a neuronal surface epitope yet to be identified. Partial improvement of the neurological symptoms following cancer removal was associated with a drastic reduction in the titer of the autoantibodies against βIV spectrin and nodal antigens in general, consistent with the autoimmune pathogenesis of the paraneoplastic lower motor neuron syndrome. The identification of βIV spectrin isoforms and surface nodal antigens as novel autoimmune targets in lower motor neuron syndrome provide new insights into the pathogenesis of this severe neurological disease.
Paraneoplastic syndromes of the central nervous system include a variety of disorders that occur in association with cancer, but which are not a result of metastasis or tumor compression of the brain tissue (1–3). Often, these disorders appear to have an autoimmune pathogenesis, as suggested by the presence of autoantibodies directed against antigens shared by neurons and cancer cells (4). The current hypothesis is that an autoimmune process originally aimed at limiting tumor growth spreads to the nervous system because of the ectopic or altered expression in cancer cells of neuronal antigens. This process, in turn, impairs neuronal functioning and possibly causes the death of neurons expressing the target antigens. The onset of the neurological symptoms often precedes the clinical manifestations of the tumor. Thus, detection of the autoantibodies against neuronal antigens can lead to the early detection of an occult cancer (5).
We have recently reported the case of a 72-year-old woman affected by a lower motor neuron syndrome (LMNS) and breast cancer, whose serum harbored autoantibodies directed against axon initial segments and nodes of Ranvier (6). These molecularly related structures are the axon compartments of myelinated neurons where action potentials originate (initial segments) and propagate (nodes of Ranvier). Therefore, it is conceivable that an autoimmune process directed against these compartments could impair nerve conduction and, ultimately, lead to neuronal degeneration. Supporting the paraneoplastic origin of LMNS in this patient was the observation that removal of the breast cancer was followed by a significant, albeit partial, improvement of the neurological symptoms.
In this study, we show that the patient's autoantibodies react with βIVΣ1 spectrin and βIV spectrin 140, two isoforms of the βIV spectrin gene that are enriched at axon initial segments and nodes of Ranvier (7). In addition, the patient autoantibodies recognize a surface neuronal epitope whose molecular identity remains to be determined.
Materials and Methods
Human Sera.
Sera from healthy subjects and from the patient with LMNS and breast cancer (patient 849 in our case load) were collected after informed consent and stored at −20°C until used.
Antibodies.
The affinity-purified rabbit antiserum directed against a C-terminal peptide in βIVΣ1 spectrin (βIV-CT) has been described (7). The following reagents were from commercial sources: mouse anti-V5 antibody (Invitrogen), anti-rabbit and anti-mouse goat IgG antibodies conjugated to Alexa 488 or Alexa 568 (Molecular Probes), rabbit IgG, peroxidase-conjugated goat anti-mouse, or goat anti-rabbit IgG and mouse anti-human IgG (Sigma).
Hippocampal Neuronal Cultures.
Primary high- and low-density cultures of rat hippocampal neurons isolated at embryonic day 18 (E18) were obtained according to established procedures (8, 9).
Western Blotting and Immunoprecipitations.
Brain tissues were collected from 18 (E18)-day-old rat embryos and adult rats and homogenized on ice (1:10 wt/vol) in homogenization buffer [HB; 10 mM Hepes, pH 7.4/5 mM EDTA/1 mM EGTA/150 mM NaCl/1 mM PMSF/10 mM benzamidine/1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml pepstatin A/1 μg/ml antipain/1% Nonidet P-40 (Roche Molecular Biochemicals), and 0.5% deoxycholic acid (Sigma)]. Postnuclear supernatant was obtained by centrifugation at 1,000 × g for 10 min and solubilized in 2% SDS sample buffer to a final concentration of ≈1.2 mg/ml. Approximately 1 × 106 1- and 2-week-old neurons in culture were solubilized in 400 μl of 2% SDS-sample buffer (≈1.2 μg/μl). Proteins (≈50 μg/lane) were separated by 6% SDS/PAGE and immunoblotted with either the preoperative serum of patient 849 (1:500), followed by mouse anti-human IgG (1:1,000) and then peroxidase-conjugated goat anti-mouse IgG (1:5,000), or with the affinity-purified βIV-CT (1:500) followed by peroxidase-conjugated goat anti-mouse IgG (1:5,000). Signals were detected by enhanced chemiluminescence (Amersham Pharmacia). For control, we repeated the same immunoblotting procedures by using as primary sources of antibodies the sera from healthy human subjects and the preimmune serum of the rabbit in which the βIV-CT antibody was developed. Protein concentration in tissue extracts was determined by using the BCA assay procedure (Pierce).
