Abstract
Enhancing cholinergic function improves performance on various cognitive tasks and alters neural responses in task specific brain regions. Previous findings by our group strongly suggested that the changes in neural activity observed during increased cholinergic function may reflect an increase in neural efficiency that leads to improved task performance. The current study was designed to assess the effects of cholinergic enhancement on regional brain connectivity and BOLD signal variability. Nine subjects participated in a double-blind, placebo-controlled crossover functional magnetic resonance imaging (fMRI) study. Following an infusion of physostigmine (1mg/hr) or placebo, echo-planar imaging (EPI) was conducted as participants performed a selective attention task. During the task, two images comprised of superimposed pictures of faces and houses were presented. Subjects were instructed periodically to shift their attention from one stimulus component to the other and to perform a matching task using hand held response buttons. A control condition included phase-scrambled images of superimposed faces and houses that were presented in the same temporal and spatial manner as the attention task; participants were instructed to perform a matching task.
Cholinergic enhancement improved performance during the selective attention task, with no change during the control task. Functional connectivity analyses showed that the strength of connectivity between ventral visual processing areas and task-related occipital, parietal and prefrontal regions was reduced significantly during cholinergic enhancement, exclusively during the selective attention task. Cholinergic enhancement also reduced BOLD signal temporal variability relative to placebo throughout temporal and occipital visual processing areas, again during the selective attention task only. Together with the observed behavioral improvement, the decreases in connectivity strength throughout task-relevant regions and BOLD variability within stimulus processing regions provide further support to the hypothesis that cholinergic augmentation results in enhanced neural efficiency.
Keywords: cholinergic system, neural efficiency, physostigmine, attention, functional magnetic resonance imaging
1. Introduction
Selective attention constitutes the ability to discriminate relevant from irrelevant stimuli (i.e. noise) and to process information within our environment preferentially (Desimone, 1998; Desimone and Duncan, 1995; Kastner et al., 1998). The need for such a selective process arises from a limited attentional capacity of the human brain, which produces a competition for representation at a neural level when multiple stimuli are presented simultaneously (Desimone, 1998; Kastner et al., 1998). The biased competition model of selective attention (Desimone, 1998; Kastner et al., 1998) argues that the processing of stimuli within our environment is based on an interaction between two mechanisms, ‘bottom-up’ processes that act through stimulus-based operations, and ‘top-down’ processes that act through cognitive or executive actions (Desimone, 1998; Duncan, 1998). Thus, the extent to which neural processing is biased towards or away from any particular stimulus in the environment is dependent on the resolution of interactions between these bottom-up and top-down attention mechanisms.
While the cholinergic system is important in multiple cognitive domains, including memory and attention, the literature suggests that the modulation of these functions may occur via stimulus processing mechanisms (Bentley et al., 2011; Furey, 2011). At a behavioral level, cholinergic modulation produced stimulus specific behavioral effects during attention tasks, consistent with the modulation of the relative salience of competing visual stimuli (Bentley et al., 2003a; Furey et al., 2008a). At a neuronal level, the direct application of acetylcholine increased the selectivity of neural response to stimulus orientation in the cat visual cortex (Sillito, 1986; Sillito and Kemp, 1983) consistent with enhancing signal, while cholinergic input to the hippocampus is inhibitory, suggesting a modulation of S/N by reducing response to noise (Buzsaki, 1989). Thus, modulation of S/N may constitute the neural mechanism through which the cholinergic system may establish the relative strengths of stimulus representations. At a regional level, functional brain imaging studies (Bentley et al., 2003a; Freo et al., 2005; Furey et al., 2000c; Furey et al., 1997; Furey et al., 2008b; Ricciardi et al., 2009) demonstrated that enhanced cholinergic activity selectively increased neural responses to task relevant stimuli (i.e. signal) in visual processing cortical regions with reduced or no change in neural responses to task-irrelevant stimuli (i.e. noise), consistent with the hypothesized improvement of S/N ratio.
The enhanced representation of signal together with a reduced representation of noise inherently is reflected in the interaction between top-down and bottom-up mechanisms (Hasselmo et al., 1996; Sarter et al., 2001; Sarter et al., 2005; Sarter and Parikh, 2005), and inherently results in shifting a processing bias among the competing stimuli. Functional brain imaging studies have demonstrated that cholinergic enhancement selectively increases neural activity in medial visual processing areas while reducing neural responses in lateral visual extrastriate cortex (Bentley et al., 2003a, b; Furey et al., 2000c; Furey et al., 2008b; Ricciardi et al., 2009) and in task-relevant prefrontal and parietal cortical regions (Bentley et al., 2004; Bentley et al., 2003a, b; Freo et al., 2005; Furey et al., 2000c; Furey et al., 1997; Furey et al., 2008b; Ricciardi et al., 2009). As these response modulations were accompanied by improved task performance, we hypothesized that these changes in brain response are associated with an enhanced visual percept that rendered the task easier to perform (Furey, 2011; Furey et al., 2000c). More specifically, the increased processing in medial visual cortex may underlie a superior representation of the stimulus being processed, which renders the task easier to perform and reduces the need to recruit prefrontal cortex.
Temporal variability in BOLD signal is thought to reflect cognitive capacity and integration of information (Garrett et al., 2010, 2011), and changes in BOLD temporal variability potentially could reflect shifts in S/N that reflect improvement in stimulus processing (Mohr and Nagel, 2010; Samanez-Larkin et al., 2010). If the hypothesis that cholinergic enhancement results in more efficient processing is correct, cholinergically mediated decreases in task difficulty may manifest as changes in BOLD temporal variability within task associated brain areas.
Changes in neural activity following cholinergic enhancement extend throughout task-relevant brain regions (Bentley et al., 2004; Bentley et al., 2003a, b; Freo et al., 2005; Furey et al., 2000c; Furey et al., 1997; Furey et al., 2008b; Ricciardi et al., 2009) and thus suggest that the regional effects are likely not independent, but rather follow changes in communication among task-important brain regions. Cholinergic activity reportedly affects interactions among prefrontal, parietal and sensory brain regions (Golmayo et al., 2003; Nelson et al., 2005), and some specifically have suggested that cholinergic transmission influences anatomically segregated but functionally interconnected regional processes (Bentley et al., 2011; Sarter et al., 2001). Thus our understanding of the neuromodulatory effects of acetylcholine on efficiency of stimulus processing may benefit by investigating changes in interregional connectivity.
Cholinergic potentiation-associated changes in neural activity that are identified based on standard GLM approaches to data analysis are relatively subtle, and are described as simple increases or decreases in neural activity associated with task related brain regions. We anticipate that cholinergically mediated changes in neural response that reflect more efficient processing can be characterized more effectively by using analysis procedures that address both neural efficiency and regional interactions. Our previous findings have lead to the hypothesis that increased cholinergic function produces enhanced visual percepts of critical stimuli, and in this way results in a more efficient processing. In the current manuscript, we explore this hypothesis by assessing functional efficiency as evaluated by the strength of functional connectivity and by indices of BOLD temporal variability in task relevant brain regions following cholinergic modulation.
2. Methods
2.1 Subjects
Nine healthy individuals (mean age±SD=31±6 years; four females/five males) participated in a randomized, double-blind, placebo-controlled crossover study. All were right-handed, normotensive, had no abnormalities on clinical examination or on laboratory tests (including routine blood and urine tests, EKG, EEG, brain magnetic resonance imaging scan, and chest X-ray), and no history of any relevant medical, neurological, or psychiatric disorder. All were free of medication, including over-the-counter medications, for 4 weeks before the study (Pietrini et al., 1996). All participants gave their written informed consent after the purpose of the study and potential side effects of the drug had been fully explained. The study was approved by the National Institute on Aging Institutional Review Board (NIH protocol 00-M-0056).
