Abstract
Humoral immune mechanisms are an important component of protective immunity to Ehrlichia species. However, the molecular basis of antibody mediated immunity is not completely defined, and the role of other molecularly characterized major immunoreactive proteins is unknown. In previous studies, we mapped major species-specific continuous epitopes in three surface exposed and secreted tandem repeat proteins (TRP32, TRP47 and TRP120). In this study, we report that protection is provided by antibodies against these molecularly defined TRP epitopes using in vitro and in vivo models. Protection was demonstrated in vitro after prophylactic and therapeutic administration of epitope-specific anti-TRP antibodies, suggesting that the protective mechanisms involve extracellular and intracellular antibody-mediated effects. In vivo passive transfer of individual epitope-specific TRP sera significantly reduced the ehrlichial load and splenomegaly, and protected mice against lethal infection. Moreover, the combination of antibodies to all three TRPs provided enhanced reduction in ehrlichial load similar to that of E. chaffeensis immune sera. IgG1 was the predominant antibody isotype in the epitope-specific TRP mouse sera. These results demonstrate that antibodies against linear epitopes in TRP32, TRP47 and TRP120 are protective during E. chaffeensis infection and involves extracellular and intracellular antibody-mediated mechanisms.
Keywords: EHRLICHIA CHAFFEENSIS, TANDEM REPEAT PROTEINS, IMMUNOREACTIVE PROTEINS, EPITOPE, ANTIBODY
1. Introduction
Ehrlichia chaffeensis is an obligately intracellular bacterium that is the causative agent of human monocytotropic ehrlichiosis, an emerging life-threatening zoonosis. Major immunoreactive proteins of E. chaffeensis and E. canis include a small subset of proteins strongly recognized by antibody [31]. Humoral immunity is essential for protection against active E. chaffeensis infection, and antibody specific for a linear epitope located in a hypervariable region of the major outer membrane protein (OMP-1g) is involved [20;44]. Antibody-mediated Fcγ receptor (FcR) dependent phagocytosis has been identified as a mechanism contributing to E. chaffeensis clearance during active infection [44]. However, the role of other major immunoreactive proteins in immunity to E. chaffeensis is unknown. The majority of the major immunoreactive proteins of E. chaffeensis and E. canis have been molecularly characterized, and many of these proteins contain tandem repeats. Molecularly defined tandem repeat protein (TRP) orthologs in E. chaffeensis and E. canis include TRP120/TRP140, TRP75/TRP95, TRP47/TRP36, and TRP32/TRP19 [6;24–26;32;33]. Several TRP ortholog pairs have similar characteristics including the fact that they are secreted, are serine/threonine-rich and highly acidic, and have a major molecularly distinct continuous antibody epitope (~20 amino acids) located within the tandem repeat regions [6;24;26].
The role of TRPs in pathogenesis is emerging, and it is well established that TRP47 and TRP120 are differentially expressed on the surface of dense-cored (infectious) ehrlichiae and TRP32 is extracellularly associated with the morular fibrillar matrix and the morula membrane and is expressed on both dense cored and reticulate cells [4;24;26]. Additionally, TRP47 and TRP32 transcripts are hyper-expressed during infection of the macrophage [16]. Recent studies have also demonstrated that E. chaffeensis TRPs are secreted effector proteins that interact with many host cell targets [22;40]. Molecular interactions recently reported between host cell proteins and TRP47 and TRP120 include targets associated with distinct cellular functions including signaling, transcriptional regulation, vesicle trafficking and cellular proliferation and differentiation [22;40]. The TRP120 is also involved in the binding and internalization of E. chaffeensis, and its expression is regulated by the second messenger cyclic di-GMP and protease HtrA [15;38]. Moreover, the TRP120 is a DNA binding protein with transcriptional activator function that targets host genes associated with biological processes known to be altered during E. chaffeensis infection [47]. Molecular host pathogen interactions between TRP32, and host targets associated with TRP47 and TRP120, have also been recently described [23].
Antibodies against a number of intracellular pathogens have been shown to mediate protection [3]. Recently, protection against intracellular bacteria, Mycobacterium and Legionella, was demonstrated by antibody mediated FcR signaling for lysosomal targeting [11]. During Legionella infection, antibodies block the downstream functions of the type 4 secretion system effectors, which include subversion the host cell trafficking system to prevent lysosomal fusion [11]. Prophylactic administration of immune serum or purified antibodies has been shown to reduce the severity and duration of disease caused by Francisella tularensis and Mycobacterium tuberculosis [9;39], and antibodies are required for protection, but not clearance of, Salmonella typhimurium [34]. Antibodies against outer membrane proteins of Rickettsia conorii and secreted Listeria monocytogenes listeriolysin O (LLO) are also protective during infection [7;8], and in the case of Listeria, neutralization of LLO by antibodies occurs intracellularly [7].
Studies to determine protection provided by major immunoreactive proteins and experimental vaccine development for Ehrlichia spp. have focused primarily on the OMP family. The objective of this investigation was to determine protection provided by epitope-specific antibody against the major immunoreactive proteins TRP 32, TRP47 and TRP120 during E. chaffeensis infection. In the present study, we demonstrate that antibodies directed at major linear epitopes of three secreted E. chaffeensis TRPs reduce ehrlichial load through extracellular and intracellular antibody mediated mechanisms. In addition, we identified IgG1 as the predominant TRP epitope-specific antibody isotype in anti-TRP sera. Furthermore, an in vitro model was developed to test the antibody-mediated protection stimulated by the major antibody epitopes of the TRPs that correlated with protection during active infection in the animal model.
2. Materials and methods
2.1. Antisera and antibody purification
Mouse and rabbit (TRP32, TRP47, TRP120 and control) antisera were generated against the synthetic keyhole limpet hemocyanin-conjugated peptides located in the epitope-containing tandem repeat regions by a commercial vendor (Bio-Synthesis, Lewisville, TX). IgG was purified from the rabbit TRP32, TRP47, TRP120, control and pre-immune antisera using Melon Gel IgG purification kit (Thermo Fisher, Rockford, IL) and were used in the in vitro experiments. A convalescent-phase anti-E. chaffeensis dog serum was derived from an experimentally infected dog (no. 2251).
