Abstract
The occipital-temporal N1 component of the event-related potential (ERP) has previously been shown to index a stimulus discrimination process. However, the N1 has not consistently been shown to be sensitive to the difficulty of stimulus discrimination. Here we manipulated the difficulty of stimulus discrimination by modulating the similarity between serially presented targets and non-targets. The same target stimulus was employed in both easy and difficult discrimination contexts, and these physically identical target stimuli elicited a larger N1 and smaller P3b in the difficult task context. Moreover, when targets were incorrectly categorized, N1 amplitude was diminished and a P3b was not elicited. These findings provide evidence that the N1 component reflects a sensory discrimination process that is modulated by executive control, and that this component can index discrimination errors when stimulus discrimination is difficult.
Introduction
The bottom-up mechanisms that differentiate a relevant object from task-irrelevant distractors (Itti & Koch, 2001) have been a major focus of the stimulus discrimination literature. Additionally, many theoretical models of visual stimulus differentiation and attentional allocation implicate top-down, executive cognitive processes (Desimone & Duncan, 1995; Hopfinger, Buonocore, & Mangun, 2000; Miller & Cohen, 2001). However, it remains unclear how these top-down influences manifest themselves in early, sensory processing. Understanding how sensory and executive control processes interact is an important step in identifying the mechanisms underlying disambiguation of visual scenes.
Top-down Executive Control
Theoretical models of executive control in the human brain suggest that difficult tasks require top-down control, which influences accurate sensory processing (Norman & Shallice, 1986; Posner & DiGirolamo, 1998). The mechanism of top-down control has been previously shown to enhance activation in specific cortical regions that process task-relevant stimulus features (Kehrer et al., 2009; Pessoa, Kastner, & Ungerleider, 2003). Lavie’s theory of perceptual load suggests that the level of attentional modulation employed depends strongly on task demands (De Fockert, Rees, Frith, & Lavie, 2001; Lavie, 1995) with more difficult tasks requiring greater top-down control. For example, enhanced attention-related neural responses are seen in higher ? visual cortical areas in line discrimination tasks when the difference between orientations is small (Boudreau, Williford, & Maunsell, 2006).
Neuroimaging studies have shown increased activation in the visual cortex in response to cues of an impending difficult task (Serences, Yantis, Culberson, & Awh, 2004). Increases in visual cortical activation have also been shown to predict performance in a difficult detection task (Ress, Backus, & Heeger, 2000). These cortical activations are independent of visual stimulus presentation, implicating top-down executive control as opposed to bottom-up sensory processing.
Visual discrimination is also influenced by executive control (Beck & Kastner, 2009). The more physically similar a target and non-target, the more difficult the discrimination between them. Because physical similarity necessarily reduces differences in bottom-up sensory processing, the intercession of top-down executive control is required to allocate attentional resources to facilitate stimulus discrimination. Given this proposed role of executive control, discriminatory perceptual processes can be expected to be modulated by task difficulty. However, the event-related potential (ERP) component most strongly linked with stimulus discrimination, the occipital-temporal N1, has not consistently been shown to be sensitive to the difficulty of task discrimination.
Occipital-temporal N1
The occipital-temporal N1 is a cortically-generated negative potential following visual stimulus presentation. It has a peak latency of 150–200 ms post-stimulus presentation, and is maximal over the lateral posterior scalp. The neural generator of the N1 component has been localized to extra striate visual cortex (Gomez-Gonzalez, Clark, Fan, Luck, & Hillyard, 1994), and specifically to inferior occipital-temporal cortex (Hopf, Vogel, Woodman, Heinze, & Luck, 2002). The occipital-temporal N1 has been shown to index a voluntary discrimination process (Ritter, Simson, & Vaughan, 1983; Ritter, Simson, Vaughan, & Macht 1982; Senkowski & Hermann, 2002; Vogel & Luck, 2000) at attended stimulus locations (Fort, Besle, Giard, & Pemier, 2005; Luck, 1995; Mangun, 1995; Mangun & Hillyard, 1991; Parasuraman, 1980). N1 amplitudes are increased at validly cued spatial locations (Luck, 1995; Luck et al., 1994) suggesting an enhancement of N1-indexed processes at attended locations as opposed to suppression at non-attended locations. This discrimination process has a limited capacity (Handy & Mangun, 2000; Hillyard, Vogel, & Luck, 1998), and modulations of N1 in discrimination tasks are seen across multiple stimulus properties (Vogel & Luck, 2000).