2.2.Experimental design
Subjects participated in two testing fMRI sessions, during which they received in random order i.v. infusions of placebo and drug, and subsequently performed a selective attention task. The physostigmine infusion schedule was as follows: a 10-minutes loading dose of 1.93 mg/h was used to quickly achieve the desired plasma levels followed by a maintenance drip of 0.816 mg/h to maintain stable drug levels to completion of the study session, for a total dose of 1.0 mg/hr. Infusions continued for 30 minutes prior to fMRI scanning to allow drug effects to stabilize (Furey et al., 2000a; Furey et al., 2000c). Glycopyrrolate, a cholinergic muscarinic antagonist that does not cross the blood-brain barrier, also was administered (0.02 mg) to minimize peripheral side effects (Mirakhur et al., 1977; Oduro, 1975). Placebo infusions followed the same schedule while using saline. Blood pressure and heart rate were monitored throughout each session for each subject.
2.3 Task design
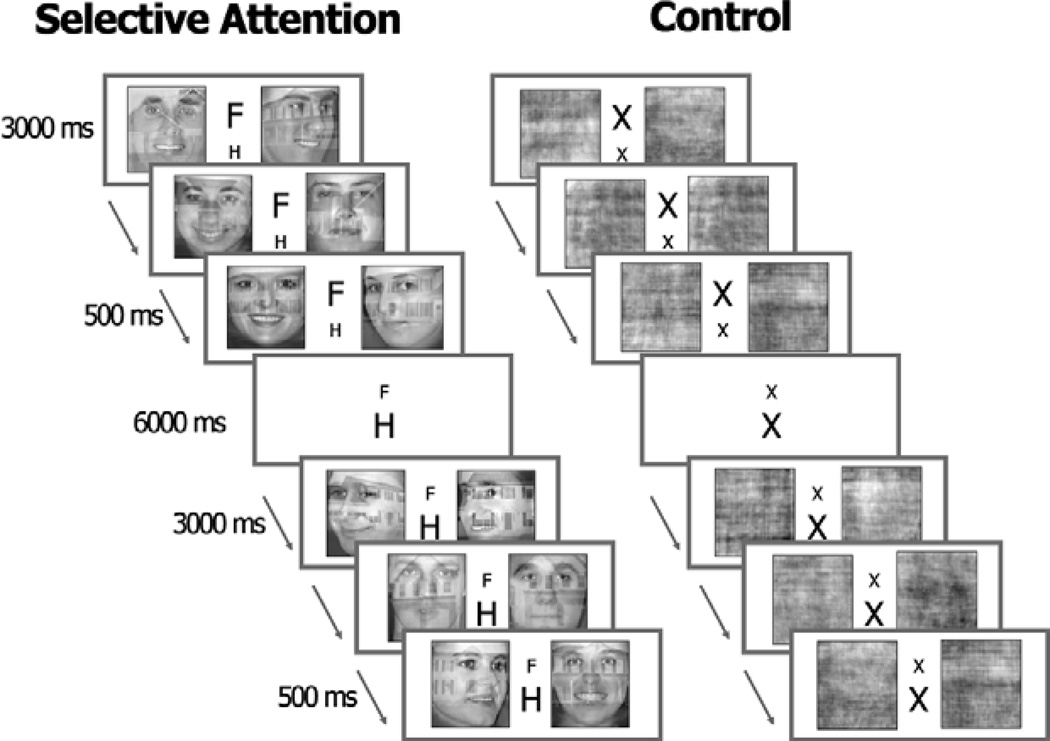
Subjects performed a matching task while viewing two stimuli shown simultaneously, side by side. In the selective attention task condition, two double-exposure pictures of faces and houses were presented. Subjects were instructed by a cue to attend to either the face or the house component of the stimuli, and to decide if the two exemplars from the attended category were of the same person/house. Pictures with different views of the same person or house were used so that subjects could not base their response on a simple pattern match. Subjects were cued to shift their attention from one stimulus component to the other every 4–7 trials (Figure 1). Stimuli were presented for 2.5 s and were followed by a 1.5 s inter-trial interval. In the control task, two stimuli were presented in the same spatial and temporal manner but here the double-exposure images were phase scrambled to create stimuli that had equivalent luminance, contrast, and spatial frequency spectra as the double-exposure pictures. The control condition required subjects to determine if the scrambled images were the same or different. To control for the presentation of the cue in the attention task, a cue comprised of one large and one small ‘x’ (i.e., X x) was presented between the two scrambled image stimuli. The cue changed every 4–7 trials by reversing the location of the large and small x (e.g., x X), with no relevance to task requirements. Performance reaction time and accuracy were recorded.
Figure 1.
The selective attention and control tasks.
2.4 Image Acquisition and Experimental Task
Gradient echo, echo-planar (GRE-EPI) images were acquired with a 3 Tesla scanner (General Electric, Milwaukee, WI). In each of 9 runs, 116 whole-brain volumes, comprised of 40 contiguous, 3.5-mm, sagittal slices (field of view = 24 cm, echo time = 40 ms, flip angle = 90, image in-plane resolution = 64×64 pixels), were obtained, resulting in a total of 1,044 volumes. High resolution T1-weighted spoiled gradient recall images were obtained for each subject to provide detailed brain anatomy. MRI data were obtained at the National Institutes of Health.
BOLD signal was measured as the selective attention task condition was performed over six runs (the initial set of stimuli – faces or houses - was randomly alternated across runs), and as the control task was performed over three runs. During the fMRI experiment, stimuli were projected onto a rear-projection screen, and subjects responded using a hand-held response button. Each run consisted of five blocks of stimuli for each type of target stimulus.
2.5 Behavioral data analysis
Mean reaction time (RT) was calculated across stimuli (faces and houses), and trials (trials 1–4) before group analyses. Mean RT as measured during placebo and during drug was compared using a t-test to determine overall drug effect. A more detailed report of the behavioral data only has been published elsewhere (Furey et al., 2008a).
2.6 Functional data analysis
We used the AFNI and SUMA package and related software plugins to analyze and view functional imaging data - http://afni.nimh.nih.gov/afni (Cox, 1996). The first four and the last volumes from each run were discarded (resulting in a total of 999 volumes). All volumes from across runs were concatenated and coregistered (3dvolreg), temporally aligned (3dTshift), and spatially smoothed (isotropic Gaussian filter, s = 4 mm). One subject was excluded from further analysis due to high level of head movement during scan sessions.
Data were normalized by calculating the mean intensity value for each voxel, and then dividing each time point within each voxel by its mean to estimate the percent signal change. Individual voxel timeseries were transformed into the Talairach and Tournoux Atlas (Talairach and Tournoux, 1988) coordinate system.
2.6.1. GLM analysis and ANOVA
Brain regions involved in the selective attention task and in the control condition were identified using multiple regression (via 3dDeconvolve). Regressors were created to model the attention shifts, and subsequent trials following each shift. Regressors for the attention conditions (attention to faces and to houses) and for the faces on scrambled and houses on scrambled control conditions were modeled separately; for all conditions only the first 4 trials following a shift were included as regressors of interest. The remaining trials (trials 5 through 7) were modeled as regressors of no interest as these trials did not occur with every shift. The cues for both the selective attention and the control task also were modeled. All regressors were convolved with a standard hemodynamic response model. The six movement parameters derived from the volume registration and the polynomial regressors to account for baseline shifts and linear/quadratic/cubic drifts in each scan series were included in the model as regressors of no interest.