2.2. E. chaffeensis and cell culture
E. chaffeensis (Arkansas strain) was propagated in DH82 (canine macrophages) cells in DMEM as previously described [30]. Cells were prophylactically treated with 0.5µg/ml of purified IgG (anti-TRP or control) for 2 hours prior to infection or therapeutically 2 and 24 hours post-infection in serum-free medium (1µg/ml). Host cell-free E. chaffeensis was purified by sonication and differential centrifugation. Briefly, infected DH82 cells were harvested and pelleted (5000 × g) for 15 min. The pellet was resuspended in sterile PBS and sonicated twice for 10 s on ice at 40W using an Ultrasonic Processor (Sonics, Newtown, CT). The suspension was centrifuged for 10 min (1500 × g) to pellet the cell debris. The supernatant containing the host cell-free ehrlichiae was pelleted for 15 min (10,000 × g). Monolayers of DH82 cells in serum-free medium were prophylactically treated with purified IgG (0.5µg/ml) for 2 hours and the cells were infected with host cell-free E. chaffeensis in the presence of IgG. The recombinant TRP32 (rTRP32) and the TR domain (first two repeats) of TRP120 (rTRP120) were expressed and purified as previously described [26;46]. In the competition experiments, recombinant proteins (1µg/ml) were added in addition to TRP IgG. The therapeutic effects of the anti-TRP IgG were tested by adding the IgG after internalization of E. chaffeensis. Cells were washed three times with sterile phosphate-buffered saline to remove extracellular E. chaffeensis and were treated with IgG (0.5µg/ml) 2 and 24 hours post-infection. Three control groups were used including IgG purified from sera generated against a control peptide, pre-immune sera and PBS. Monolayers were infected with same amount of host cell-free E. chaffeensis and were harvested 1, 2, and 3 days post infection.
2.3. Western immunoblotting
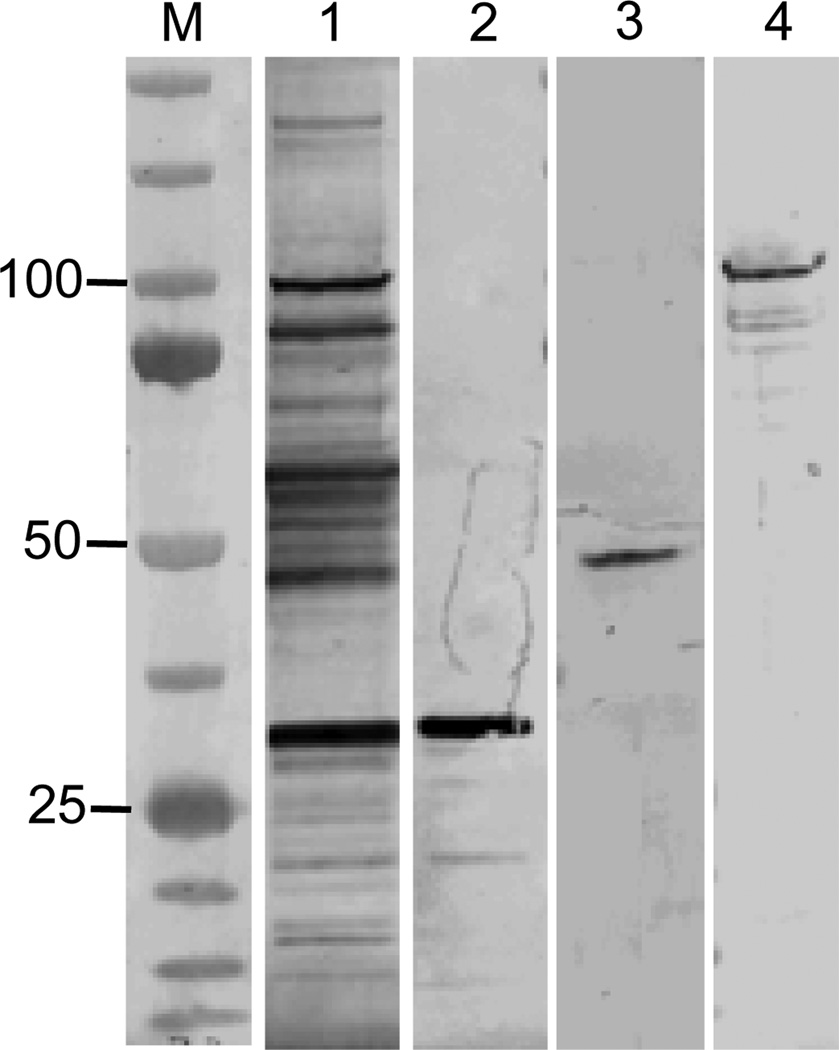
E. chaffeensis whole cell lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and western immunoblotting was performed as previously described [31]. The dog anti-E. chaffeensis serum was diluted 1:100, and mouse anti-TRP sera were diluted 1:500 (Fig. 1). Bound primary antibodies were detected with alkaline phosphatase-conjugated anti-rabbit IgG (H+L) secondary antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) and visualized after incubation with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium) substrate.
FIG. 1.
Identification of immunodominant E. chaffeensis TRPs with epitope-specific mouse antisera used for passive protection experiments. Western immunoblot of whole-cell lysate from E. chaffeensis-infected DH82 cells probed with anti-E. chaffeensis dog (no. 2251) serum (lane 1; positive control) and, mouse anti-TRP32 (lane 2), anti-TRP47 (lane 3) and anti-TRP120 (lane 4) sera. M, molecular mass marker (kilodaltons).
2.4. Mice and infection
C57BL/6-scid (B6.CB17-Prkdcscid) and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed and cared for in the Animal Research Center at the University of Texas Medical Branch in accordance with the Institutional Animal Care and Use Committee guidelines under whose review and approval the experiments were conducted. Six to 12-week-old, sex-matched mice were infected intraperitoneally with approximately 2×106 infected DH82 cells (>95% infected) as previously described [43]. Immune serum was produced in C57BL/6 mice as previously described [44]. Mice were intraperitoneally administered mouse anti-TRP (individually or in combination), E. chaffeensis-immune, or control sera (0.1 ml) on days 7 and 17 post-infection for the C57BL/6-scid mice and on day 3 for the C57BL/6 mice. Mice were sacrificed 21 days (C57BL/6-scid) or 5 days (C57BL/6) post-infection and the liver and spleen were collected. The control groups were administered sera generated against a control peptide, pre-immune sera or PBS. Three mice were used per group, and each experiment was repeated at least three times.
2.5. Quantification of E. chaffeensis
Total DNA was extracted from infected DH82 cells or homogenized mouse liver with DNeasy Blood and Tissue Kit (Qiagen). Recombinant plasmids were constructed by cloning the E. chaffeensis dsb gene using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA). The size of the plasmid (with the insert) was determined, and the concentration of the double-stranded DNA was calculated with a NanoDrop ND-1000 spectrometer (Wilmington, DE). The plasmid copy number for the standards was calculated using the following formula: plasmid copy/µl = [(plasmid concentration g/µl) / (plasmid length in basepairs × 660)] × 6.022 × 1023. E. chaffeensis was amplified with primers Dsb-321 forward, 5′ TTG CAA AAT GAT GTC TGA AGA TAT GAA ACA 3′, and Dsb-671 reverse, 5′ GCT GCT CCA CCA ATA AAT GTA TCT CCT A 3′ [5]. The thermal cycling protocol consisted of an initial denaturation step of 95°C for 2 min and 40 cycles of 95° for 10 s, 55°C for 30 s, and 65°C for 30 s. The absolute E. chaffeensis dsb copy number in the cells (in vitro) and liver was determined by real-time qPCR and plotted against the standard curve.