While the influence of discriminative processes on N1 is well established, the influence of task difficulty on occipital-temporal N1 amplitude has been inconsistent. Early work failed to show a modulation of N1 with manipulations of discrimination difficulty (Senkowski & Hermann, 2002; Vogel & Luck, 2000). Conversely, increased perceptual load, either during stimulus presentation with distractor background stimuli (Fu et al., 2008) or with a post-stimulus mask (Handy & Mangun, 2000), has been shown to increase occipital-temporal N1 amplitude in spatially-cued attention tasks. However, the use of spatial cueing in these studies makes it difficult to separate the effects of spatial attention from those of feature-based attention.
Present Study
The present study was designed to modulate the difficulty of stimulus discrimination and observe the changes in the related sensory processes under the influence of top-down executive control. If N1 is an index of stimulus discrimination processing, it is predicted to be sensitive to discrimination task difficulty. Task difficulty was modulated via manipulation of the similarity between serially presented targets and non-targets.
Manipulating distractor characteristics with a serial presentation of targets and non-targets achieves two goals. First, serial presentation of an individual target or non-target allows for physically identical targets to be used for both easy and difficult discriminations, thus allowing for an assessment of the influence of discrimination difficulty independent of physical stimulus differences. Second, this manipulation is expected to increase executive control processes associated with attentional modulation across multiple trials as opposed to within a single trial, as is the case when targets and non-targets are presented simultaneously.
Thus, serial presentation allows for an examination of the effects of contextual differences in task difficulty on early neural processing of the same target. For the purposes of the current study, we defined the task context based on the properties of the non-target stimuli preceding or following a target stimulus. When the target was preceded or followed by dissimilar non-target stimuli, the target was viewed in a context where discrimination is easy. Conversely, when the target was preceded or followed by similar non-target stimuli, the target was viewed in a context where discrimination is difficult.
The current experimental design isolated task context (easy or difficult) as the sole parameter influencing top-down executive control. The influence of bottom-up processing was controlled across task contexts with the use of physically identical target stimuli in both easy and difficulty task contexts. In addition, target stimuli were presented at the same spatial locations as non-target stimuli, removing any requirement of shifts in spatial attention during task performance. Modulations of N1 and the subsequent ability to predict accurate task performance are predicted to be related solely to an index of discriminatory processing under the influence of executive control.
An additional ERP component, the parietal P3b, can also be used to characterize the role of top-down executive control on discriminatory processes. P3b amplitude has been shown to index the efficacy of stimulus categorization in oddball tasks (Johnson, 1986; Kok, 2001). The magnitude of the P3b has been shown to index the certainty with which the target and non-target stimuli are appropriately categorized. Both greater uncertainly (Parasuraman & Beatty, 1980) and increases in discrimination task difficulty (Comerchero & Polich, 1999) are associated with reduced P3b amplitude. Changes in P3b amplitude should be correlated with changes in N1 amplitude. Specifically, a reduction in P3b amplitude, when target discrimination is difficult, is expected to follow an increase in N1 amplitude. Thus, the well-characterized antecedent conditions of P3b should be useful in supporting the functional interpretation of N1.
Finally, we hypothesized that the index of discriminatory processes provided by N1 should also be predictive of errors in the identification of rare targets, especially in the difficult task context. In a discrimination task, a failure of the discriminatory process should be reflected in sequence by decreased occipital-temporal N1 amplitude, a reduction in P3b, and an incorrect behavioral response.
Methods
Participants
Fourteen healthy participants (11 female) from the undergraduate student population of George Mason University took part in the study in exchange for course credit. Participants were between 18 and 39 years of age (mean age 23.9 years), right-handed, had normal or corrected-to-normal vision and no reported history of neurological illness. Informed consent was obtained from all participants prior to the experiment.
Stimuli
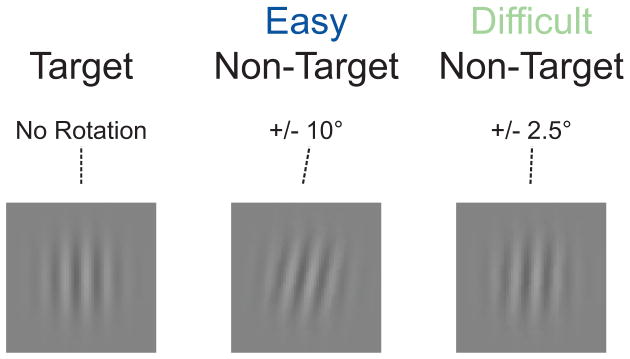
Figure 1 contains representative target and non-target stimuli from both the easy and difficult task contexts. Visual stimuli consisted of Gabor patches (6 cycles per image, 2°×2° visual angle subtended) presented 1.5° above a fixation dot (0.3°×0.3°) at the center of the screen on a gray background. Task difficulty was manipulated via the degree of rotation of the non-target stimuli as compared to the vertical target stimuli. Easy task context non-targets were rotated 10° to the left or right of vertical, difficult task context non-targets were rotated 2.5° to the left or right of vertical. The degree of rotation for the difficult task context non-targets was determined via separate pilot testing. Identical target stimuli, with vertically oriented Gabor patches, were used in both task contexts.