The mean functional response associated with the selective attention conditions was obtained by averaging response measures across trials 1–4, and across the attention to faces and to houses conditions. This resulted in an average response to the selective attention task, without separating across trial or attention conditions.
A three-way mixed model ANOVA (task: selective attention vs. control condition; drug: physostigmine vs. placebo; subjects as random a variable) was conducting using unthresholded β-weights of each condition. BOLD estimates for task conditions were based on an inherent, resting baseline condition. T-contrasts were calculated to identify significant differences between task conditions (selective attention vs. control condition) and between infusion conditions (physostigmine vs. placebo). An ANOVA was p< 0.05 conducted to test for a task × drug interaction. Voxelwise significance of p< 0.05 was used, and a whole brain correction was applied using 3dClustSim in AFNI to control for multiple comparisons to result in a corrected p< 0.05, and requires a minimum cluster volume of 837 µL.
2.6.2. ROI-based functional connectivity analysis
For the functional connectivity analysis, two procedures were used to identify regions of interest (ROIs) based on peak task-dependent activity (selective attention and the control tasks were combined; voxel-wise p<0.05, uncorrected) as measured separately during the saline and physostigmine conditions (thus producing ‘placebo ROIs’ and ‘drug ROIs’, respectively). The following analyses were conducted separately on each of the two sets of ROIs.
Infusion conditions were considered separately for two reasons. First, in our previous functional brain studies of cholinergic modulation, we reported drug-induced, task-related increases and decreases in brain activity (e.g. Freo et al., 2005; Furey et al., 2000c; Furey et al., 1997; Furey et al., 2008b; Ricciardi et al., 2009). Selecting ROIs from the saline infusion condition only could, therefore, potentially exclude regions that selectively responded to drug in some manner. Second, the selection of representative ROIs is a critical step in functional connectivity analyses. When different methods of ROI selection have been compared, quantitatively different results have been reported (Marrelec and Fransson, 2011; Poldrack, 2007). In our study, we employed different ROI selection procedures to rule out the possibility that our findings might be due merely to a bias in ROI selection. The results obtained using ROIs as defined by the drug activity maps are provided as Supplementary material only.
Preprocessed data (as defined in 2.6) were low-pass filtered (3dFourier) at 0.15 Hz to remove high frequency physiological artifacts including cardiac and respiratory pulsatility, with no effects on the features of BOLD signal change related to the task (Birn et al., 2006). The influence of the six motion correction parameters derived from the volume registration and the polynomial regressors that account for baseline shifts and linear/quadratic/cubic drifts in each scan series were mathematically removed from the voxel timeseries (3dSynthesize) (Lund et al., 2006).
We identified ROI peaks by combining structural and functional information. For each of the infusion conditions separately, the task-related activity maps were superimposed onto the anatomical regions of a probabilistic cytoarchitectonic atlas (Eickhoff et al., 2005). Any potential ROI was required to have an arbitrary minimum volume of 200 µL to limit the number of anatomical regions included in the analysis (Marrelec and Fransson, 2011; Poldrack, 2007). A 4 mm radius sphere was placed onto the positive activity peak of each ROI (see Figure S2). The average BOLD signal was extracted for each ROI separately for the selective attention and control conditions, under placebo and physostigmine. Pearson’s r correlation coefficients were calculated among ROIs, to derive four correlation matrices for each subject. To test the strength of each functional connection at an individual level, each r coefficient within each correlation matrix was tested against 500 surrogates generated by randomizing the Fourier phases of the original signal (Theiler, 1992) using the TISEAN software package (Hegger et al., 1999; Schreiber and Schmitz, 2000). The surrogates had the same power spectrum and autocorrelation function as the original timeseries to test the null hypothesis that the correlations between ROIs depend on linearly filtered Gaussian noise (Hlinka et al., 2011). A one sided rank-order test was performed to obtain a p-value associated with the original Pearson’s r correlation coefficient. The correlation p-values for each ROI were combined across subjects using Fisher’s combined probability test (Fisher, 1925). Only those correlations retaining significance in the group analysis using a False Discovery Rate (FDR) correction (p < 0.05) were included in subsequent analyses (Benjamini and Yekutieli, 2001).
To conduct group analyses, correlation coefficients for each subject were transformed into Z scores using Fisher’s Z transformation, and paired two-sample two-tailed t-tests were performed between drug and task conditions (p<0.05). We then compared the number of the observed significant correlations at p<0.05 with the number of significant correlations to be expected using a Poisson distribution as the model. All functional connectivity coefficients were positive as the selected ROIs resulted from t-tests against baseline.
2.6.3. Voxel-wise functional connectivity analysis on representative regions
Given the effects on neural activity in prefrontal and extrastriate visual areas following cholinergic modulation (Bentley et al., 2003a, b; Furey et al., 2000c; Furey et al., 2008a; Ricciardi et al., 2009), two representative regions were selected from the placebo ROIs, one in ventro-temporal and another in prefrontal cortex, and used as ‘seeds’ to perform a whole brain functional connectivity analysis (3dfim+). In brief, average BOLD signal of each selected ROI was calculated for the selective attention condition under both placebo and physostigmine, and was correlated with each individual voxel timeseries across the brain. Individual correlation coefficient maps were transformed into Z score maps using Fisher’s Z transformation, and a paired two-sample two-tailed t-test was performed between the two infusion conditions to identify brain areas showing drug-associated differences in connectivity strength (p<0.05, whole brain corrected).
While the previous ROI-based functional connectivity analysis described drug-related changes in specific ROIs distributed throughout the brain, this voxelwise approach incorporates whole brain information regarding changes in functional connectivity. Furthermore, the behavior of two representative prefrontal and extrastriate visual areas, implicated in the cholinergic enhancement of attention processes, has been specifically characterized.
2.6.4. Temporal brain variability analysis
Temporal brain variability was measured using the mean squared successive difference (MSSD) as calculated on individual voxel timeseries using the preprocessed data that was used for the functional connectivity analysis (e.g. concatenated and coregistered, temporally aligned, spatially smoothed and normalized volumes for each task type, as described in Section 2.6). We applied an additional preprocessing step by regressing out white matter (WM) and cerebrospinal fluid (CSF) timeseries (Garrett et al., 2010; Samanez-Larkin et al., 2010). WM and CSF time courses were extracted from two small (1 voxel radius) ROIs located in the corpus callosum and lateral ventricle, respectively, and were identified on a common template, which was obtained by merging spatially normalized anatomical images of all subjects. Temporal BOLD signal variability measures were calculated on the whole brain volume, within-subject, for each task type, thus concatenating selective attention task runs and control condition runs, separately. Paired t contrasts were used to compare the MSSD indices between task (selective attention vs. control condition) and drug (physostigmine vs. placebo) conditions, and to determine the task × drug interaction. The correction for multiple comparisons was made using Monte-Carlo simulations run via 3dClustSim in AFNI with a voxelwise threshold of p < 0.05, whole brain corrected to p< 0.05.
All statistical results were anatomically localized on Talairach-transformed T1-weighted images, and visualized using normalized SUMA surface templates.