2.6. ELISA
Plates (MaxiSorp; NUNC, Roskilde, Denmark) were coated with synthetic peptides (1 µg/well; 50 µl) suspended in phosphate-buffered saline (pH 7.4) as previously described [24]. Plates were blocked with 100 µl of 10% equine serum (Sigma) in TBST for 1 h at room temperature with agitation and washed. Mouse immune sera or anti-TRP sera (1:100 or 1:6400) diluted in 5% equine serum-TBST were added to each well (50 µl) and incubated at room temperature for 1 h with gentle agitation. The plates were washed four times and incubated in 50 µl of rat anti-mouse IgG1, IgG2a, IgG2b or IgG3 (BD Biosciences, Sparks, MD) (1:1000) for 1 h at room temperature. The plates were washed and incubated in 50 µl of horseradish peroxidase-labeled goat anti-rat IgG antibody (Southern Biotech, Birmingham, AL) (1:2000) for 1 h at room temperature. Substrate (100 µl, TMB Liquid Substrate System, Sigma) was added to each well, and plates were incubated in the dark for 30 min with agitation. Optical density was determined on a microplate reader (VersaMax, Molecular Devices, Sunnyvale, CA) at A650 and data analyzed by SoftmaxPro v4.0 (Molecular Devices). Optical density (OD) readings represent the mean OD for three wells (± standard deviations) after subtracting the OD value of the negative peptide control.
2.7. Statistical Analysis
Data are expressed as means ± standard deviations (SD), and the statistical significance was determined using a one-way analysis of variance followed by Tukey’s multiple comparison tests were performed using Prism 5 software (GraphPad). P values of < 0.05 were considered significant.
3. Results
3.1. Antibodies against TRP32, TRP47 and TRP120
Major immunoreactive epitopes of tandem repeat proteins 32, 47, 120 have been previously determined [6;24;26]. Mouse and rabbit antisera against these epitopes were generated and tested to confirm recognition of the ehrlichial proteins (Fig. 1, Table 1).
Table 1.
Antibody titers of TRP mouse and rabbit sera generated against epitope-containing peptides of TRP32, TRP47 and TRP120 as determined by three immunoassays.
| Sera | IFA | ELISA | Western Blotting | |||
|---|---|---|---|---|---|---|
| Mouse | Rabbit | Mouse | Rabbit | Mouse | Rabbit | |
| Anti-TRP32 | 1:1000 | 1:500 | 1:6400 | 1:25600 | nd | 1:5000 |
| Anti-TRP47 | 1:100 | 1:100 | 1: 12800 | 1:512000 | nd | 1:100 |
| Anti-TRP120 | 1:1000 | 1:1000 | 1:12800 | 1: 6400 | nd | 1:1000 |
| Immune Sera | 1:1000 | nd | nd | |||
nd, not determined.
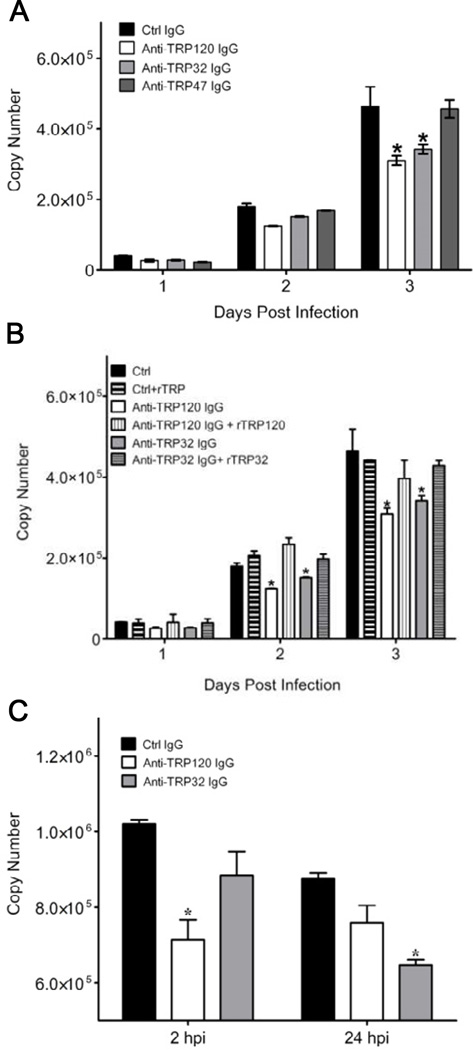
3.2. Prophylactic protection by TRP32 and TRP120 antibody in vitro
To determine the effect of antibodies prior to ehrlichial entry, DH82 monolayers were treated with purified rabbit anti-TRP32 and anti-TRP120 IgG prior to infection. A lower ehrlichial load was observed in anti-TRP32 and anti-TRP120 IgG treated cells compared to the controls with a significant reduction (p < 0.05) on day three (Fig. 2A). However, there was no difference observed with anti-TRP47 IgG. The reduction in bacterial load observed with anti-TRP32 and anti-TRP120 IgG was completely reversed by addition of recombinant TRP32 or TRP120 as a competitor (Fig. 2B). No significant difference in bacterial copy number was observed in the control peptide IgG, pre-immune IgG or PBS treated cells; thus, pre-immune sera or PBS were used in all subsequent experiments.
FIG. 2.
Prophylactic and therapeutic effect of anti-TRP IgG on E. chaffeensis infection in vitro. (A) Cells were treated with IgG (anti-TRP or control) 2 hrs prior to infection and ehrlichial loads determined 1, 2, and 3 days post infection by qPCR. (B) Reduction in ehrlichial load in the presence of anti-TRP32 and anti-TRP120 IgG is protein-specific. Cells were incubated with anti-TRP IgG alone or in combination with recombinant protein (rTRP32 and rTRP120) to neutralize the effect of specific antibody for 2 hrs prior to infection and ehrlichial loads determined by qPCR. (C) Infected cells were treated with IgG (anti-TRP or control) 2 and 24 hrs post-infection, and ehrlichial load determined 3 dpi by qPCR. Bar graphs represent means ± SD, (*p <0.05).