Figure 1.
Procedure
The experiment consisted of an oddball task, with rare target stimuli (20% of trials) and frequent non-target stimuli (80% of trials) presented on a standard CRT computer monitor in pseudorandom order. Each experimental block consisted of 70 trials (14 targets, 56 non-targets). Brief, self-timed breaks were permitted after every block. Task context easy (E) or difficult (D) alternated every 4 blocks (e.g., EEEE DDDD EEEE…), and the starting block difficulty was counterbalanced across participants. The experiment consisted of 40 blocks of the task. Participants were presented a total of 560 target and 2240 non-target stimuli (1120 easy, 1120 difficult) across all blocks. The entire experiment lasted approximately 90 minutes.
The E-Prime software package (Psychology Software Tools, Pennsylvania, USA) was used to present stimuli. Participants were instructed to respond to the target stimuli by pressing the first key on a button box, while withholding a response when a non-target was presented. Response accuracy and speed were emphasized equally. Gabor patch stimuli were presented for 100 ms. Participants were given a 700 ms response window from stimulus onset, followed by an inter-trial interval randomly jittered between 500 and 900 ms.
Electroencephalographic Data Acquisition
The SCAN 4.3 software package (Compumedics, North Carolina, USA) was used to record EEG. Thirty-eight channels of EEG and EOG were recorded from the scalp with an electrode cap. Standard 10–20 sites were FPZ, FP1, FP2, FZ, F3, F4, F7, F8, FCZ, FC3, FC4, FT7, FT8, CZ, C3, C4, T7, T8, CPZ, CP3, CP4, TP7, TP8, PZ, P3, P4, P7, P8, POZ, PO3, PO4, PO7, PO8, OZ, O1, O2, M1 and M2. The physical reference electrode was approximately 2 cm posterior to CZ, and the EEG data were re-referenced to the average of M1 and M2 (left and right mastoid) offline. Horizontal eye movements (HEOG) were monitored by placing two electrodes lateral to the left and right orbits. Vertical eye movements (VEOG) and eye blinks were measured by placing two electrodes 1.5 cm below and above the left eye. The EEG from each electrode site was digitized at 500 Hz and was filtered with a high pass of 0.1 Hz and a low pass of 40 Hz.
Artifact rejection was performed to discard epochs contaminated by eye blinks, body movements and muscle activity. The rejection criterion was voltage change of more than +/−75 μV on the HEO or VEO channels. ERP epochs were calculated using a 200 ms pre-stimulus interval to 550 ms post-stimulus presentation. More than 150 epochs were used to compute participants’ grand average waveform for each of the correct experimental conditions (easy task context targets (M=214.2 epochs, SD=57.6) and non-targets (M=812.5, SD=212.9), difficult task context targets (M= 158.8, SD=60.8) and non-targets (M= 803.1, SD=209.3)). Difficult target error waveforms were computed from an average of 68 (SD=12.8) epochs per participant.
Data Analysis
Data were analyzed with EEGLAB (Delorme and Makeig, 2004), a toolbox for the MATLAB environment (MathWorks, Massachusetts, USA). For trials resulting in a button press, responses between 150–650 ms of stimulus presentation were considered valid. For trials without a button press, all trials were considered valid. Target trial errors occurred when participants failed to press the response box button within the allotted response window. Non-target trial errors occurred when participants pressed the response box button when presented with a non-target stimulus.
The grand average waveforms of all 14 participants were used to determine the ERP component peak amplitudes. Mean amplitudes for sensory components were then computed using 20 ms windows centered on the respective grand average peak for each component: Occipital-temporal P1 (128–148 ms), occipital-temporal N1 (168–188 ms). A 100 ms window was used to compute mean amplitude for the parietal P3b (450–550 ms). Overall scalp topographies in each of the time windows were inspected to determine the locus of activation for each component. Representative electrodes were selected based on maximal component amplitude (posterior P1 and N1: PO7, PO8; parietal P3b: PZ), and data from these electrodes were used for statistical analysis. Post-hoc analyses in each analysis of variance (ANOVA) were conducted with the Bonferroni correction for multiple comparisons.
