Abstract
The capsaicin (vanilloid) receptor, VR1, is a sensory neuron-specific ion channel that serves as a polymodal detector of pain-producing chemical and physical stimuli. It has been proposed that ATP, released from different cell types, initiates the sensation of pain by acting predominantly on nociceptive ionotropic purinoceptors located on sensory nerve terminals. In this study, we examined the effects of extracellular ATP on VR1. In cells expressing VR1, ATP increased the currents evoked by capsaicin or protons through activation of metabotropic P2Y1 receptors in a protein kinase C-dependent pathway. The involvement of Gq/11-coupled metabotropic receptors in the potentiation of VR1 response was confirmed in cells expressing both VR1 and M1 muscarinic acetylcholine receptors. In the presence of ATP, the temperature threshold for VR1 activation was reduced from 42°C to 35°C, such that normally nonpainful thermal stimuli (i.e., normal body temperature) were capable of activating VR1. This represents a novel mechanism through which the large amounts of ATP released from damaged cells in response to tissue trauma might trigger the sensation of pain.
Pain is initiated when noxious thermal, mechanical, or chemical stimuli excite the peripheral terminals of specialized primary afferent neurons called nociceptors (1–4). Many different kinds of ionotropic and metabotropic receptors are known to be involved in this process (5–7). Tissue damage associated with infection, inflammation, or ischemia produces an array of chemical mediators that activate or sensitize nociceptor terminals to elicit pain at the site of injury. An important component of this proalgesic response is ATP released from different cell types (8–13). ATP is released from microvascular endothelial cells during hyperemia, from nociceptive terminals after noxious stimulation (e.g., with capsaicin), and from sympathetic nerve terminal varicosities as a cotransmitter with norepinephrine and neuropeptide Y. ATP also is released from tumor cells during abrasive activity and from damaged tissues after trauma or surgery. Extracellular ATP excites the nociceptive endings of nearby sensory nerves, evoking a sensation of pain (9, 13). In these neurons, the most widely studied targets of extracellular ATP have been ionotropic ATP (P2X) receptors (11–13). Indeed, several P2X receptor subtypes have been identified in sensory neurons, including one (P2X3) whose expression is largely confined to these cells (14, 15). Our understanding of purinergic contributions to pain sensation may be incomplete, however, given that the potential involvement of widely distributed metabotropic ATP (P2Y) receptors has not yet been well investigated.
Vanilloid receptors are nociceptor-specific cation channels that serve as the molecular target of capsaicin, the pungent ingredient in hot chili peppers (16, 17). We have shown that, when expressed in heterologous systems, the cloned capsaicin receptor (VR1) also can be activated by noxious heat (with a thermal threshold of >43°C) or protons (acidification), both of which cause pain in vivo (18, 19). Furthermore, analyses of mice lacking VR1 have shown that VR1 is essential for selective modalities of pain sensation and for tissue injury-induced thermal hyperalgesia (20, 21). These data suggest a critical role for VR1 in the detection or modulation of pain. To address whether metabotropic P2Y receptors are involved in VR1-mediated nociceptive responses, we examined the effects of extracellular ATP on VR1 expressed in human embryonic kidney-derived HEK293 cells and rat dorsal root ganglion (DRG) neurons. We report that extracellular ATP potentiates or sensitizes VR1 responsiveness by any of three different stimuli through P2Y1 receptors in a protein kinase C (PKC)-dependent pathway, resulting in the activation of VR1 at normal body temperature.
Materials and Methods
Mammalian Cell Culture.
HEK293 cells were maintained in DMEM (supplemented with 10% FBS, penicillin, streptomycin, and l-glutamine) and transfected with 1 μg of plasmid DNA by using lipofectamine plus reagent (GIBCO). VR1 cDNA was prepared as described (16). M1 muscarinic acetylcholine receptor cDNA was given generously by Huai-hu Chuang (Univ. of California, San Francisco). Primary cultures prepared from male adult Wistar rat DRG (22) were incubated overnight (37°C, 5% CO2) in medium containing nerve growth factor (100 ng/ml). All procedures involving the care and use of rats were carried out in accordance with institutional guidelines.