3.3. Therapeutic protection of TRP32 and TRP120 antibody in vitro
To determine the effect of antibodies post ehrlichial entry, infected DH82 cells were treated with TRP32, TRP120 or control IgG 2 and 24 hrs post-infection. A significant (p < 0.05) reduction in bacterial load was observed when TRP120 IgG was added 2 hrs post-infection and TRP32 IgG was added 24 hrs post-infection (Fig. 2C).
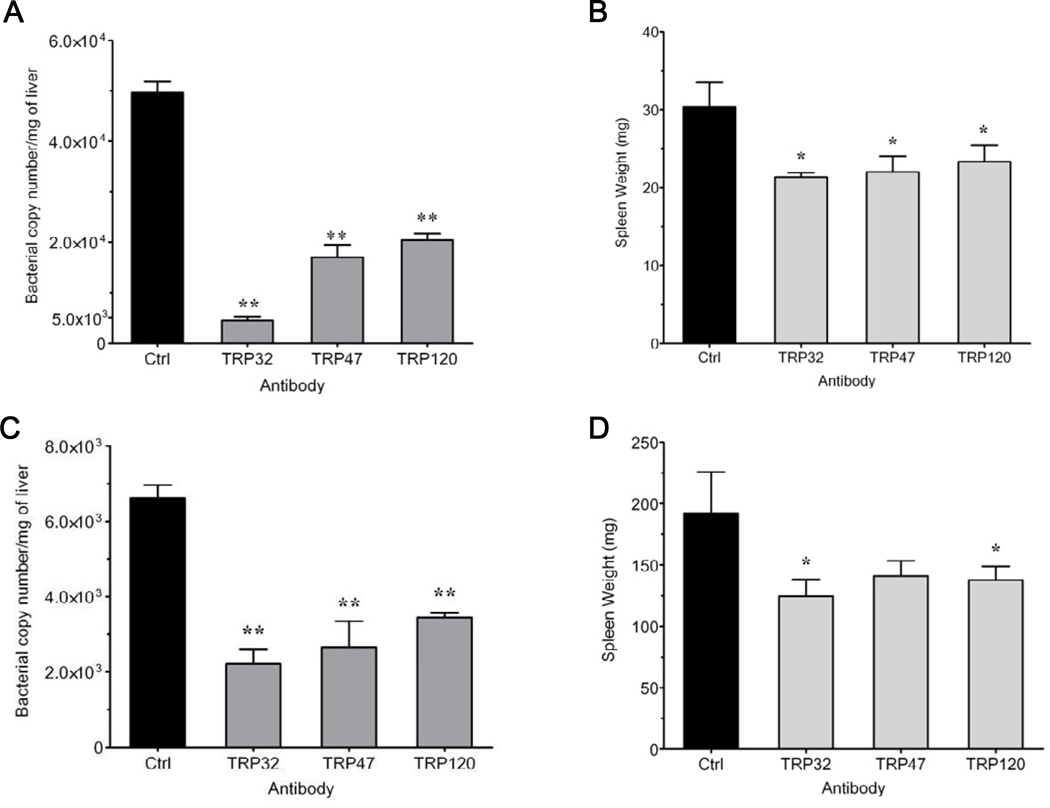
3.4. Passive protection of TRP antibodies individually in vivo
To determine the in vivo protection of anti-TRP sera, we used a previously developed murine model of E. chaffeensis infection [43]. In several initial experiments, no significant differences were observed in control peptide sera, pre-immune sera or PBS-treated mice (data not shown); therefore, in subsequent experiments, pre-immune sera or PBS were used as controls. Immunocompetent C57BL/6 mice develop a transient infection and inflammation but clear ehrlichiae within two weeks and immunocompromised SCID mice, lacking T and B lymphocytes develop persistent infection and disease and become moribund within three weeks after infection [43]. Immunocompetent C57BL/6 mice and susceptible SCID mice were infected with E. chaffeensis and were treated with anti-TRP32, TRP47 or TRP120 sera to test their protective capacity. Ehrlichial burden in the liver, one of two principal sites of ehrlichial infection in mice [43], was determined by qPCR. Since E. chaffeensis-infected mice exhibit pronounced splenomegaly, the spleen weights of the anti-TRP sera-treated mice were compared to controls.
SCID mice were infected and treated with sera on days 7 and 17 post-infection, and the livers and spleens were harvested 21 days post-infection. A significant (p < 0.005) reduction in ehrlichial copy number was observed when TRP32, TRP47 or TRP120 antisera were administered to SCID mice during the course of the infection (Fig. 3A). Furthermore, anti-TRP antibody treated mice had significantly (p < 0.05) lower spleen weights than the controls (Fig. 3B).
FIG. 3.
Reduction of ehrlichial burden in C57BL/6-scid and C57BL/6 mice treated with anti-TRP32, anti-TRP47 or anti-TRP120 sera. Mice (SCID and immunocompetent) were inoculated intraperitoneally with 2×106 E. chaffeensis- infected DH82 cells on day 0. SCID mice were administered anti-TRP32, anti-TRP47, anti-TRP120 or control sera on days 7, 17, and mice were sacrificed 21 days post-infection. Immunocompetent C57BL/6 mice were administered anti-TRP32, anti-TRP47, anti-TRP120 or control sera on day 3 and sacrificed 5 days post-infection. E. chaffeensis dsb copy number in the liver was determined by qPCR (A and C) and spleen weights (B and D) were compared to controls. Three mice were used per group and data are representative of one of three independent experiments. Graphs represent means ± SD, (*p<0.05, **p <0.005).
To examine the anti-TRP protection in immunocompetent mice, C57BL/6 mice were infected and treated with TRP32, TRP47 or TRP120 antisera three days post infection, and organs were harvested five days post-infection. Similar to the SCID mice, significant reductions in the E. chaffeensis burden (p < 0.005) and spleen weights (p < 0.05) were observed in the immunocompetent mice compared to controls (Fig. 3C and D).