Results
Behavioral
Table 1 presents reaction time (RT) and accuracy data for targets and non-targets in the easy and hard task contexts. Incorrect response RTs were not analyzed.
Table 1.
Behavioral Measures of Task Performance
| RT (ms) | SEM | Accuracy (%) | SEM | |
|---|---|---|---|---|
|
|
||||
| Easy Task Context | ||||
| Target | 347 | 11.6 | 93.4 | 6.78 |
| Non Target | -- | -- | 99.9 | 0.18 |
| Difficult Task Context | ||||
| Target | 383 | 11.9 | 70.9 | 20.0 |
| Non Target | -- | -- | 92.5 | 9.24 |
Reaction time
Mean RT differences between easy and difficult task context were examined with a two-tailed t-test. RTs were faster for easy task context targets than for difficult task context targets, t(13)=3.67, p=.011.
Accuracy
A 2 (task context) × 2 (stimulus type) repeated measures ANOVA was performed on the task accuracy data. Response accuracy was higher in the easy task (M=.97) than in the difficult task (M=.82), F(1,13)=31.52, p<.001, ηp2= .71, revealing a significant task context effect. Response accuracy was lower for targets (M=.82) than for non-targets (M=.96), F(1,13)=18.92, p.001, ηp2= .59, revealing a significant effect of stimulus type. A task context by stimulus type interaction was observed, F(1,13)=10.62, p=.006, ηp2= .45. A follow up one-way repeated measures ANOVA of the difference between stimulus type accuracy as a function of task context showed a significantly larger difference in accuracy for the target stimuli as compared to the non-target stimuli, F(1,13)= 20.55, p=.001, ηp2= .69.
ERP Waveforms
Occipital-temporal P1 and N1
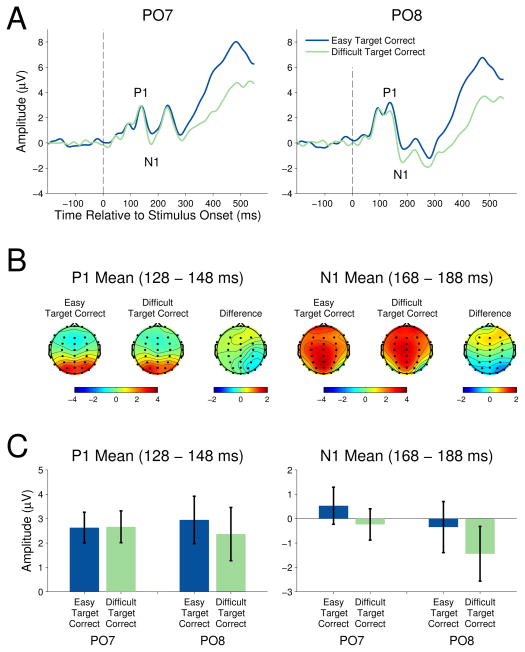
Figure 2 shows the grand average ERP waveforms for targets in the easy and difficult task contexts at the occipital-temporal electrodes PO7 and PO8 (left and right locations, respectively). ERPs elicited by non-target stimuli were not analyzed, as the degree of rotation of the non-targets was not identical across easy and difficult task contexts. Thus, any differences in the ERP amplitudes could be related to the physical difference between stimuli as opposed to executive control of attentional allocation. The target stimulus-elicited ERPs contain a P1, clearly visible as a positive deflection peaking around 130 ms post-stimulus onset. The subsequent negative deflection peaking around 175 ms post-stimulus onset is the N1. Scalp topographies included in the figure (see Figure 2B) verify the occipital-temporal maxima of each component. The mean occipital-temporal P1 and N1 amplitudes are shown in figure 2C.
Figure 2.
P1
The relationship between task difficulty and target P1 amplitude was examined with a 2 (task context) × 2 (electrode location) repeated measures ANOVA. No amplitude difference was seen between easy and difficult task context targets, F(1,13)=1.42, p=.26, ηp2= .10, or between left and right occipital-temporal electrode position. The task context by electrode location interaction was not significant, F(1,13)=2.09, p=.17, ηp2= .14.