Electrophysiology.
Whole-cell patch-clamp recordings were carried out at 1 or 2 days after transfection of VR1 cDNA to HEK293 cells or dissociation of the DRG neurons (16). Standard bath solution contained 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 5 mM EGTA, 10 mM Hepes, and 10 mM glucose, pH 7.4 (adjusted with NaOH). Bath solution was buffered to different pH values with either 10 mM Hepes (pH 6.7) or 10 mM Mes (pH 6.2, 5.8, 5.6, 5.3, 4.7, and 4.3). Pipette solution contained 140 mM CsCl (or KCl), 5 mM EGTA, and 10 mM Hepes, pH 7.4 (adjusted with CsOH or KOH). All patch-clamp experiments were performed at room temperature (25°C) unless otherwise noted. When examining the heat-evoked current responses, bath temperature was increased by using a preheated solution with the rate of 1.5–2.0°C/sec (about 20 sec). When the heat-activated currents started to inactivate, the preheated solution was changed to a 25°C one. Chamber temperature was monitored with a thermocouple (accuracy ± 0.1°C) placed within 4 mm of the patch-clamped cell. The solutions containing drugs were applied to the chamber (180 μl) by a gravity at a flow rate of 5 ml/min.
Reverse Transcription–PCR.
Total RNA was isolated from male adult rat DRG and HEK293 cells and reverse-transcribed by using Superscript II (GIBCO). The gene-specific primers used in this study (5′-GTTCAATTTGGCTCTGGCCG-3′ and 5′-CTGATAGGTGGCATAAACCC-3′ for rat P2Y1; 5′-GATCTGTATCAGCGTGCTGG-3′ and 5′-CTTGTGCCTTCACAGGCTTG-3′ for human P2Y1) were designed from rat and human P2Y1 sequences (23, 24). PCR was performed with 30 cycles of the following amplification protocol: 94°C, 30 sec; 56.5°C, 30 sec; and 72°C, 90 sec.
Results
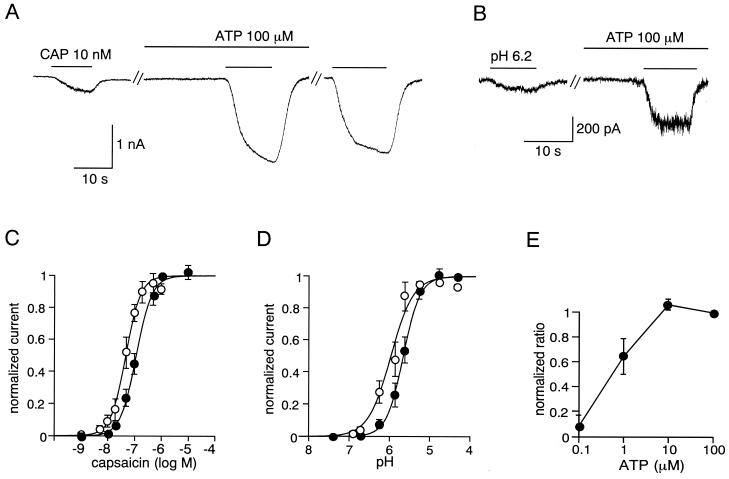
In voltage-clamp experiments, low doses of capsaicin (10 or 20 nM) evoked small inward currents in the HEK293 cells expressing VR1. In the absence of extracellular calcium, no change was observed in the magnitude of responses evoked by repetitive capsaicin applications. In contrast, after a 2-min pretreatment with 100 μM extracellular ATP, the same doses of capsaicin produced much larger current responses [6.42 ± 1.01-fold (mean ± SEM), n = 52] (Fig. 1A). Other electrophysiological properties of these capsaicin-evoked responses, including an outwardly rectifying current–voltage relationship, antagonist (capsazepine) sensitivity, and extracellular Ca2+-dependent desensitization, were unchanged by the presence of extracellular ATP (data not shown). A similar potentiating effect of extracellular ATP was observed on proton-evoked activation of VR1 (5.68 ± 0.92-fold, n = 32) (Fig. 1B). ATP appears to serve as a modulator, rather than a direct activator of VR1, because no current was observed upon application of ATP alone (Fig. 1 A and B). In addition, the ATP-stimulated increase in capsaicin-evoked currents persisted for several minutes after removal of ATP from the bath (Fig. 1A), suggesting the involvement of cytosolic second messengers in this process.