3. Results
3.1 Behavioral findings
Mean reaction time while performing the selective attention task was significantly faster during the physostigmine as compared to placebo condition (mean RT ± S.D.: physostigmine = 1755 ± 259 s, placebo: 1819 ± 257 s; p= 0.027). No drug-related RT changes occurred during the control task (mean RT ± S.D.: physostigmine = 1284 ± 275 s, placebo: 1267 ± 242 s; p> 0.3). For a detailed report of the behavioral results see Furey et al., 2008a.
3.2 Drug -related BOLD response during the selective attention and control tasks
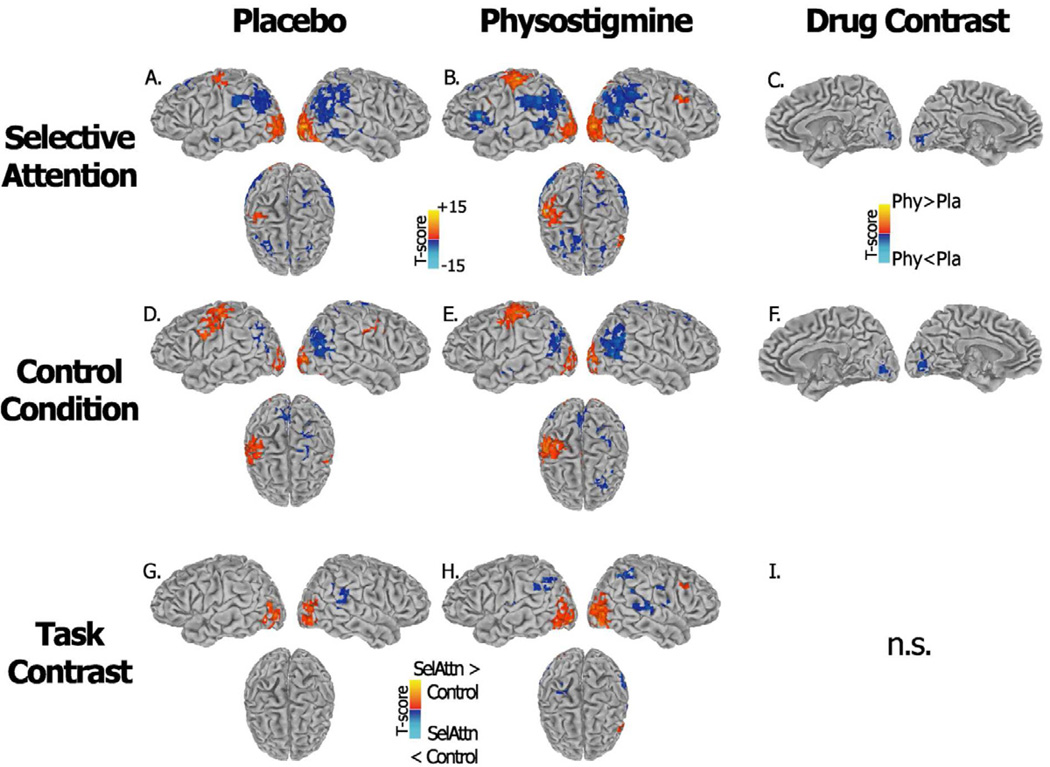
During placebo and physostigmine, significant (p<0.05 whole brain corrected) increases in BOLD during the selective attention task as compared to a rest baseline were observed bilaterally in occipital and temporal visual extrastriate regions - extending into fusiform, lingual and parahippocampal giri - superior parietal and intraparietal regions, premotor (BA6), middle prefrontal (BA9) and anterior insula/inferior prefrontal areas. Additional activations were found in left sensorimotor and inferior parietal cortex and bilateral thalamus. Selective attention task-related decreases were found in default-mode regions, including bilateral medial frontal areas, precuneus, supramarginal and inferior parietal cortex, posterior portion of superior temporal gyrus, and temporal poles (Figures 2A and 2B). Significant drug-associated differences were observed only in early visual cortical areas where activity decreased during cholinergic enhancement as compared to saline infusion (Figure 2C).
Figure 2.
Brain regions showing increased (red) and decreased (blue) BOLD response during performance of the selective attention task (A–B–C) and the control task (D–E–F) relative to a crosshair baseline condition are shown. Panels A–D and panels B–E show results as measured during placebo and physostigmine respectively. Panels G and H show the interaction between the selective attention and controls tasks under each infusion condition; panels C and F show the interaction between infusion conditions for each task condition separately. T-score maps are projected onto a standard Talairach template which includes lateral views and a top view, results are thresholded at a whole brain corrected significance level of p < 0.05.
During the control condition, a similar task-related pattern in occipital and temporal visual extrastriate regions, superior parietal and intraparietal areas was observed (Figures 2D and E). An additional bilateral recruitment of the sensorimotor region was seen. Similarly, cholinergic enhancement induced significant decreases only in early visual areas (Figure 2F).
During placebo, the selective attention task produced larger increases in BOLD than the sensorimotor control task in bilateral middle occipital and fusiform cortex, and a larger reduction in BOLD in right inferior parietal/supramarginal cortex (Figure 2G). During physostigmine, the selective attention task produced larger increases in BOLD response in bilateral ventral and dorsal extrastriate regions, intraparietal and superior parietal cortex, and in right middle frontal cortex, and the control task produced larger BOLD increases in the right sensorimotor cortex (Figure 2H). During cholinergic enhancement, larger decreases in BOLD were seen bilaterally in inferior parietal/supramarginal cortex and postcentral cortex during the selective attention task as compared to the control task.
No cluster survived whole brain correction in the task × drug interaction.
3.3 Functional connectivity
The superimposition of the task-related activity maps with the anatomical regions of a probabilistic cytoarchitectonic atlas (Eickhoff et al., 2005) resulted in 42 ROIs for the placebo condition and 42 ROIs for the physostigmine condition.
3.3.1 Placebo ROIs
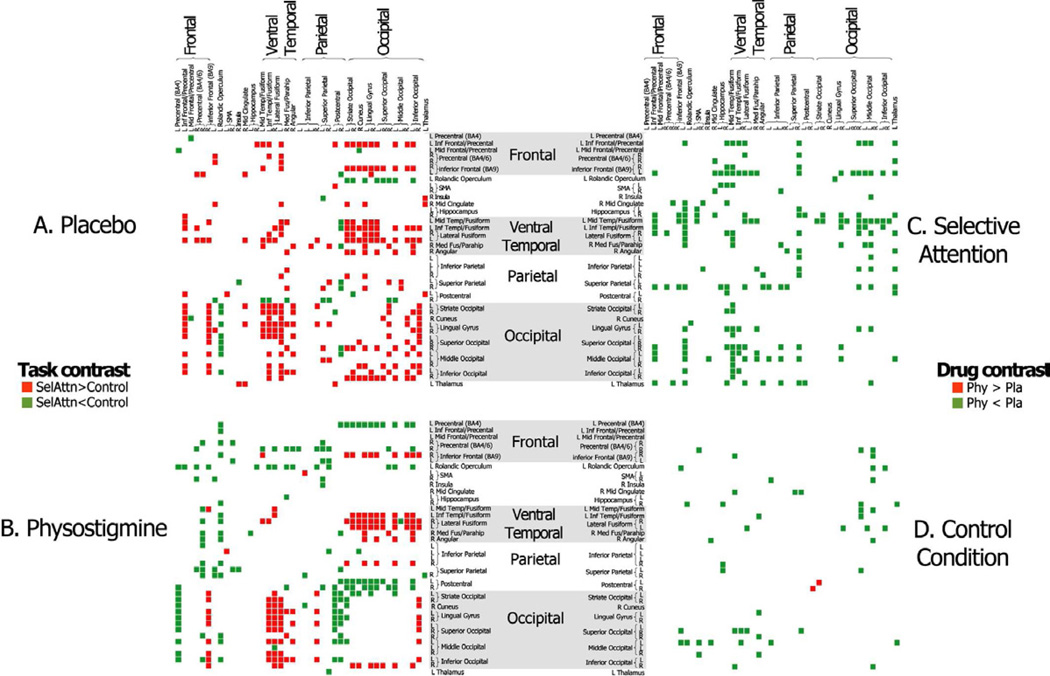
In a task comparison during placebo, the functional connectivity strength of striate and extrastriate visual cortical regions was significantly greater during the selective attention task than the control task within the ventral and dorsal temporal and occipital visual areas, including fusiform and lingual cortex, as well as bilateral inferior parietal and precentral cortical areas, and inferior and middle frontal regions (Figure 3A). During physostigmine, functional connectivity strength within occipital visual regions diminished during the selective attention task, but the same occipital areas maintained significantly higher correlations with ventrotemporal extrastriate visual areas, including fusiform and parahippocampal areas, and with inferior frontal areas (Figure 3B). Additionally, ventrotemporal regions showed no difference in connectivity strength with prefrontal and parietal regions between the two experimental conditions, as was seen during placebo. Indeed, during cholinergic enhancement, the functional connectivity strength of visual occipital ROIs with sensorimotor and precentral areas was higher during the control condition than during the selective attention task. In both task contrasts, the number of observed correlations corresponded to a p<0.00001 when compared to the expected number of correlations based on the Poisson distribution.