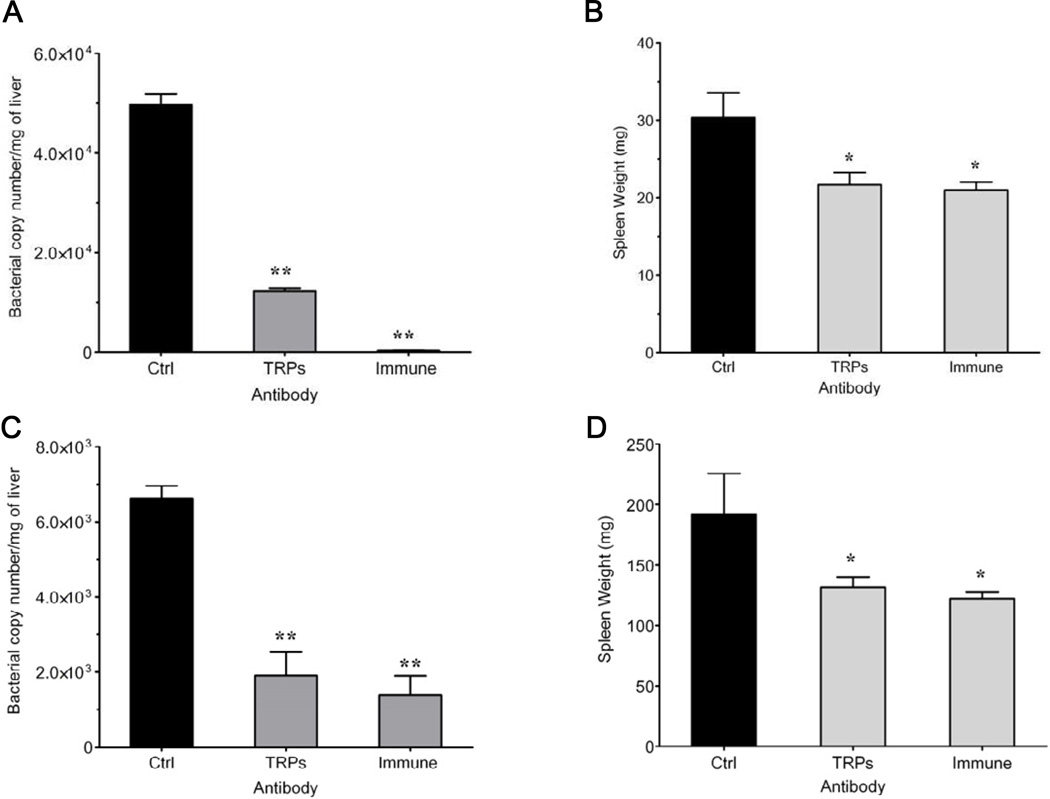
3.5. Passive protection with TRP antibodies and E. chaffeensis immune sera
In a previous study, significant protective effect was observed in SCID mice that received immune sera from immunocompetent mice during E. chaffeensis infection [44]. To compare the protection mediated by antibodies directed at TRP epitopes compared to the protection previously observed with immune sera, immunocompetent and SCID mice were treated with a combination of anti-TRP32, TRP47 and TRP120 sera or E. chaffeensis-immune sera. SCID mice treated with the combination of TRP antisera and immune sera had a significantly (p < 0.005) lower ehrlichial burdens compared to the control group (Fig. 4A). Furthermore, the mice that received E. chaffeensis immune sera had fewer than 10 ehrlichiae/250 ng of total DNA. The spleen weights of mice that received immune sera were similar to the mice treated with the combination of anti-TRP sera, and both groups’ spleen weights were significantly (p < 0.05) lower than the control group (Fig. 4B). In the immunocompetent mice, immune sera-treated mice and the mice treated with the combination of TRP antisera had significantly lower ehrlichial loads (p < 0.005) than controls. Most notable were the similar bacterial loads and spleen weights observed in mice treated with immune sera and combination of TRP antisera (Fig. 4C and D).
FIG. 4.
Reduction of ehrlichial burden in C57BL/6-scid and C57BL/6 mice after treatment with a combination of anti-TRP sera compared to immune sera. Mice were infected intraperitoneally with 2×106 E. chaffeensis infected DH82 cells on day 0. SCID mice were administered a combination of anti-TRP32, anti-TRP47 and anti-TRP120 sera (TRPs), immune or control sera on days 7, 17, and mice were sacrificed 21 days post-infection. Immunocompetent C57BL/6 mice were administered a combination of anti-TRP32, anti-TRP47 and anti-TRP120 sera (TRPs), immune or control sera on day 3 and sacrificed 5 days post-infection. E. chaffeensis dsb copy number in the liver was determined by quantitative real-time PCR (A and C) and spleen weights (B and D) were compared to control mice. Three mice were used per group, and data are representative of one of three independent experiments. Graphs represent means ± SD, (*p<0.05, **p <0.005).
3.6. IgG isotypes in anti-TRP and immune sera
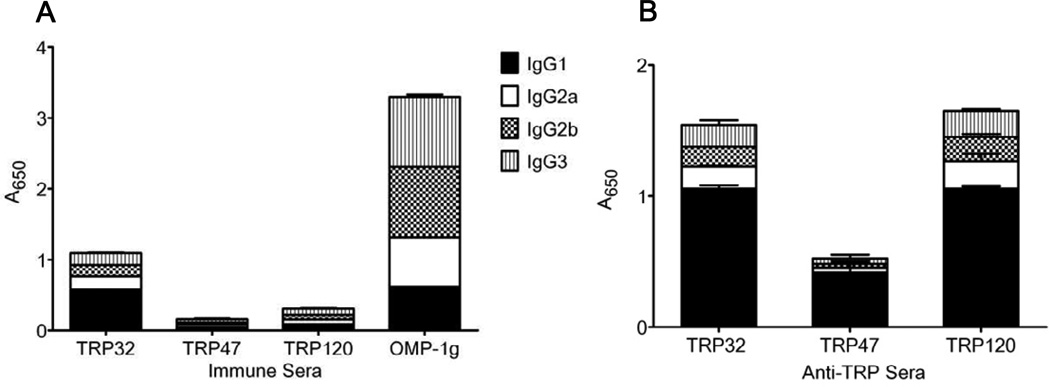
The TRP32, TRP47 and TRP120 epitope-specific IgG isotypes in the E. chaffeensis-immune sera were quantified by ELISA (Fig. 5A). Previously described protective OMP-1g (p28-19) epitope was also measured for comparison [20]. In the E. chaffeensis-immune sera, all IgG isotypes (IgG1, 2a, 2b and 3) were detected against the three protective TRP epitopes and IgG1 was the predominant isotype. TRP32 has the highest antibody levels of the three TRPs in immune serum obtained from E. chaffeensis-infected mice and used in passive transfer experiments. In hyperimmune mouse serum generated against peptides representing TRP epitopes, IgG1 was also the predominant isotype. The concentration the other isotypes (IgG2a, 2b, and 3) were detected in similar concentrations, but lower than IgG1 (Fig. 5B).
FIG. 5.
Quantification of antibody isotypes detected in E. chaffeensis-immune and TRP epitope-specific sera by ELISA. (A) Epitope-specific TRP32, TRP47, TRP120 and OMP-1g IgG isotypes in E. chaffeensis-immune sera and (B) mouse anti-TRP32, TRP47 and TRP120 serum.