N1
The relationship between task difficulty and target N1 amplitude was examined with a 2 (task context) × 2 (electrode location) repeated measures ANOVA. Targets in the easy task context elicited a smaller amplitude N1 when compared to targets in the difficult task context, F(1,13)=11.36, p=.005, ηp2= .47, reflecting a main effect of task context. Neither the effect of electrode location, F(1,13)=1.58, p=.231, ηp2= .11, nor the task context by electrode interaction, F(1,13)=0.65, p=.43, ηp2= .05, were significant.
Error-related processing
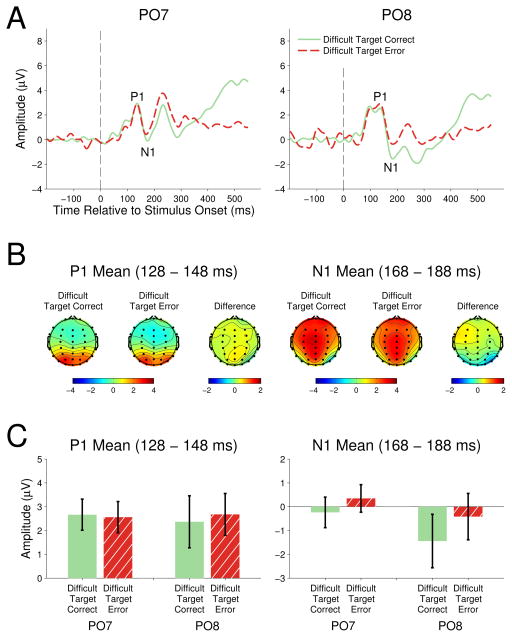
Figure 3 presents the grand average waveforms for correct and erroneous target responses in the difficult task context. The high accuracy (>.93) in the easy task did not produce sufficient error trials to carry out electrophysiological analysis.
Figure 3.
P1
The relationship between target response accuracy and P1 amplitude in the difficult task context was examined with a 2 (accuracy) × 2 (electrode location) repeated measures ANOVA. No P1 amplitude differences were observed between correct and erroneous target responses, F(1,13)<1.00, ηp2< .01, electrode location, F(1,13)<1.00, ηp2< .01, or the accuracy by electrode interaction, F(1,13) <1.00, ηp2= .05.
N1
The relationship between target response accuracy and N1 amplitude in the difficult task context was examined with a 2 (accuracy) × 2 (electrode location) repeated measures ANOVA. Correct target responses in the difficult task context elicited larger amplitude N1 (M=−0.84) than erroneous target responses (M= −0.03), F(1,13)=13.67, p=.003, ηp2= .51. Neither the effect of electrode location, F(1,13)=1.60, p=.23, ηp2= .11, nor the accuracy by electrode interaction, F(1,13)=0.97, p=.34, ηp2= .07, were significant.
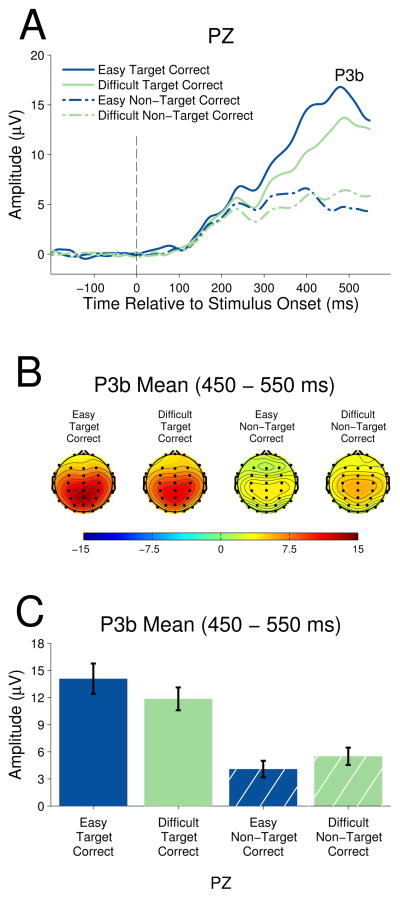
Parietal P3b
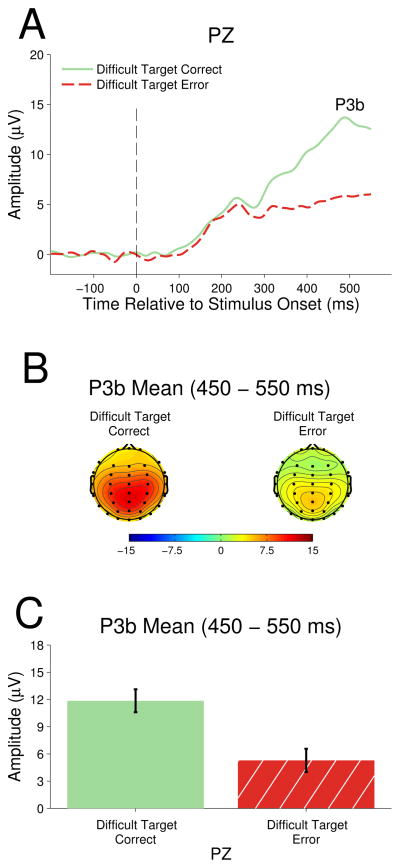
Figure 4 shows the grand average ERP waveforms for target and non-target stimuli in the easy and difficult task contexts at parietal electrode PZ. Since P3b is not associated with sensory processing, the component amplitudes of both targets and non-targets can be compared simultaneously. P3b is clearly visible as a positive deflection peaking around 450 ms post-stimulus onset.