Figure 3.
Forty-two ROIs that were recruited to perform the selective attention and control tasks were identified under placebo. Connectivity matrices are shown to identify all correlations among the 42 ROIs that differ significantly between the selective attention and control task conditions under placebo (A) and physostigmine (B), correlations that are larger during selective attention are shown in red and correlation that are larger during the control task are shown in green. Connectivity matrices also are shown to identify significant differences in connectivity strength between placebo and physostigmine during the selective attention task (C) and the control task (D) with correlations that are larger during placebo shown in green and correlations that are larger during physostigmine shown in red. SelAttn= Selective Attention; Phy= Physostigmine condition; Pla= Placebo condition.
The drug comparison during the attention selective task confirmed significant diffuse reductions in functional connectivity during cholinergic enhancement as compared to placebo (p<0.00001 when compared to the expected number of correlations based on the Poisson distribution), primarily among ventrotemporal, occipital and prefrontal ROIs (Figure 3C). The drug comparison during the control condition showed no major change in functional connectivity, and the number of observed significant differences was lower than expected from the Poisson distribution (Figure 3D).
3.3.2 Drug ROIs
When estimating the strength of the functional connectivity in task-related brain regions identified during cholinergic enhancement, the results of task and drug comparisons overlapped considerably with results provided above (3.3.1). Nonetheless, the results are provided in supplemental material for completeness (Figure S2).
3.3.3 Voxel-vise analysis
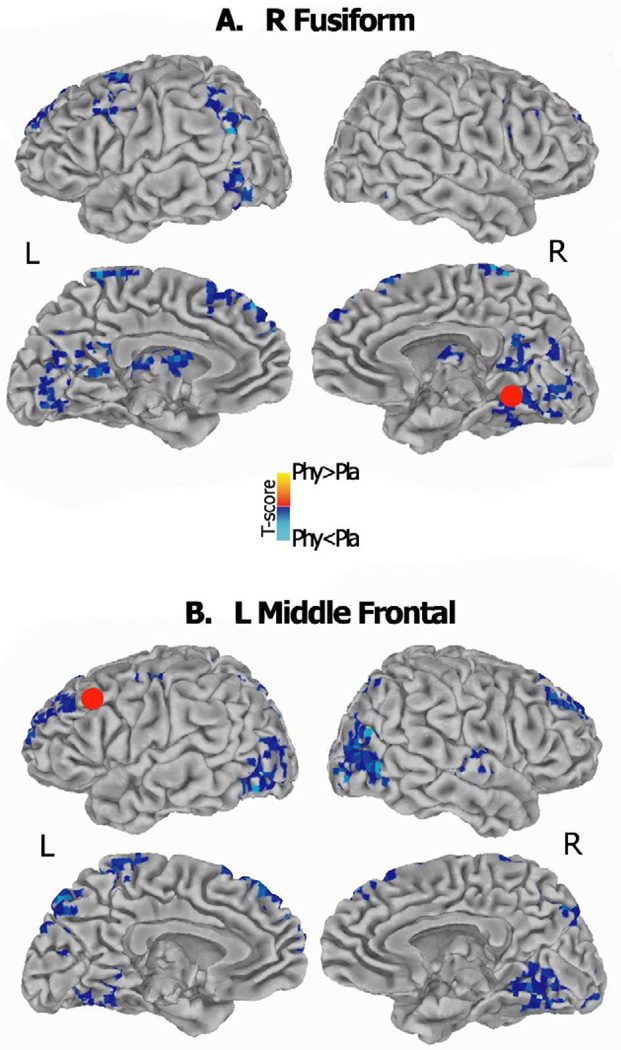
In Figure 4, functional connectivity maps for the two representative ROIs, located in the left middle frontal and in the right fusiform cortex, are shown to supplement the previous results (reported in section 3.3.1). Consistently, during selective attention the functional connectivity strength was significantly reduced during physostigmine (relative to placebo) between the right fusiform seed and the fronto-parietal regions (p<0.05, whole brain correction). Similarly, the left middle frontal seed showed reduced functional connectivity with bilateral prefrontal regions and with dorsal and ventral extrastriate clusters following cholinergic enhancement.
Figure 4.
Functional connectivity maps of two representative ROIs, located in right fusiform cortex (A) and the left middle frontal cortex (B) are shown. Brain areas showing larger correlations with the seed ROI (identified by the red spheres) during placebo (blue) and physostigmine (red) are presented. T-score maps are projected onto a standard Talairach template which includes lateral and medial views of both hemispheres; results are thresholded at a whole brain corrected significance level of p < 0.05.