For immunoprecipitations, adult rat brain was homogenized in HB (1:10 wt/vol) and centrifuged, and the resulting postnuclear supernatant was solubilized for 2 h at 4°C. After removal of the insoluble material by a high-speed spin (100,000 × g for 30 min at 4°C), the Nonidet P-40 soluble material was precleared with a control human serum (10 μl/ml), normal rabbit IgG (10 μg/ml), and 50% slurry protein A + G Sepharose beads (50 μl/ml; Amersham Pharmacia) for 4 h. One-milliliter aliquots of the extract were incubated overnight at 4°C either with 10 μl of the preoperative patient serum or with 10 μl of the βIV-CT antibody, followed by 50% slurry protein A + G Sepharose beads for 1 h. Beads were pelleted, washed three times in HB, and resuspended in 40 μl of 1 × SDS sample buffer. Immunoprecipitates were separated on 6% SDS/PAGE and immunoblotted with the preoperative patient serum (1:500) or with the affinity-purified βIV-CT (1:500). Control immunoprecipitates were performed with 10 μl of a control human serum or 10 μg of control rabbit IgG.
Transient Transfection of Chinese Hamster Ovary (CHO) Cells.
The cDNA encoding for the C-terminal domain of βIV spectrin (amino acids 2117–2559) plus a starting methionine was amplified by PCR (5′ primer, GCACCATGGTGCGGCCACGACCGGAGCGCCAGGAG; 3′ primer, CTTCCTGCGCCCGCTGGCCCTGCGATCTCCGCCTTCCC) and subcloned upstream of the V5 epitope tag into pcDNA3.1/V5-His-TOPO/lacZ (Invitrogen). Transfection of this construct into CHO cells with the Lipofectin reagent (Life Technologies, Rockville, MD) was performed as described (10).
Immunocytochemistry.
Male Sprague–Dawley rats (150–175 g) were fixed by trans-cardiac perfusion with 1% paraformaldehyde in 120 mM sodium phosphate buffer (PBS). Tissues of interest were collected, fixed for an additional 3 h, and then infiltrated with 30% sucrose in PBS. Two-week-old hippocampal cultures were briefly washed in PBS, fixed with 1% paraformaldehyde and 1% sucrose in PBS for 30 min, and stored in PBS until used. Single and double immunolabeling on 12-μm cryostat tissue sections and transfected CHO cells was performed as previously described (11, 12). The preoperative and postoperative sera of patient 849, the serum of a healthy subject, and the anti-βIV-CT antibody were used at a concentration of 1:500, whereas the anti-V5 antibody was used at a concentration of 1:200. Confocal microscopy was performed by using a Bio-Rad MRC 1024 station attached to a Zeiss Axiovert microscope. For the surface staining of hippocampal neurons, 2-week-old cultured neurons were briefly washed and incubated on ice with cold PBS for 10 min with the preoperative or postoperative serum of the patient (dilution 1:1,000) and the βIV-CT antibody (1:1,000). In parallel experiments, the cultured neurons were exposed to human control sera (1:1,000). Next, the neurons were washed three times for 5 min in ice-cold PBS and then fixed immediately in ice-cold 1% paraformaldehyde. The neurons were stained with Alexa 568-conjugated goat anti-human IgG for the human sera and Alexa 488 goat-anti rabbit IgG for anti-βIV-CT.
RIA.