4. Discussion
It is well established in that antibodies against the obligately intracellular pathogen, E. chaffeensis, are essential for immunity [18;44]. However, during E. chaffeensis infection, many proteins elicit strong antibody responses, yet only antibodies against a linear epitope in the hypervariable region of OMP-1g protein primarily of the isotype IgG2a have been demonstrated to provide protection [20;44]. This antibody mediated protection appears to involve Fc receptor-dependent mechanisms [45]. The purpose of this study was to expand our knowledge of immunoprotective components of Ehrlichia and have a better understanding of the antibody-mediated immune mechanisms involved. We have previously identified and molecularly characterized a subset of immunodominant ehrlichial proteins that contain tandem repeats and determined that each of these TRPs contains a single major continuous species-specific antibody epitope within the TR region [4;24;26]. In this study, we conclusively demonstrated antibody-mediated protection against E. chaffeensis infection by passively transferring TRP epitope-specific antibody against three E. chaffeensis TRPs (TRP32, TRP47 and TRP120).
Humoral immunity to bacteria has been associated with proteins localized on the cellular surface such as OspA of Borrelia. burgdorferi and PsaA of Yersinia. pestis [1;17]. In Anaplasma marginale, antibodies against the highly immunogenic multifamily major surface proteins (Msps) that are components of the outer membrane provide protection against infection, anemia and high-level bacteremia [36;37]. In addition, the alpha C protein of the group B Streptococcus is a surface-expressed TRP that elicits protective antibody-mediated immunity [35]. Monoclonal antibodies to the alpha C protein induce opsonic killing of the bacterium and protect mice from lethal challenge, and altered tandem repeat numbers are associated with immune evasion [14;27;28]. However, unlike the MSPs of A. marginale, many of the major immunoreactive proteins of E. chaffeensis are secreted TRPs that are involved in molecular interactions with the host cell. Secreted effector proteins of several pathogens have been shown to be immunogenic, such as the recently described Chlamydia trachomatis Tarp protein or LLO from Listeria monocytogenes [7;42]. Although the C. trachomatis Tarp is a secreted TRP effector that is strongly recognized by antibodies in immune patient sera, its role in inducing protective antibodies is unknown [41;42]. In contrast, antibodies against the secreted effector LLO have been demonstrated to provide protection when administered prophylactically [7]. Prophylactic and therapeutic administration of antibodies is also protective against respiratory infection with F. tularensis [39].
In this investigation, the effect of E. chaffeensis TRPs linear epitope specific antibodies during infection was determined using an in vitro model which was validated and supported using immunocompetent and SCID murine models. In the in vitro model, anti-TRP120 IgG was protective when administered prophylactically and therapeutically at two hours post-infection, but not at 24 hours post infection. TRP120 is differentially expressed on the infectious dense-cored ehrlichiae and has been previously associated with ehrlichial binding and internalization [15;38]. Moreover, it has recently been identified as a DNA binding protein and interacts with other defined host cell targets [22;47]. The prophylactic efficiency of the anti-TRP120 IgG during the early stages of infection in the in vitro model when the antibody is in direct contact with the host cell-free ehrlichiae suggests that the antibody is involved in neutralization or other extracellular antibody mediated mechanism. Recently, antibodies were shown to protect by altering the intracellular trafficking of Legionella pneumophila into lysosomal compartments via Fc receptor (FcR) mediated signaling in macrophages [12]. Antibody mediated activation of the FcR blocks the functions of bacterial effector proteins secreted into the host cell cytoplasm which include avoiding fusion to the lysosome, making the host cell nonpermissive for intracellular replication, and this was observed both in vitro and in vivo [12]. During ehrlichial infection of immunocompetent mice, FcR common γ chain and FcγRI have been previously demonstrated to be required for clearance of bacteria [45], consistent with the FcR mediated clearance of L. pneumophila.
A reduction in bacterial burden was observed when anti-TRP32 IgG was administered prophylactically and therapeutically at 24 hours post-infection, but not two hours post-infection. TRP32 is expressed on both dense-cored and replicating reticulate cells and is associated with morular fibrils and membrane [26]. TRP32 was recently identified as a secreted ehrlichial effector protein that interacts with several host cell proteins involved in protein synthesis, iron acquisition, immune signaling and transcriptional regulation [23]. The protection mediated by therapeutically administered anti-TRP32 IgG also likely involves extracellular neutralization, since both TRP32 and TRP120 are found on the surface of DC ehrlichiae. However, the therapeutic protection mediated by anti-TRP32 and anti-TRP120 antibodies two and 24 hours post-infection, after ehrlichiae have entered the host cell, suggests that intracellular antibody-mediated immune mechanisms are involved. TRP32, TRP47 and TRP120 are found extracellularly in inclusion matrix and associated with the inclusion membrane [6;26;38]. Additionally, TRP47 and TRP32 are highly upregulated during ehrlichial infection of the macrophage and TRP120 is translocated to the host cell nucleus [16;47]. The subcellular localization of and TRP120 and the molecular interactions between TRP32, TRP47 and TRP120 with host proteins [22;23;40] suggest that the TRPs and associated effector functions may be neutralized by cytoplasmic antibodies that block the effector functions of these proteins at different stages of infection. Antibody directly transfected into cells blocked interactions of type IV secreted effector protein, AnkA, resulting in reduction of the A. phagocytophilum burden [21]. Moreover, antibodies against LLO, a secreted effector protein of L. monocytogenes provided protection through intracellular mechanisms post-internalization of the bacterium [7]. Additionally, monoclonal antibodies against intracellular oncoproteins have been shown to enter host cells and inhibiting tumor cell growth [10]. Cytosolic IgG receptors such as the recently described TRIM21 could be involved in the intracellular antibody mediated immunity [13]. TRIM21 is expressed on all cell types, binds all IgG isotypes, and mediates intracellular immunity by recruiting antibody-bound antigen for proteasomal degradation [29].
In the in vivo mouse model, the three TRP antisera were protective when therapeutically administered individually and in combination to immunocompetent and SCID mice after establishment of infection. In the SCID mice, anti-TRP32 was the most protective of the three TRP antisera. Interestingly, in the immunocompetent mice, when treated with the combination of the three antisera, mice had similar correlates of protection as immune sera treated mice. Similarly, Winslow et. al. previously reported that extremely low copy numbers of bacterial DNA was detected in immune sera-treated immunocompetent mice four and seven days post-infection [44]. These results suggest that the TRP epitopes contribute to a significant fraction of antibody mediated protection in the immune sera in the immunocompetent mice in addition to previously characterized outer membrane protein epitopes.