3.4 Temporal variability
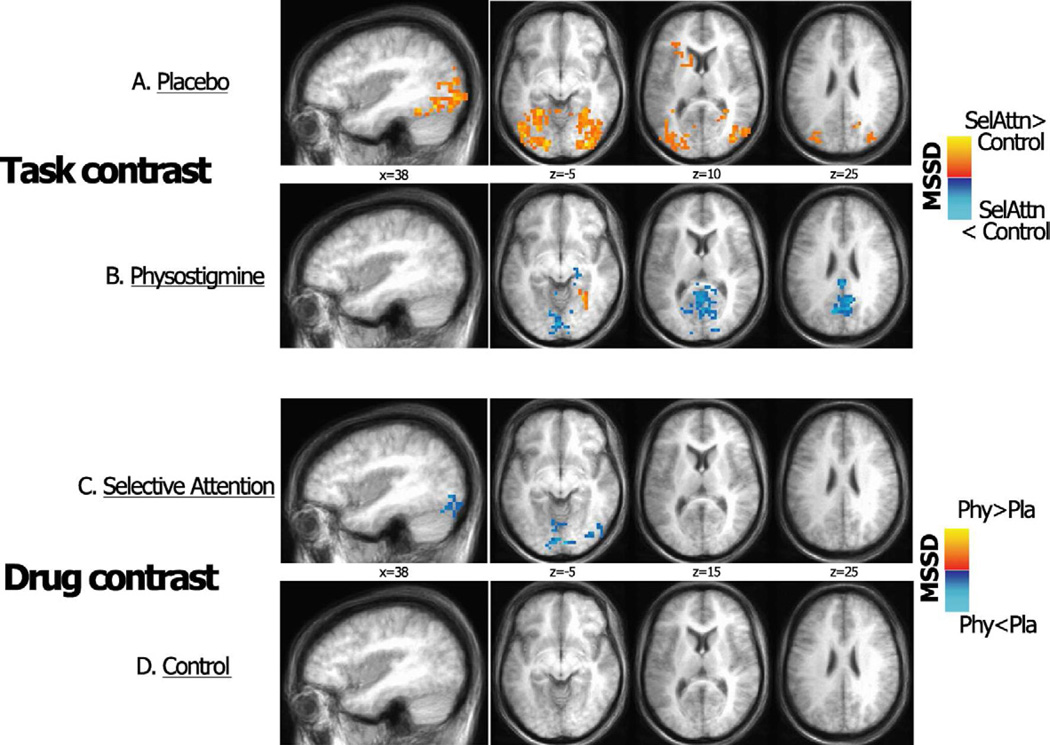
The task contrast during placebo showed a higher BOLD temporal variability during the selective attention task as compared to the control condition in bilateral ventro-temporal, and occipito-temporal and dorsal occipital extrastriate regions (p< 0.05, whole brain corrected) (Figure 5A). An additional cluster of higher BOLD temporal variability was located in the left inferior frontal cortex (Figure 5A). During cholinergic enhancement, higher temporal variability in ventral extrastriate regions during the selective attention task was limited to a right medial temporal/fusiform cluster (Figure 5B) (p< 0.05, whole brain corrected), consistent with drug-associated reductions in functional connectivity strength reported above (sections 3.3.1 and 3.3.2). Additionally, during physostigmine administration, significantly higher MSSD during the control condition was found bilaterally in the posterior cingulate/precuneus and calcarine cortex (Figure 5A).
Figure 5.
Significant differences in BOLD signal variability between the selective attention and control tasks are shown for the placebo (A) and physostigmine (B) conditions; regions with higher BOLD variability are indicated in red-yellow for the selective attention task and blue for the control task. Differences in BOLD signal variability between the placebo and physostigmine conditions also are shown for the selective attention task (C) and the control task (D). T-score maps are projected onto a standard talairach template including a lateral view and three representative axial slices; results are thersholded at a whole brain corrected significance level of p < 0.05. SelAttn= Selective Attention; Phy= Physostigmine condition; Pla= Placebo condition.
Drug contrasts showed a significant drug-associated decrease in MSSD in bilateral early visual regions and lingual cortex, and in right ventral extrastriate cortex during physostigmine administration (Figure 5C). No significant drug-related change was found in the control condition (Figure 5D).
4. Discussion
The results of the current study support the hypothesis that enhanced cholinergic function may lead to a more efficient stimulus processing, which renders tasks less demanding. Exclusively during the selective attention task, and not during the control condition, cholinergic modulation induced by physostigmine reduced both the strength of connections and the BOLD temporal variability in task-related areas, particularly implicating visual processing extrastriate regions. Cholinergic enhancement also improved performance exclusively during the selective attention task, again with no change during the control task.
Specifically, the results of the functional connectivity analysis showed that cholinergic enhancement reduces the strength of connections between the visual processing regions and task-related occipital, parietal and prefrontal regions. The absence of effects during the control task indicates that the changes in connectivity are related specifically to conditions that retain multiple stimuli and thus compete for neural processing resources. Of note, the drug-associated reductions in connectivity strength made the pattern of connectivity under cholinergic enhancement to become more sparse, and thus more specific, among ventral temporal and occipital loci during the selective attention task, and among frontal, parietal and visual occipital regions during the control task (please refer to Figures 3A and 3B). Importantly, the connections between prefrontal and parietal regions with visual processing areas were reduced during the selective attention task as compared to the placebo condition. Moreover, the results of the seed-based correlation analysis are consistent with the ROI-based connectivity analysis, lending further support to these findings.
These specific task-related reductions in the strength of connectivity between stimulus processing visual extrastriate areas and prefrontal and parietal regions may be related directly to previous work showing that cholinergic enhancement improves behavioral performance on visual tasks while reducing neural responses in frontoparietal regions (Bentley et al., 2004; Bentley et al., 2003a, b; Freo et al., 2005; Furey et al., 2000c; Furey et al., 1997; Furey et al., 2008b; Ricciardi et al., 2009). We hypothesized that cholinergic potentiation may enhance the efficiency of perceptual processing, and thereby produce an enhanced visual percept of task-relevant stimuli. The improved percept accordingly decreases the effort required to perform the task, and indirectly diminishes the need to allocate attentional resources (Furey, 2011). Consistent with this hypothesis, studies that investigated functional correlates of learning and expertise observed that increased automaticity and improved performance are reflected in both reduced recruitment of attention regions and a downscaling of connectivity strength between these attention regions and task-related sensory areas (Rypma et al., 2006; Toni et al., 2002). Furthermore, studies evaluating the influence of cognitive load on connectivity suggest that, as task difficulty decreases, the strength of connections among task-relevant regions also decreases (Fu et al., 2006). Consequently, the reduced strength in functional connectivity between visual processing regions and frontal and parietal areas, together with the observed behavioral improvement, may reflect both a more efficient processing of task-significant stimuli and a reduction in the effort required to perform the task (Neubauer and Fink, 2009; Rypma and Prabhakaran, 2009).
We also observed extensive cholinergically mediated change in BOLD temporal variability throughout ventral temporal, occipital and parietal cortices. Under placebo, a higher BOLD variability was observed broadly in the lateral extents of visual processing areas during the selective attention as compared to the control task condition. Cholinergic enhancement resulted in a widespread decrease in BOLD variability specifically during the selective attention task, so that BOLD variability differences were virtually absent in lateral visual processing extrastriate areas, and medial occipital areas showed significantly less BOLD variability during the selective attention task versus the control condition. Thus, the influence of cholinergic enhancement on BOLD temporal variability is limited to the selective attention condition only, and does not exert any specific influence during the control condition.
Indices of BOLD signal temporal variability are associated with information processing and integration mechanisms, which relate directly to neural efficiency (Garrett et al., 2010, 2011, 2012b; Mohr and Nagel, 2010; Samanez-Larkin et al., 2010; Leo et al., in press). While the meaning of these physiological fluctuations in BOLD signal remains empirical, a decrease in variability also has been related to reductions in connectivity (Garrett et al., 2012b; Misic et al., 2011; Vakorin et al., 2011). In our study, the lateral visual processing areas showed a higher variability during the attention task (vs. control) under placebo, but showed no variability difference under cholinergic enhancement. Importantly, these lateral visual processing regions overlap with the ventro-temporal extrastriate ROIs that showed reduced functional connectivity with prefrontal and parietal regions. Therefore, the reductions in MSSD indices are observed in the same regions showing changes in functional connectivity strength between visual processing regions and prefrontal and parietal areas.