The cDNA encoding the C-terminal domain of βIV spectrin (amino acids 1651–2559) plus a starting methionine was amplified by PCR (5′ primer, ATGACCCTGCAGCTGCTCCAAGAAA; 3′ primer, GATGTGTCAGGTCCTGGGGGTGG) and subcloned into Bluescript KS II. Escherichia coli DH5α cells were used for amplification and purification of the plasmid. 35S-βIV spectrin was prepared by using a TNT T7 quick-coupled transcription/translation kit (Promega). Before performing the RIA, unincorporated [35S]methionine was removed from the 35S-βIV spectrin by separation on a MicroSpin G-25 column (Amersham Pharmacia). Autoantibodies were measured by using an RIA based on a method described by Yu et al. (13). In brief, 35S-βIV spectrin was incubated with the patient serum at 4°C overnight followed by immunoprecipitation with protein A-Sepharose (Amersham Pharmacia) in 96-well MultiScreen plates (Millipore). Each sample was run in triplicate, and data were collected by using a TopCount microplate scintillation counter (Packard). Results were expressed as an index by using the following formula: Index = (sample cpm − negative control cpm)/(positive control cpm − negative control cpm). The patient serum sample before the tumor removal (5/98) was used as the positive control, and the negative control was a randomly selected normal human serum. This formula generates an index of 1 for the initial patient serum sample.
Results
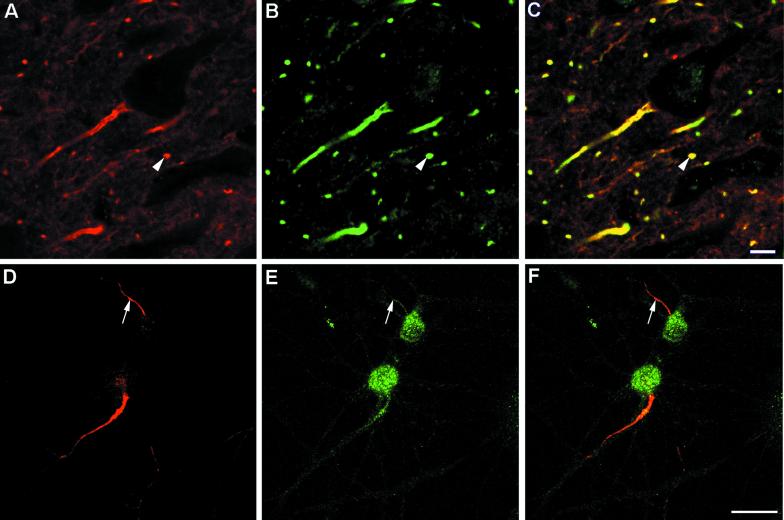
Our recent studies on the localization of βIV spectrin at axon initial segments and nodes of Ranvier (7) prompted us to test whether this molecule may also be the autoantigen recognized by the autoantibodies of the patient with paraneoplastic LMNS and breast cancer. As previously shown (6), the preoperative serum of this patient contained autoantibodies that specifically reacted by immunocytochemistry with the axon initial segments and nodes of Ranvier of myelinated neurons in rat brain (Fig. 1A). By confocal microscopy, this staining appeared virtually identical to the immunolabeling obtained with the affinity-purified antibody raised against the C-terminal peptide of βIVΣ1 spectrin (βIV-CT) (Fig. 1 B and C). A similar overlapping staining was also observed at the axon initial segments of 2-week-old primary cultured rat hippocampal neurons. In addition, the βIV-CT antibody, but not the patient serum, strongly reacted with neuronal cell bodies (Fig. 1 D–F). In tissue sections, a similar staining of the perikarya of large myelinated neurons, including pyramidal cells of the hippocampus and Purkinje cells of the cerebellar cortex, was also observed with the βIV-CT antibody (see Fig. 6 herein; see also figures 9 and 11 in ref. 7).
Figure 1.
Double immunolabeling and confocal microscopy of a section of rat cerebral cortex (A–C) and cultured hippocampal neurons (D and E) immunostained with the preoperative patient serum (A and D, pseudo-red) and the βIV-CT antibody (B and E, pseudo-green). (C and F) Merged images of A and B, or D and E, respectively. The arrowheads in A–C point to a transversely cut axon initial segment/node or Ranvier in situ costained by the patient autoantibodies and the βIV-CT antibody, whereas the arrows in D–F indicate a double-labeled axon initial segment of a cultured hippocampal neuron. (Bars: A–C, 100 μm; D–F, 25 μm.)