Figure 4.
A 2 (stimulus type) × 2 (task context) repeated measures ANOVA showed target stimuli elicited larger amplitude P3b (M=12.97) than did non-target stimuli (M=4.79), F(1,13)= 74.19, p< .001, ηp2= 0.85, indicating a main effect of stimulus type. There was no difference between easy task stimuli P3b (M=9.08) and difficult task context stimuli (M=8.68), F(1,13)= 0.49, p=0.50, ηp2=0.04. However, the stimulus type by task context interaction was significant, F(1,13)=20.41, p<.01, ηp2=0.61. Follow up one-way repeated measures ANOVAs showed that target P3b was significantly larger in the easy task context as compared to the difficult task context, F(1,13) = 6.44, p = .025, ηp2 = .33, whereas non-target P3b was significantly larger in the difficult task context as compared to the easy task context, F(1,13) = 8.79, p = .011, ηp2 =.40.
Error-related processing
Figure 5 presents the grand average waveforms for correct and erroneous difficult task context target responses. The high accuracy (>93%) in the easy task context did not produce sufficient error trials to carry out electrophysiological analysis. For the difficult task context, a two-tailed t-test to examine the difference in P3b amplitude for correctly categorized targets and target errors was performed. There was a significant decrease in P3b amplitude for incorrectly categorized targets (M=5.29) as compared to correctly categorized targets (M=11.85)at electrode PZ, t(13)=6.88, p<.001.
Figure 5.
Discussion
The present study examined the effects of discrimination difficulty on neural processes associated with visual discrimination and stimulus categorization. Easy and difficult task contexts were created by manipulating the similarity between target and non-target stimuli, thus increasing the requirement of top-down executive control over sensory processes. Both behavioral and electrophysiological indices of stimulus uncertainty showed that participants had difficulty performing the stimulus discrimination in the difficult task context.
Behavioral responses to targets in the difficult task context were slower and less accurate than responses to physically identical targets in the easy task context. Targets in the difficult task context also elicited a larger occipital-temporal N1 and a reduced parietal P3b when compared to the easy task context. These findings are in agreement with previous work showing that the occipital-temporal N1 is an index of a stimulus discrimination process (Vogel & Luck 2000). Moreover, the results provide evidence that this process is modulated by top-down executive control as a function of the difficulty of stimulus discrimination.
Attention to a stimulus can be driven by involuntary, bottom-up or goal-directed, top-down mechanisms (Corbetta & Shulman, 2002; Knudsen, 2007). In the current paradigm, the difficulty of the required stimulus discrimination was manipulated not via a cluttered visual field as in previous studies, but instead by manipulating the similarity between serially presented targets and non-targets. This manipulation of the context in which a target stimulus is viewed implicates top-down executive control as opposed to bottom-up sensory processing in the observed electrophysiological modulations.
Target Salience
The increased amplitude of N1 in the difficult task context is inconsistent with a bottom-up source of the observed selective attention effects. The salience of target stimuli has previously been shown to increase bottom-up attentional capture (Theeuwes, 1991, 1994). By design, target salience in the current study was reduced in the difficult task context as compared to the easy task context. Thus, bottom-up attentional capture due to differences in the physical properties of the target and non-target stimuli is predicted to be greater in the easy task context. However, N1 amplitude showed an inverse relationship with target salience. The amplitude of this component was greater in the difficult task context, where target salience was reduced and discrimination difficulty was increased.