Recently, decreases in signal variability have been related specifically to both a reduction of long-range functional connections and thus to a reduction in the extent of distributed networks (Vakorin et al., 2011). These decreases in variability were interpreted as a reflection of more homogeneous inputs resulting in more local processing of information (Misic et al., 2011). Two main consequences may be derived from this. First, the decreases in BOLD signal variability during cholinergic potentiation may reflect a less distributed and less connected network subserving selective attention processes. This less distributed network also is reflected in the more sparse pattern of connectivity under physostigmine, observed among ventral temporal and occipital loci during selective attention. Second, as the reductions in signal variability are mainly located in ventral extrastriate areas, these may also reflect both a reduction in noise and thus an enhanced signal (Garrett et al., 2010, 2011, 2012b; Mohr and Nagel, 2010; Samanez-Larkin et al., 2010). As physostigmine enhances the cholinergically-mediated effects on S/N processing (Furey, 2011; Furey et al., 2000c), the presence of less variable dynamics should lead to an increased homogeneity of sensory representations in visual extrastriate regions (Garrett et al., 2012b; Misic et al., 2011; Vakorin et al., 2011).
Finally, as a greater brain signal variability often reflects improved and more stable cognitive performance (Garrett et al., 2010, 2011, 2012b), the prediction would be that improved behavioral performance following cholinergic enhancement would be accompanied by an increased BOLD signal variability. Although, recent fMRI data during a face matching task in healthy young participants (Garrett et al., 2012a) confirmed a positive linear relation between signal variability and task performance, this relation was evident only at a certain degree of task difficulty. No differences as compared to the control condition were found in those conditions that did not reach that given difficulty threshold, and resulted simpler to perform. In our study, during physostigmine infusion signal variability in visual processing regions did not differ between the selective attention and the control task, consistent with the hypothesis that cholinergic enhancement may lead to a more efficient stimulus processing that renders the selective attention task less demanding. Indeed, differences between the task and control conditions in visual processing regions were found selectively during placebo.
In the current study, the neuromodulatory effects of cholinergic enhancement were associated with pervasive changes in interregional connectivity and BOLD variability, rather than with small localized regional changes in neural responses. The standard GLM analysis presented here showed higher levels of activity in visual extrastriate and frontoparietal regions during the selective attention task as compared to the control condition, consistent with previous studies (Haxby et al., 1994; Kastner et al., 1998). Drug effects were observed exclusively in early visual areas, in which neural activity decreased aspecifically during cholinergic enhancement.
Previously, we and others have reported both increases and decreases in neural activity following cholinergic enhancement in visual processing areas, as reflected also by both changes in the extent of cortex that responds to task and in the magnitude of the neural response (Bentley et al., 2004; Freo et al., 2005; Furey et al., 2000b; Furey et al., 2000c; Furey et al., 1997; Furey et al., 2008b; Ricciardi et al., 2009). Decreases in response were non-selective for task condition, and thus reflected a more aspecific effect of cholinergic enhancement. Increases in BOLD response in visual cortex were stimulus dependent, and thus were not merely an aspecific effect of the drug. As the current study was not designed to evaluate stimulus specific (only task specific) drug effects, stimulus specific increases in BOLD response would not have been detected. Thus, the absence of BOLD increases in visual cortex likely is a reflection of the specific analysis conducted here, which was designed to suit the purposes of the current study.
In summary, several findings in the literature indicate that the effects of cholinergic potentiation on task performance may be due to enhanced processing of task relevant stimuli, as reflected by specific changes in the magnitude of the BOLD response. In the current paper, we used functional connectivity and estimates of BOLD signal variability to evaluate neural efficiency, and the results suggest that increasing cholinergic function leads to more efficient neural responses. These effects are observed in task-relevant stimulus processing regions specifically during the selective attention task, with no such changes evident during the control task.
Supplementary Material
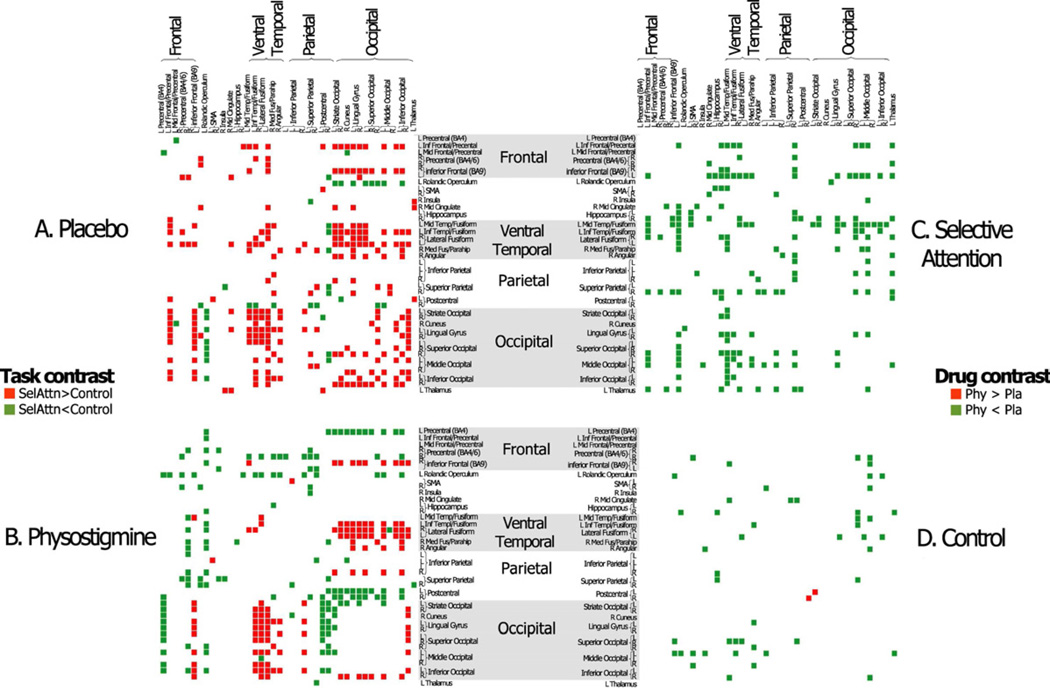
Forty-two ROIs that were recruited to perform the selective attention and control tasks were identified under physostigmine. Connectivity matrices are shown to identify all correlations among the 42 ROIs that differ significantly between the selective attention and control task conditions under placebo (A) and.physostigmine (B), correlations that are larger during selective attention are shown in red and correlation that are larger during the control task are shown in green. Connectivity matrices also are shown to identify significant differences in connectivity strength between placebo and physostigmine during the selective attention task (C) and the control task (D) with correlations that are larger during placebo shown in green and correlations that are larger during physostigmine shown in red.
‘Placebo ROIs’ and ‘drug ROIs’, as defined in the Methods section, are projected onto a standard Talairach inflated template which includes lateral and medial views of both hemispheres. The ROIs of the two drug conditions have been defined by combining structural and functional information (as described in the Methods) and are shown here in different colors. Four mm radius spheres were placed onto the positive activity peaks of each ROI, and are shown here with a yellow circle.
Highlights.
-
–
Physostigmine improves performance and alters brain response to cognitive tasks
-
–
We evaluated cholinergic effects on neural efficiency during selective attention
-
–
Physostigmine reduced connectivity and BOLD variability of visual areas during task
-
–
Cholinergic enhancement improves task-specific neural efficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emiliano Ricciardi, Email: emiliano.ricciardi@bioclinica.unipi.it.
Giacomo Handjaras, Email: giacomo.handjaras@gmail.com.
Giulio Bernardi, Email: giulio.bernardi@for.unipi.it.
Pietro Pietrini, Email: pietro.pietrini@med.unipi.it.