Figure 6.
(A–F) Immunolabeling and confocal microscopy of sections of rat cerebellar cortex immunostained with the preoperative patient serum (A, pseudo-red) or the 1-year postoperative patient serum (D, pseudo-red). The same sections were double stained with the βIV-CT antibody (B and E, pseudo-green). C and F show the merged images of A and B, or D and E, respectively. (Bar, 100 μm.) (G) Titration of anti-βIV spectrin autoantibodies in the serum of the patient by RIA using an in vitro transcribed and translated 35S-labeled C-terminal fragment of βIV spectrin. Aliquots of the patient serum obtained before (■) and 1-month (▴) or ≈1-year (×) after the removal of the breast cancer were tested at the dilutions indicated on the horizontal axis. The vertical axis shows the autoantibody titer expressed in arbitrary units.
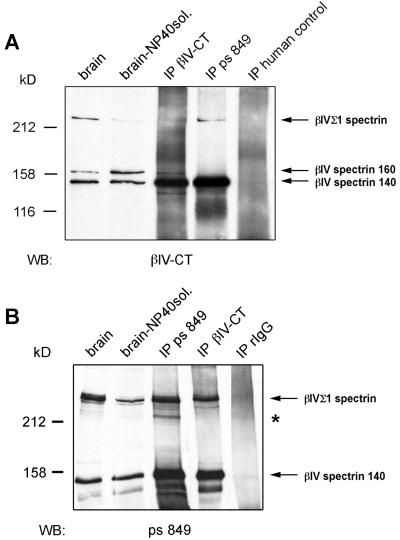
By Western blotting the patient's autoantibodies, we detected two major autoantigens of ≈250 kDa and ≈140 kDa in both adult rat brain and 2-week-old cultured hippocampal neurons (Fig. 2A, lanes 1 and 2). The ≈250-kDa and ≈140-kDa autoantigens were expressed at similar levels in adult brain, whereas the 250-kDa autoantigen was the predominant autoantigen expressed in 2-week-old cultured hippocampal neurons. These autoantigens had an identical electrophoretic mobility and comparable expression levels to the spectrin isoforms βIVΣ1 and βIV 140 recognized by the βIV-CT antibody (Fig. 2A, lanes 3 and 4). The βIV-CT antibody, but not the patient autoantibodies, recognized a third βIV spectrin isoform of 160 kDa (βIV spectrin 160) (Fig. 2) in both brain and 2-week-old cultured hippocampal neurons. Reactivity against this isoform may account for the immunostaining of neuronal perikarya with the βIV-CT antibody, but not with the serum of the patient with paraneoplastic LMNS. Taken together, the immunomicroscopy and immunoblotting data raised the possibility that the 250-kDa and the 140-kDa autoantigens recognized by the patient autoantibodies correspond to βIVΣ1 spectrin and βIV spectrin 140, respectively. Because the primary amino acid sequence of βIV spectrin 160 is still unknown, no information could be drawn from these results regarding the location of potential epitopes recognized by the patient autoantibodies in βIVΣ1 spectrin and βIV spectrin 140.
Figure 2.
(A) Western blotting with the patient serum (ps 849) and the βIV-CT antibody of homogenates from adult rat brain and rat hippocampal neurons after 2 weeks in culture. The arrows indicate the three isoforms of βIV spectrin detected with the βIV-CT antibody: βIV Σ1 spectrin, βIV spectrin 160, and βIV spectrin 140. (B and C) Western blotting on hippocampal tissue at embryonic day 18 (hp E18) and hippocampal neurons after 1 and 2 weeks in culture. The asterisks are aligned with βIV spectrin 140, which becomes detectable in the 2-week-old cultured neurons.