The observed decrease in P3b for targets in the difficult task context is also consistent with decreased stimulus salience in this condition. When target salience is increased, perceptual load is reduced and certainty of stimulus categorization is greater. The greater P3b amplitude in the easy context supports the notion that higher stimulus salience resulted in greater certainty of stimulus category (Kok, 2001; Polich & Comerchero, 2003). The observed dissociation in modulation of N1 and P3b is inconsistent with increased bottom-up attentional capture in the difficult task context. Thus, top-down executive control is implicated in the observed effects.
Sensitivity to Task Difficulty
The current results show that an increase in the difficulty of stimulus discrimination elicits a larger occipital temporal N1. Although N1 has been characterized as an index of stimulus discrimination, previous attempts to show that N1 is modulated by discrimination difficulty have not met with success when spatial attention was controlled (Senkowski & Hermann, 2002; Vogel & Luck, 2000). One potential explanation for this lack of sensitivity to discrimination difficulty is that the manipulation of stimulus discriminability in previous studies was insufficient to significantly alter perceptual load. The current experiment was expressly designed to increase the need for this discriminatory process by creating a small (+/− 2.5°) orientation difference between the target and non-targets in the difficult task context.
In previous studies of spatial attention, perceptual load has been shown to modulate both the P1 and N1 components (Fu et al., 2008; Handy & Mangun, 2000). In the current study, the allocation of spatial attention was controlled across conditions, and perceptual load was modulated by altering the similarity between serially presented targets and non-targets in separate blocks. Under these conditions, N1 was the earliest sensory component to exhibit sensitivity to the differences in task difficulty; the earlier P1 component was not affected by discrimination difficulty.
Given the explicit control of bottom-up influences on target processing in the two task contexts, our results implicate a top-down attentional control signal in response to increased task difficulty. This top-down control signal is observable as an increase in a sensory process associated with stimulus discrimination. The findings are consistent with the previously described mechanism for selective, top-down attentional control of sensory gain and/or tuning in visual cortex (Beck & Kastner, 2009; Desimone & Duncan, 1995; Knudsen, 2007), and they are in line with previous evidence from other paradigms showing competition occurs at the level of representation at which competition between stimulus features occurs (Beck & Kastner, 2009).
Failure of Discrimination
Failure of the discriminatory process indexed by N1 led to target categorization errors and a predictable relationship among discriminatory and categorization processes. For erroneous target responses in the difficult task context—those trials where subjects categorized a target as a non-target—both the occipital-temporal N1 and parietal P3b were significantly reduced. Thus, the failure of top-down executive control led to three consistent events: a reduced N1, indicative of an impaired stimulus discrimination; the absence of a P3b, indicative of incorrect stimulus categorization; and the absence of a motor response, indicative of failed task performance. This is the first evidence of an error-related modulation of the occipital-temporal N1 component.
Alternative Explanations
In the current study, the specificity of the observed modulations of visual cortical activation distinguishes these modulations from enhancements due to general arousal. The effects of top-down executive control have been shown to be directed at specific areas of visual cortex (Beck & Kastner, 2009; Corbetta, Miezin, Dobmeyer, Shulman, & Petersen, 1991; Williford & Maunsell, 2006). Arousal facilitates an overall increase in activation, whereas top-down executive control facilitates a focused increase in specific cortical areas underlying processing of relevant stimulus features.
In the current task, the P1 component, which has been shown to be sensitive to arousal (Eason, Harter, & White, 1969), was not modulated by task difficulty, whereas the N1 component was modulated. This enhancement of a specific sensory process, namely stimulus discrimination, is consistent with selective, top-down executive control. In addition, the pre-stimulus baseline was flat in both task contexts. This suggests there was no difference in anticipatory processing (Gomez & Flores, 2011) between the easy and difficult context stimulus blocks.
Conclusion
In the present study, exogenous differences between targets in the easy and difficult task contexts were directly controlled. The use of identical targets in both task contexts removed any contribution of bottom-up processes to the observed modulation of N1. Thus, the present study provides evidence that the context in which an individual target stimulus is perceived can elicit top-down modulation of early sensory processing, as indexed by well-characterized ERP components. This top-down modulation is consistent with theoretical models of both executive control (Miller & Cohen, 2001; Posner & DiGirolamo, 1998) and sensory processing (Desimone & Duncan, 1995; Kastner & Ungerleider, 2000).
Acknowledgments
This research was supported by NIH grant AG19653 and AFOSR/AFRL grant FA9550-10-1-0385, the Center of Excellence in Neuroergonomics, Technology, and Cognition (CENTEC), to RP.