Maura L. Furey, Email: mfurey@mail.nih.gov.
References
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple hypothesis testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94:360–388. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003a;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J Neurophysiol. 2003b;90:1171–1181. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B Biol Sci. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J. Converging levels of analysis in the cognitive neuroscience of visual attention. Philos Trans R Soc Lond B Biol Sci. 1998;353:1307–1317. doi: 10.1098/rstb.1998.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Edinburgh: Oliver and Boyd; 1925. [Google Scholar]
- Freo U, Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Pharmacological modulation of prefrontal cortical activity during a working memory task in young and older humans: a PET study with physostigmine. Am J Psychiatry. 2005;162:2061–2070. doi: 10.1176/appi.ajp.162.11.2061. [DOI] [PubMed] [Google Scholar]
- Fu CH, McIntosh AR, Kim J, Chau W, Bullmore ET, Williams SC, Honey GD, McGuire PK. Modulation of effective connectivity by cognitive demand in phonological verbal fluency. Neuroimage. 2006;30:266–271. doi: 10.1016/j.neuroimage.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Furey ML. The prominent role of stimulus processing: cholinergic function and dysfunction in cognition. Curr Opin Neurol. 2011;24:364–370. doi: 10.1097/WCO.0b013e328348bda5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Alexander GE, Mentis MJ, Szczepanik J, Shetty U, Greig NH, Holloway HW, Schapiro MB, Freo U. Time course of pharmacodynamic and pharmacokinetic effects of physostigmine assessed by functional brain imaging in humans. Pharmacol Biochem Behav. 2000a;66:475–481. doi: 10.1016/s0091-3057(00)00186-6. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Alexander GE, Schapiro MB, Horwitz B. Cholinergic enhancement improves performance on working memory by modulating the functional activity in distinct brain regions: a positron emission tomography regional cerebral blood flow study in healthy humans. Brain Res Bull. 2000b;51:213–218. doi: 10.1016/s0361-9230(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000c;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Alexander GE, Lee HC, VanMeter J, Grady CL, Shetty U, Rapoport SI, Schapiro MB, Freo U. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci U S A. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective Effects of Cholinergic Modulation on Task Performance during Selective Attention. Neuropsychopharmacology. 2008a;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Ricciardi E, Schapiro MB, Rapoport SI, Pietrini P. Cholinergic Enhancement Eliminates Modulation of Neural Activity by Task Difficulty in the Prefrontal Cortex during Working Memory. J Cogn Neurosci. 2008b;20:1342–1353. doi: 10.1162/jocn.2008.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D, McIntosh AR, Grady CL. Moment-to-moment brain variability is experimentally "tunable”. 18th Annual Meeting of the Organization for Human Brain Mapping; Beijing, China. 2012a. p. 572WTh. [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. 2011;31:4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The Modulation of BOLD Variability between Cognitive States Varies by Age and Processing Speed. Cereb Cortex. 2012b doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Wallenstein GV. Encoding and retrieval of episodic memories: role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegger R, Kantz H, Schreiber T. Practical implementation of nonlinear time series methods: The TISEAN package. CHAOS. 1999;9:413–435. doi: 10.1063/1.166424. [DOI] [PubMed] [Google Scholar]
- Hlinka J, Palus M, Vejmelka M, Mantini D, Corbetta M. Functional connectivity in resting-state fMRI: is linear correlation sufficient? Neuroimage. 2011;54:2218–2225. doi: 10.1016/j.neuroimage.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Leo A, Bernardi G, Handjaras G, Bonino D, Ricciardi E, Pietrini P. Increased BOLD Variability in the Parietal Cortex and Enhanced Parieto-Occipital Connectivity during Tactile Perception in Congenitally Blind Individuals. Neural Plasticity. doi: 10.1155/2012/720278. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TE, Madsen KH, Sidaros K, Luo WL, Nichols TE. Non-white noise in fMRI: does modelling have an impact? Neuroimage. 2006;29:54–66. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Fransson P. Assessing the influence of different ROI selection strategies on functional connectivity analyses of fMRI data acquired during steady-state conditions. PLoS One. 2011;6:e14788. doi: 10.1371/journal.pone.0014788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakhur RK, Dundee JW, Clarke RS. Glycopyrrolate-neostigmine mixture for antagonism of neuromuscular block: comparison with atropine-neostigmine mixture. Br J Anaesth. 1977;49:825–829. doi: 10.1093/bja/49.8.825. [DOI] [PubMed] [Google Scholar]
- Misic B, Vakorin VA, Paus T, McIntosh AR. Functional embedding predicts the variability of neural activity. Front Syst Neurosci. 2011;5:90. doi: 10.3389/fnsys.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PN, Nagel IE. Variability in brain activity as an individual difference measure in neuroscience? J Neurosci. 2010;30:7755–7757. doi: 10.1523/JNEUROSCI.1560-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Oduro KA. Glycopyrrolate methobromide: 2. comparison with atropine sulphate in anaesthesia. Can Anaesth Soc J. 1975;22:466–473. doi: 10.1007/BF03004861. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Furey ML, Graff-Radford N, Freo U, Alexander GE, Grady CL, Dani A, Mentis MJ, Schapiro MB. Preferential metabolic involvement of visual cortical areas in a subtype of Alzheimer's disease: clinical implications. Am J Psychiatry. 1996;153:1261–1268. doi: 10.1176/ajp.153.10.1261. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Cholinergic modulation of visual working memory during aging: a parametric PET study. Brain Res Bull. 2009;79:322–332. doi: 10.1016/j.brainresbull.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D'Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Kuhnen CM, Yoo DJ, Knutson B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. J Neurosci. 2010;30:1426–1434. doi: 10.1523/JNEUROSCI.4902-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Schmitz A. Surrogate time series. Physica D. 2000;142:346–382. [Google Scholar]
- Sillito AM. Visual cortex. Cholinergic input and plasticity? Nature. 1986;320:109–110. doi: 10.1038/320109a0. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983;289:143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publisher, Inc.; 1988. [Google Scholar]
- Theiler J. Testing for nonlinearity in time series: the method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
- Toni I, Rowe J, Stephan KE, Passingham RE. Changes of cortico-striatal effective connectivity during visuomotor learning. Cereb Cortex. 2002;12:1040–1047. doi: 10.1093/cercor/12.10.1040. [DOI] [PubMed] [Google Scholar]
- Vakorin VA, Lippe S, McIntosh AR. Variability of brain signals processed locally transforms into higher connectivity with brain development. J Neurosci. 2011;31:6405–6413. doi: 10.1523/JNEUROSCI.3153-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forty-two ROIs that were recruited to perform the selective attention and control tasks were identified under physostigmine. Connectivity matrices are shown to identify all correlations among the 42 ROIs that differ significantly between the selective attention and control task conditions under placebo (A) and.physostigmine (B), correlations that are larger during selective attention are shown in red and correlation that are larger during the control task are shown in green. Connectivity matrices also are shown to identify significant differences in connectivity strength between placebo and physostigmine during the selective attention task (C) and the control task (D) with correlations that are larger during placebo shown in green and correlations that are larger during physostigmine shown in red.
‘Placebo ROIs’ and ‘drug ROIs’, as defined in the Methods section, are projected onto a standard Talairach inflated template which includes lateral and medial views of both hemispheres. The ROIs of the two drug conditions have been defined by combining structural and functional information (as described in the Methods) and are shown here in different colors. Four mm radius spheres were placed onto the positive activity peaks of each ROI, and are shown here with a yellow circle.