To further test the identity of the 250-kDa and 140-kDa autoantigens with βIVΣ1 spectrin and βIV spectrin 140, we compared the expression profiles of these molecules in brain and cultured hippocampal neurons. We have previously shown that in rat brain βIVΣ1 spectrin is first detected at embryonic day 19, when axon initial segments of hippocampal pyramidal neurons start to react with the βIV-CT antibody (7). βIV spectrin 140 appears at a later developmental stage in postnatal brain, in parallel with the progressive myelination of axons and the formation of nodes of Ranvier. Brain expression of βIV spectrin 160, on the other hand, precedes the appearance of βIVΣ1 spectrin and does not significantly change during development. βIV spectrin isoforms had a similar expression pattern in cultured hippocampal neurons, in the absence of direct contacts with oligodendrocytes and myelin formation. In particular, βIV spectrin 160, but virtually no βIVΣ1 spectrin, was found at E18, when the hippocampal tissue was dissociated for the preparation of neuronal cultures (Fig. 2B, lane 1). In 1-week-old neuronal cultures, βIVΣ1 spectrin began to appear, and it was well expressed in 2-week-old cultured neurons (Fig. 2B, lanes 2 and 3). At this time, βIV spectrin 140 could also be detected. The same expression profile was observed in the case of the 250-kDa and 140-kDa autoantigens recognized by the serum of the patient with paraneoplastic LMNS, except that a weak reactivity against a protein doublet of 250 kDa was already apparent in hippocampal extracts at E18.
Additional evidence that the patient autoantibodies recognize βIVΣ1 spectrin and βIV spectrin 140 was obtained by immunoprecipitation assays from adult rat brain extracts (Fig. 3). Specifically, the 250-kDa and 140-kDa autoantigens immunoprecipitated by the patient autoantibodies were recognized by Western blotting with the βIV-CT antibody (Fig. 3A, lane 4). Likewise, βIVΣ1 spectrin and βIV spectrin 140 immunoprecipitated by the βIV-CT antibody were detected by Western blotting with the patient serum (Fig. 3B, lane 4). In contrast, neither one of these βIV spectrin isoforms was immunoprecipitated by a control human serum or by purified control rabbit IgG (Fig. 3, lanes 5). An additional antigen of ≈220 kDa, which was recognized by the patient autoantibodies but not by the βIV-CT antibody, was enriched in the immunoprecipitates obtained with the patient serum (asterisk in Fig. 3B). Whether this protein is a cleavage product of βIVΣ1 spectrin lacking the C-terminal epitope reacting with the βIV-CT antibody, another βIV spectrin isoform, or yet an unrelated antigen remains to be determined. Even in this assay, we could not reveal any reactivity of the patient autoantibodies with βIV spectrin 160.
Figure 3.
About 50 μg of total brain postnuclear supernatant (brain), detergent-soluble brain postnuclear supernatant (brain-NP40sol.), and immunoprecipitates obtained with the βIV-CT antibody (IP βIV-CT), the preoperative patient serum (IP ps 849), a human control serum, or rabbit IgG (IP rIgG) were immunoblotted with the βIV-CT antibody (A) or the patient serum (B). Whereas the βIV-CT antibody detected three βIV spectrin isoforms (arrows in A), the patient serum recognized only βIVΣ1 spectrin and βIV spectrin 140 (arrows in B). Asterisk (B) indicates a protein that was only immunoprecipitated with the patient serum.
To conclusively prove the recognition of βIV spectrin isoforms by the patient autoantibodies, a polypeptide including the last 497 residues of βIVΣ1 (amino acids 2117–2559) was transiently expressed in CHO cells. Double immunolabeling showed that transfected CHO cells, but not adjacent nontransfected cells, were strongly costained by the βIV-CT antibody and the patient autoantibodies (Figs. 4 A and B). Conversely, a human control serum did not recognize the βIVΣ1 spectrin fragment in transfected fibroblasts (Fig. 4 C and D).
Figure 4.
Double immunolabeling of CHO cells transiently transfected with the V5-tagged C-terminal domain of βIVΣ1 spectrin. (A and B) Double immunostaining with the anti-V5 monoclonal antibody (A) and the preoperative patient serum. (C and D) Double immunostaining with the anti-V5 monoclonal antibody (C) and the serum of a healthy control subject (D). (Bar, 25 μm.)