References
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Research. 2009;49:1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau CE, Williford TH, Maunsell JH. Effects of task difficulty and target likelihood in area V4 of macaque monkeys. Journal of Neurophysiology. 2006;96:2377–2387. doi: 10.1152/jn.01072.2005. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clinical Neurophysiology. 1999;110:24–30. doi: 10.1016/S0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. Journal of Neuroscience. 1991;11:2383–402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- De Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Eason RG, Harter MR, White CT. Effects of attention and arousal on visually evoked cortical potentials and reaction time in man. Physiology & Behavior. 1969;4:283–289. doi: 10.1016/0031-9384(69)90176-0. [DOI] [Google Scholar]
- Fort A, Besle J, Giard M-H, Pernier J. Task-dependent activation latency in human visual extrastriate cortex. Neuroscience Letters. 2005;379:144–148. doi: 10.1016/j.neulet.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Fu S, Zinni M, Squire PN, Kumar R, Caggiano DM, Parasuraman R. When and where perceptual load interacts with voluntary visuospatial attention: An event-related potential and dipole modeling study. Neuroimage. 2008;39:1345–1355. doi: 10.1016/j.neuroimage.2007.09.068. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Flores A. A neurophysiological evaluation of a cognitive cycle in humans. Neuroscience and Biobehavioral Reviews. 2011;35:452–461. doi: 10.1016/j.neubiorev.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalez CM, Clark VP, Fan S, Luck SJ, Hillyard SA. Sources of attention-sensitive visual event-related potentials. Brain Topography. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- Handy TC, Mangun GR. Attention and spatial selection: Electrophysiological evidence for modulation by perceptual load. Perception & Psychophysics. 2000;62:175–186. doi: 10.3758/BF03212070. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society B. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Vogel E, Woodman G, Heinze HJ, Luck SJ. Localizing visual discrimination processes in time and space. Journal of Neurophysiology. 2002;88:2088–2095. doi: 10.1152/jn.2002.88.4.2088. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modeling of visual attention. Nature Reviews Neuroscience. 2001;2:194–204. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:267–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kehrer S, Kraft A, Irlbacher K, Koch SP, Hagendorf H, Kathmann N, et al. Electrophysiological evidence for cognitive control during conflict processing in visual spatial attention. Psychological Research. 2009;73:751–761. doi: 10.1007/s00426-008-0194-y. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annual Review of Neuroscience. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/S0048577201990559. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037/0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Multiple mechanisms of visual-spatial attention: recent evidence from human electrophysiology. Behavioural Brain Research. 1995;71:113–123. doi: 10.1016/0166-4328(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037/0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulation of sensory-evoked brain potentials provide evidence for changes in perceptual processing during visual-spatial priming. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:1057–1074. doi: 10.1037/0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. New York, NY: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Parasuraman R. Effects of information processing demands on slow negative shift latencies and N100 amplitude in selective and divided attention. Biological Psychology. 1980;11:217–233. doi: 10.1016/0301-0511(80)90057-5. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Beatty J. Brain events underlying detection and recognition of weak sensory signals. Science. 1980;210:80–83. doi: 10.1126/science.7414324. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: From modulation of sensory processing to top-down control. Journal of Neuroscience. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Comercho MD. P3a from visual stimuli: Typicality, task, and topography. Brain Topography. 2003;15:141–152. doi: 10.1023/A:1022637732495. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: The MIT Press; 1998. pp. 401–423. [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nature Neuroscience. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HG. Event-related potential correlates of two stages of information processing in physical and semantic discrimination tasks. Psychophysiology. 1983;20:168–179. doi: 10.1111/j.1469-8986.1983.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HG, Macht M. Manipulation of event-related potential manifestations of information processing stages. Science. 1982;218:909–911. doi: 10.1126/science.7134983. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Hermann CS. Effects of task difficulty on evoked gamma activity and ERPs in a visual discrimination task. Clinical Neurophysiology. 2002;113:1742–1753. doi: 10.1016/S1388-2457(02)00266-3. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, Awh E. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. Journal of Neurophysiology. 2004;92:3538–3545. doi: 10.1152/jn.00435.2004. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: The effect of visual onsets and offsets. Perception & Psychophysics. 1991;49:83–90. doi: 10.3758/BF03211619. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: Selective search for color and visual abrupt onsets. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:799–806. doi: 10.1037/0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. doi: 10.1111/1469-8986.3720190. [DOI] [PubMed] [Google Scholar]
- Williford T, Maunsell JH. Effects of spatial attention on contrast response functions in macaque area V4. Journal of Neurophysiology. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]