The in vivo studies were supportive of the in vitro model data for TRP32 and TRP120 antibodies. The difference in protection mediated by TRP47 antibody in vitro and in vivo is potentially due to differences in antibody titer (as measured by IFA), or suggestive of a mechanistic deficiency that is reconstituted in vivo. Higher concentrations of anti-TRP47 antibody were not tested to determine if in vitro protection could be achieved, but based our in vivo findings, anti-TRP47 antibodies are protective. Results from the in vitro model of antibody protection correlated well with the in vivo model of protection for the E. chaffeensis TRPs, supporting the in vitro model as a valid screening alternative for defining immunoprotective proteins. During infection with A. marginale, an intracellular bacterium closely related to E. chaffeensis, a similar assay was used to demonstrate the inhibition of infection mediated by antibodies against immunogenic major surface proteins (Msp) of A. marginale during infection of tick cells [2].
Murine E. chaffeensis-specific mononclonal antibodies generated in a previous study were of the IgG2a, 2b and 3 isotypes [20], and protective antibodies identified were directed at the hypervariable region of OMP-1g and were of the IgG2a isotype [18]. IgG2a is capable of activating complement and binding to Fc receptors. Thus, consistent with antibody mediated mechanisms associated with bacterial OMPs and protective ehrlichial OMP IgG2a antibodies have been proposed to opsonize E. chaffeensis and involve FcγRI-mediated phagocytosis of antibody opsonized bacteria that either escaped from infected host cells or were released during cell lysis [19;45]. In this study, we demonstrated that TRP-specific antibodies provide protection similar to OMP antibodies demonstrating that both TRP and OMP specific antibodies are important for protection. However, the functional differences in these proteins and the cellular localization (membrane versus secreted) suggest that protective antibodies may confer protection through distinct mechanisms.
In the present study, IgG1 was the predominant isotype detected in the protective TRP-peptide antisera, but other antibody isotypes (IgG2a, 2b, and 3) were also present in substantially lower concentrations. Interestingly, although we detected similar antibody concentrations of all four isotypes in sera from mice infected with E. chaffeensis, monoclonal antibodies developed by Winslow et. al represented primarily IgG2a and 2b isotypes, and IgG1 monoclonal antibodies were not obtained [18]. The predominance of the IgG1 isotype (which does not activate complement or bind Fc receptors) in the murine epitope-specific anti-TRP antisera, suggests that mechanisms other than complement activation and Fc receptor mediated killing could also be involved. Furthermore, higher concentrations of IgG1 antibody in anti-TRP peptide serum and immune serum (TRP32) from E. chaffeensis- infected mice suggest that antibodies to TRPs are more likely to be of the IgG1 isotype. Nevertheless, multiple antibody isotypes are likely to play a role in TRP specific antibody mediated immunity through different mechanisms. Characterization of antibody isotypes directed at TRPs and OMPs during E. chaffeensis human infections would also help define that antibody isotypes produced during infection and the antibody mediated mechanisms are associated with anti-TRPs and anti-OMP antibodies.
Our findings support the role of antibodies in host defense during ehrlichial infection. In this study we have demonstrated that antibodies against the linear epitopes of immunodominant TRPs (32, 47 and 120) are protective and appear to involve extracellular and intracellular antibody-mediated mechanisms. These epitopes should be included in development of vaccines and therapies for ehrlichial diseases and further studies need to be done to elucidate the mechanism of anti-TRP antibody-mediated protection during E. chaffeensis infection.
Acknowledgments
This work was supported by grant R01 AI 071145 from the National Institute of Allergy and Infectious Diseases (NIAID) and by the Clayton Foundation for Research. Jeeba Kuriakose was supported by NIAID Biodefense training grant T32 AI060549. We thank David Walker and Xuejie Yu for reviewing the manuscript and providing helpful suggestions.
Abbreviations
- TRP
tandem repeat protein
- OMP
outer membrane protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Benach JL, Coleman JL, Golightly MG. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J.Immunol. 1988;140:265–272. [PubMed] [Google Scholar]
- 2.Blouin EF, Saliki JT, de la Fuente J, Garcia-Garcia JC, Kocan KM. Antibodies to Anaplasma marginale major surface proteins 1a and 1b inhibit infectivity for cultured tick cells. Vet.Parasitol. 2003;111:247–260. doi: 10.1016/s0304-4017(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 4.Doyle CK, Cardenas AM, Aguiar DM, Labruna MB, Ndip LM, Yu XJ, McBride JW. Molecular characterization of E canis gp36 and E chaffeensis gp47 tandem repeats among isolates from different geographic locations. Ann.N.Y.Acad.Sci. 2005;1063:433–435. doi: 10.1196/annals.1355.079. [DOI] [PubMed] [Google Scholar]
- 5.Doyle CK, Labruna MB, Breitschwerdt EB, Tang YW, Corstvet RE, Hegarty BC, Bloch KC, Li P, Walker DH, McBride JW. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J.Mol.Diagn. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle CK, Nethery KA, Popov VL, McBride JW. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect.Immun. 2006;74:711–720. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 8.Feng HM, Whitworth T, Olano JP, Popov VL, Walker DH. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect.Immun. 2004;72:2222–2228. doi: 10.1128/IAI.72.4.2222-2228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin.Microbiol.Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo K, Tang JP, Tan CP, Wang H, Zeng Q. Monoclonal antibodies target intracellular PRL phosphatases to inhibit cancer metastases in mice. Cancer Biol.Ther. 2008;7:750–757. doi: 10.4161/cbt.7.5.5764. [DOI] [PubMed] [Google Scholar]
- 11.Joller N, Weber SS, Muller AJ, Sporri R, Selchow P, Sander P, Hilbi H, Oxenius A. Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. Proc.Natl.Acad.Sci.U.S.A. 2010;107:20441–20446. doi: 10.1073/pnas.1013827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joller N, Weber SS, Oxenius A. Antibody-Fc receptor interactions in protection against intracellular pathogens. Eur.J.Immunol. 2011;41:889–897. doi: 10.1002/eji.201041340. [DOI] [PubMed] [Google Scholar]