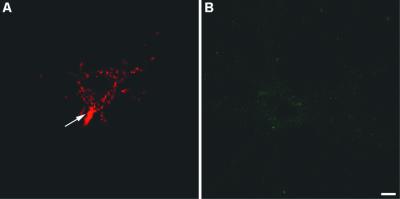
Next, we tested whether the patient serum contained autoantibodies directed against neuronal surface antigens. To this aim, 2-week-old cultured hippocampal neurons were incubated with the preoperative serum of the patient and the βIV-CT antibody. Parallel experiments were performed in which living neurons were incubated instead with the serum of healthy subjects. Fig. 5A shows that incubation of living neurons with the preoperative serum of the patient produced a strong staining of the neuronal surface. This immunoreactivity was mostly restricted to the axon initial segment, although a lower signal was also present in the cell body and proximal segments of the dendrites. On the contrary, no surface labeling was observed with the βIV-CT antibody (Fig. 5B), consistent with the intracellular localization of βIV spectrins. Likewise, there was no labeling on neurons incubated with the serum of healthy subjects (not shown).
Figure 5.
Surface labeling of living, nonpermeabilized 2-week-old cultured hippocampal neurons with the preoperative serum of the patient (A) and the βIV-CT antibody (B). (Bar, 25 μm.)
In our initial case report of this patient, we indicated that removal of the breast cancer was followed by a significant, albeit limited, improvement of the neurological symptoms (6). To assess whether this amelioration correlated with a reduced autoimmune response against axon initial segments and nodes of Ranvier, rat brain sections were immunostained with the serum of the patient obtained either before or 1 year after surgery. As an internal control, the same sections were double stained with the βIV-CT antibody. As shown in Fig. 6, the postoperative patient serum barely reacted with axon initial segments (compare A with D in Fig. 6), indicating indeed a significant decrease in the levels of circulating autoantibodies against nodal antigens. Likewise, the postoperative patient serum did not react with surface epitopes of cultured neurons (not shown). The titer of anti-βIV spectrin autoantibodies was quantitatively assessed by RIA (Fig. 6G). This analysis demonstrated that 1 month after surgery the titer of anti-βIV spectrin autoantibodies was reduced by 24%, whereas 1 year after surgery the titer was reduced by ≈70% compared with the preoperative serum.
Discussion
In this study, we have shown that βIVΣ1 spectrin and βIV spectrin 140, two isoforms of the recently identified βIV spectrin gene, are the targets of autoimmunity in a patient with paraneoplastic LMNS and breast cancer. They are enriched at initial segments and nodes of Ranvier, the related specialized compartments that are essential for nerve conduction along the axon of myelinated neurons. An immune attack toward these structures could conceivably cause a functional impairment, and eventually the death, of motoneurons. Because βIV spectrins are cytosolic proteins, however, they are not expected to be accessible to autoantibodies in living cells. Thus, the humoral autoimmune response against βIV spectrins is unlikely to be directly responsible for the neurological symptoms of the patient. Surface staining of living, nonpermeabilized hippocampal neurons, on the other hand, suggests that the serum of the patient, in addition to anti-βIV spectrin antibodies, contains autoantibodies directed against a surface antigen(s) that is (are) also enriched at axon initial segments. These autoantibodies, differently from the anti-βIV spectrin antibodies, could directly contribute to the pathogenesis of LMNS. Notably, removal of the breast cancer not only halted the rapid progression of LMNS, but it was accompanied by a significant neurological improvement, concomitantly with a conspicuous decrease in the concentration of the autoantibodies directed against βIV spectrins and the surface autoantigen at the axon initial segment. These data are consistent with the paraneoplastic nature of LMNS in this patient and suggest that autoimmunity against nodal antigens may affect motoneuron viability.