Figure 1.
Extracellular ATP potentiates capsaicin- and proton-activated currents in HEK293 cells. (A and B) Representative traces of increase of capsaicin (CAP)-activated (A) or proton-activated (pH 6.2) (B) current in transfected HEK293 cells expressing VR1. ATP (100 μM) treatment greatly increased both currents. The third trace in A was obtained 2 min after washing out of extracellular ATP. Similar effects were observed in 52 cells for the capsaicin- and 32 cells for the proton-activated currents. Whole-cell patch-clamp recordings were carried out with a holding potential of −60 mV as described previously (16). Cells were perfused for 2 min with solution containing ATP before exposure to capsaicin or acidic solution. (C) Capsaicin dose-response curves for VR1 in the absence (●) and presence (○) of 100 μM extracellular ATP. Currents were normalized to the currents maximally activated by 1 μM capsaicin in the absence of ATP. Figure shows averaged data fitted with the Hill equation. EC50 = 114.7 nM and Hill coefficient = 1.48 in the absence of ATP. EC50 = 49.3 nM and Hill coefficient = 1.56 in the presence of ATP. Values represent the mean ± SEM from 34 different cells. (D) Proton dose-response curves for VR1 in the absence (●) and presence (○) of 100 μM extracellular ATP. Currents were normalized to the currents maximally activated by pH 4.3 solution (saturating dose of proton) in the absence of ATP. Figure shows averaged data fitted with the Hill equation. EC50 = pH 5.64 and Hill coefficient = 2.30 in the absence of ATP. EC50 = pH 5.92 and Hill coefficient = 1.72 in the presence of ATP. Values represent the mean ± SEM from 21 different cells. (E) ATP dose-response curve for increase of the 20 nM capsaicin-activated currents. Values were normalized to the effects of 100 μM ATP and represent the mean ± SEM from eight cells.
To examine how ATP changes VR1 responsiveness, we measured VR1 currents in single cells by serially applying a range of concentrations of capsaicin or protons in the absence or presence of ATP. The currents were normalized to the maximal current produced by the application of 1 μM capsaicin or pH 4.3 solution without ATP to each cell. In both cases, maximal currents in the presence of ATP were almost the same as those obtained in the absence of ATP. The resultant dose-response curves clearly demonstrate that ATP enhances capsaicin and proton action on VR1 by lowering EC50 values without altering maximal responses (EC50 from 114.7 nM to 49.3 nM for capsaicin-activated currents; from pH 5.64 to pH 5.92 for proton-activated currents) (Figs. 1 C and D). VR1 potentiation by ATP could be observed at concentrations as low as 1 μM and saturated at ≈10 μM (Fig. 1E), a concentration attainable in the context of tissue damage (25).
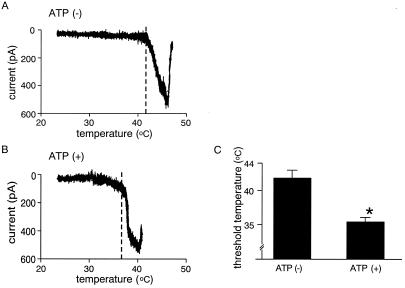
Potentiating effects of extracellular ATP also were examined on heat-evoked responses in HEK293 cells expressing VR1. For this analysis, heat-evoked current responses were compared between different cells, rather than within the same cell, because repetitive heat-evoked currents show significant desensitization even in the absence of extracellular Ca2+ (18) and because the thermal sensitivity of VR1 increases with repeated heat application (26). It is known that heat-evoked VR1 responses desensitize in an extracellular Ca2+-independent manner during relatively long heat applications (18, 27) although brief heat stimuli do not seem to cause this phenomenon (28, 29). This Ca2+-independent desensitization also has been reported in native DRG neurons (30). When temperature ramps were applied to HEK293 cells expressing VR1 in the absence of ATP, heat-evoked currents developed at about 42°C with an extremely steep temperature dependence (Fig. 2A). ATP treatment lowered the threshold temperature for VR1 activation significantly (41.7 ± 1.1°C, n = 7, and 35.3 ± 0.7°C, n = 5, without and with ATP treatment, respectively, P < 0.001) (Fig. 2 A–C). Thus, in the presence of ATP, normally nonpainful thermal stimuli (even body temperature) are capable of activating VR1, a finding consistent with the observation that exogenous ATP induces pain in human and rats (31, 32) at nonelevated body temperatures. These data clearly show that VR1 currents evoked by any of three different stimuli (capsaicin, proton, or heat) are potentiated or sensitized by extracellular ATP.