- 13.Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc.Natl.Acad.Sci.U.S.A. 2008;105:6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kling DE, Gravekamp C, Madoff LC, Michel JL. Characterization of two distinct opsonic and protective epitopes within the alpha C protein of the group B Streptococcus. Infect.Immun. 1997;65:1462–1467. doi: 10.1128/iai.65.4.1462-1467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumagai Y, Matsuo J, Hayakawa Y, Rikihisa Y. Cyclic di-GMP signaling regulates invasion by Ehrlichia chaffeensis of human monocytes. J.Bacteriol. 2010;192:4122–4133. doi: 10.1128/JB.00132-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuriakose JA, Miyashiro S, Luo T, Zhu B, McBride JW. Ehrlichia chaffeensis transcriptome in mammalian and arthropod hosts reveals differential gene expression and post transcriptional regulation. PLoS.One. 2011;6:e24136. doi: 10.1371/journal.pone.0024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Jiang L, Song Q, Yang J, Chen Z, Guo Z, Zhou D, Du Z, Song Y, Wang J, Wang H, Yu S, Wang J, Yang R. Protein microarray for profiling antibody responses to Yersinia pestis live vaccine. Infect.Immun. 2005;73:3734–3739. doi: 10.1128/IAI.73.6.3734-3739.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JS, Chu F, Reilly A, Winslow GM. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J.Immunol. 2002;169:1419–1425. doi: 10.4049/jimmunol.169.3.1419. [DOI] [PubMed] [Google Scholar]
- 19.Li JS, Winslow GM. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect.Immun. 2003;71:4229–4237. doi: 10.1128/IAI.71.8.4229-4237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JS, Yager E, Reilly M, Freeman C, Reddy GR, Reilly AA, Chu FK, Winslow GM. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J.Immunol. 2001;166:1855–1862. doi: 10.4049/jimmunol.166.3.1855. [DOI] [PubMed] [Google Scholar]
- 21.Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 22.Luo T, Kuriakose JA, Zhu B, Wakeel A, McBride JW. Ehrlichia chaffeensis TRP120 interacts with a diverse array of eukaryotic proteins involved in transcription, signaling, and cytoskeleton organization. Infect.Immun. 2011;79:4382–4391. doi: 10.1128/IAI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo T, McBride JW. Ehrlichia chaffeensis TRP32 interacts with host cell targets that influence intracellular survival. Infect.Immun. 2012 doi: 10.1128/IAI.00154-12. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo T, Zhang X, McBride JW. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E canis p140 orthologs in surface-exposed tandem repeat regions. Clin.Vaccine Immunol. 2009;16:982–990. doi: 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo T, Zhang X, Nicholson WL, Zhu B, McBride JW. Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin.Vaccine Immunol. 2010;17:87–97. doi: 10.1128/CVI.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo T, Zhang X, Wakeel A, Popov VL, McBride JW. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect.Immun. 2008;76:1572–1580. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madoff LC, Michel JL, Gong EW, Kling DE, Kasper DL. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc.Natl.Acad.Sci.U.S.A. 1996;93:4131–4136. doi: 10.1073/pnas.93.9.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madoff LC, Michel JL, Kasper DL. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect.Immun. 1991;59:204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc.Natl.Acad.Sci.U.S.A. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBride JW, Corstvet RE, Breitschwerdt EB, Walker DH. Immunodiagnosis of Ehrlichia canis infection with recombinant proteins. J.Clin.Microbiol. 2001;39:315–322. doi: 10.1128/JCM.39.1.315-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride JW, Corstvet RE, Gaunt SD, Boudreaux C, Guedry T, Walker DH. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect.Immun. 2003;71:2516–2524. doi: 10.1128/IAI.71.5.2516-2524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride JW, Doyle CK, Zhang X, Cardenas AM, Popov VL, Nethery KA, Woods ME. Identification of a glycosylated Ehrlichia canis 19-kilodalton major immunoreactive protein with a species-specific serine-rich glycopeptide epitope. Infect.Immun. 2007;75:74–82. doi: 10.1128/IAI.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride JW, Zhang X, Wakeel A, Kuriakose JA. Tyrosine phosphorylated Ehrlichia chaffeensis and Ehrlichia canis tandem repeat orthologs contain a major continuous cross-reactive antibody epitope in lysine-rich repeats. Infect.Immun. 2011;79:3178–3187. doi: 10.1128/IAI.01347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect.Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel JL, Madoff LC, Kling DE, Kasper DL, Ausubel FM. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect.Immun. 1991;59:2023–2028. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noh SM, Brayton KA, Brown WC, Norimine J, Munske GR, Davitt CM, Palmer GH. Composition of the surface proteome of Anaplasma marginale and its role in protective immunity induced by outer membrane immunization. Infect.Immun. 2008;76:2219–2226. doi: 10.1128/IAI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noh SM, Zhuang Y, Futse JE, Brown WC, Brayton KA, Palmer GH. The immunization-induced antibody response to the Anaplasma marginale major surface protein 2 and its association with protective immunity. Vaccine. 2010;28:3741–3747. doi: 10.1016/j.vaccine.2010.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popov VL, Yu X, Walker DH. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb.Pathog. 2000;28:71–80. doi: 10.1006/mpat.1999.0327. [DOI] [PubMed] [Google Scholar]
- 39.Savitt AG, Mena-Taboada P, Monsalve G, Benach JL. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin.Vaccine Immunol. 2009;16:414–422. doi: 10.1128/CVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect.Immun. 2009;77:1734–1745. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Chen L, Chen F, Zhang X, Zhang Y, Baseman J, Perdue S, Yeh IT, Shain R, Holland M, Bailey R, Mabey D, Yu P, Zhong G. A chlamydial type III-secreted effector protein (Tarp) is predominantly recognized by antibodies from humans infected with Chlamydia trachomatis and induces protective immunity against upper genital tract pathologies in mice. Vaccine. 2009;27:2967–2980. doi: 10.1016/j.vaccine.2009.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Zhang Y, Yu P, Zhong G. Immunodominant regions of a Chlamydia trachomatis type III secretion effector protein, Tarp. Clin.Vaccine Immunol. 2010;17:1371–1376. doi: 10.1128/CVI.00218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winslow GM, Yager E, Shilo K, Collins DN, Chu FK. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect.Immun. 1998;66:3892–3899. doi: 10.1128/iai.66.8.3892-3899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winslow GM, Yager E, Shilo K, Volk E, Reilly A, Chu FK. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect.Immun. 2000;68:2187–2195. doi: 10.1128/iai.68.4.2187-2195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yager E, Bitsaktsis C, Nandi B, McBride JW, Winslow G. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect.Immun. 2005;73:8009–8016. doi: 10.1128/IAI.73.12.8009-8016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu XJ, Crocquet-Valdes P, Cullman LC, Walker DH. The recombinant 120-kilodalton protein of Ehrlichia chaffeensis, a potential diagnostic tool. J.Clin.Microbiol. 1996;34:2853–2855. doi: 10.1128/jcm.34.11.2853-2855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu B, Kuriakose JA, Luo T, Ballesteros E, Gupta S, Fofanov Y, McBride JW. Ehrlichia chaffeensis TRP120 binds a G+C-rich motif in host cell DNA and exhibits eukaryotic transcriptional activator function. Infect.Immun. 2011;79:4370–4381. doi: 10.1128/IAI.05422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]