Spreading of humoral autoimmunity to multiple antigens that are part of the same macromolecular complex is a common finding in autoimmune disorders (14). In the case of neurological disorders, this phenomenon has been recently proposed to account for the coexistence of autoantibodies directed against surface epitopes of glutamate receptor 3 and the cytosolic protein munc-18/nSec-1 in a patient with Rasmussen's encephalitis (15). An intriguing possibility is that the surface neuronal antigen recognized by our patient autoantibodies may be a trans-membrane protein associated with βIV spectrin. Surface proteins enriched at axon initial segments and nodes of Ranvier and whose cytoplasmic domains interact with the ankyrin/spectrin cytoskeleton include voltage-gated sodium channels (16), the cell-adhesion molecules neurofascin and Nr-CAM (17), and receptor protein tyrosine phosphatase β/ζ (18). The very limited supply of the patient preoperative serum prevented us from determining whether any of these proteins, or yet another molecule, was the surface target of the patient autoantibodies. For the same reason, we could not investigate whether exposure of cultured neurons to the patient serum affects their function and/or viability. Finally, no cancer tissue of the patient was available to determine whether it expressed βIV spectrin, as one would expect according to the idea that autoimmune paraneoplastic neurological disorders arise from loss of tolerance toward neuronal antigens that are ectopically expressed in neoplastic cells. The breast tumor of this patient was indeed removed before the discovery of the antineuronal autoantibodies (6).
Despite these limits, our findings point to nodal antigens, and βIV spectrins in particular, as potential novel targets of autoimmunity in LMNS. Autoimmunity has been previously implicated in the pathogenesis of various motor neuron syndromes. Anti-GM1 ganglioside antibodies are found in the large majority of patients with multifocal motor neuropathy and in some patients with distal-lower motor neuron syndrome in the absence of an associated cancer (19). Converging clinical and experimental evidence suggests that anti-GM-1 autoantibodies can directly affect nerve conduction and motoneuron function (20). Autoantibodies directed against gangliosides, including GM-1, asialo GM-1, and GD-1b, however, were not detected in the serum of our patient (6). The serum of the patient was also negative for autoantibodies directed against myelin-associated glycoprotein, which are characteristic of demyelinating neuropathies with a preponderant sensory deficit over the loss of motor function (21). Additional investigations will be required to determine whether reactivity against nodal antigens is a specific feature of paraneoplastic LMNS or can occur also in motor neuron syndromes even in the absence of an associated tumor.
An additional finding of our study is that the temporal expression of various βIV spectrins in cultured hippocampal neurons closely resembles the developmental expression of these proteins in brain (7). βIV spectrin 160, in particular, is abundant even in the early stages of neuronal differentiation and seems to be confined to the perikarya. The levels of βIVΣ1 and βIV spectrin 140, in contrast, progressively increase in parallel with the extension of axonal processes. Our data also show that compartmentalization of βIV spectrins to axon initial segments may occur even in the absence of contacts between neurons and oligodendrocytes and myelination. This is in contrast to the dependence from myelination for the compartmentalization of sodium channels and ankyrinG 480/270 at nodes of Ranvier in the peripheral nervous system (22, 23).
Acknowledgments
We thank Laurie Daniell and Sunghoe Chang for helping us to establish the hippocampal neuronal cultures. We also thank Drs. Ira Mellman, Bettina Winkler, Joel Black, and Sulayman Dib-Hajj for helpful discussion. This work was supported by grants from the National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases (to M.S.) and the American Diabetes Association. S.B. was supported by a postdoctoral fellowship from the Juvenile Diabetes Foundation. Immunomicroscopy was supported by a National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases grant to the Yale Diabetes Endocrinology Research Center.
Abbreviations
- LMNS
lower motor neuron syndrome
- CHO
Chinese hamster ovary
References
- 1.Posner J B, Dalmau J. Curr Opin Immunol. 1997;9:723–729. doi: 10.1016/s0952-7915(97)80055-6. [DOI] [PubMed] [Google Scholar]
- 2.Darnell R B. N Engl J Med. 1999;340:1831–1833. doi: 10.1056/NEJM199906103402311. [DOI] [PubMed] [Google Scholar]
- 3.Younger D S, Rowland L P, Latov N, Hayes A P, Lange D J, Sherman W, Inghirami G, Pesce M A, Knowles D M, Powers J, et al. Ann Neurol. 1991;29:78–86. doi: 10.1002/ana.410290114. [DOI] [PubMed] [Google Scholar]
- 4.Floyd S, Butler M H, Cremona O, David C, Freyberg Z, Zhang X, Solimena M, Tokunaga A, Ishizu H, Tsutsui K, et al. Mol Med. 1998;4:29–39. [PMC free article] [PubMed] [Google Scholar]
- 5.Folli F, Solimena M, Cofiell R, Austoni M, Tallini G, Fassetta G, Bates D, Cartlidge N, Bottazzo G F, Piccolo G, et al. N Engl J Med. 1993;328:546–551. doi: 10.1056/NEJM199302253280805. [DOI] [PubMed] [Google Scholar]
- 6.Ferracci F, Fassetta G, Butler M, Floyd S, Solimena M, De Camilli P. Neurology. 1999;53:852–855. doi: 10.1212/wnl.53.4.852. [DOI] [PubMed] [Google Scholar]
- 7.Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel J-M, Zhang J-P, Philbrick W, Slepnev V, Ort T, Solimena M. J Cell Biol. 2000;151:985–1001. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goslin K, Banker G. In: Culturing Nerve Cells. 2nd Ed. Banker G, Goslin K, editors. Cambridge MA: MIT Press; 1998. pp. 339–370. [Google Scholar]
- 9.Matteoli M, Takei K, Perin M S, Sudhof T C, De Camilli P. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solimena, Aggujaro M, D, Muntzel C, Dirkx R, Butler M, De Camilli P, Hayday A. Proc Natl Acad Sci USA. 1993;90:3073–3077. doi: 10.1073/pnas.90.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron P L, Südhof T C, Jahn R, De Camilli P. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Camilli P, Cameron R, Greengard P. J Cell Biol. 1983;96:1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Kingsmith G, Erlich H, Norris J, Eisenbarth G. J Clin Endocrinol Metab. 1996;81:4264–4426. doi: 10.1210/jcem.81.12.8954025. [DOI] [PubMed] [Google Scholar]
- 14.McCluskey J, Farris A D, Keech C L, Purcell A W, Rischmueller M, Kinoshita G, Reynolds P, Gordon T P. Immunol Rev. 1998;164:209–229. doi: 10.1111/j.1600-065x.1998.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang R, Puranam R S, Butler L S, Qian W H, He X P, Moyer M B, Blackburn K, Andrews P I, McNamara J O. Neuron. 2000;28:375–383. doi: 10.1016/s0896-6273(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 16.Salzer J L. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 17.Bennett V, Lambert S. J Neurocytol. 1999;28:303–318. doi: 10.1023/a:1007005528505. [DOI] [PubMed] [Google Scholar]
- 18.Ratcliffe C F, Qu Y, McCormick K A, Tibbs V C, Dixon J E, Scheuer T, Catterall W A. Nat Neurosci. 2000;3:437–444. doi: 10.1038/74805. [DOI] [PubMed] [Google Scholar]
- 19.Pestronk A. Neurology. 1998;51:S22–S24. doi: 10.1212/wnl.51.6_suppl_5.s22. [DOI] [PubMed] [Google Scholar]
- 20.Takigawa T, Yasuda H, Kikkawa R, Shigeta Y, Saida T, Kitasato H. Ann Neurol. 1995;37:436–442. doi: 10.1002/ana.410370405. [DOI] [PubMed] [Google Scholar]
- 21.Latov N. Ann Neurol. 1995;37:S32–S42. doi: 10.1002/ana.410370705. [DOI] [PubMed] [Google Scholar]
- 22.Vabnick I, Novakovic S D, Levinson S R, Schachner M, Shrager J. J Neurosci. 1996;16:4914–4922. doi: 10.1523/JNEUROSCI.16-16-04914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ching W, Zanazzi G, Levinson S R, Salzer J L. J Neurocytol. 1999;28:295–301. doi: 10.1023/a:1007053411667. [DOI] [PubMed] [Google Scholar]