Figure 2.
The thermal sensitivity of VR1 increases in the presence of extracellular ATP. (A and B) Representative temperature-response profiles of heat-activated currents obtained by a temperature ramp in the absence (A) and presence (B) of 100 μM extracellular ATP. Temperature-response profiles were made only with current responses during the heat stimulation. Dashed lines show the threshold temperature for heat activation of VR1. Holding potential was −60 mV. (C) Temperature threshold for activation of VR1 in the presence of ATP (35.3 ± 0.7°C, n = 5) was significantly lower than that in the absence of ATP (41.7 ± 1.1°C, n = 7) (*, P < 0.001). Threshold was defined as a temperature at which clear current increase was observed in the temperature-response profile.
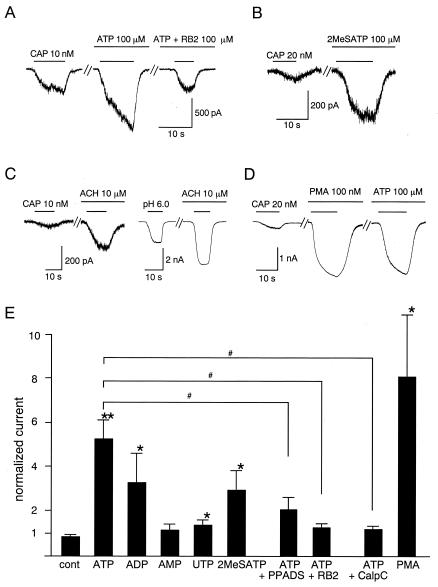
To explore the identity of the ATP receptors responsible for VR1 sensitization, we first examined the effects of two P2 receptor antagonists. In the presence of 100 μM pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (33), the enhancement of capsaicin-evoked currents by ATP was decreased significantly (2.05 ± 0.26-fold increase, n = 13) compared with ATP alone (5.25 ± 0.83-fold, n = 25) (P < 0.05) (Fig. 3E). Another P2 antagonist, reactive blue 2 (RB2, 100 μM) (12), almost completely abolished the effect of ATP (1.36 ± 0.13-fold, n = 8, P < 0.05 vs. ATP alone) (Fig. 3 A and E), indicating that P2 receptors mediate the enhancement of capsaicin-activated currents by ATP. Moreover, we conclude that ATP effect is mediated by metabotropic ATP (P2Y) receptors, because we failed to observe ATP-evoked currents indicative of ionotropic ATP (P2X) receptors in HEK293 cells, consistent with previous reports (34).
Figure 3.
P2Y1 receptor mediates ATP-induced potentiation of VR1 response through a PKC-dependent pathway. (A) Representative traces of the inhibition of ATP-induced potentiation of capsaicin (CAP)-activated current by 100 μM reactive blue 2 (RB2). Holding potential was −60 mV. Cells were perfused for 2 min with solution containing ATP or ATP + RB2 before exposure to capsaicin. (B) Representative traces of increase of the capsaicin-activated current by 100 μM of 2-methylthio ATP (2MeSATP). (C) Representative traces of increase of the capsaicin- or proton-activated currents by extracellular acetylcholine (ACH, 10 μM) in transfected HEK293 cells expressing VR1 and M1 muscarinic acetylcholine receptors (5.30 ± 0.96-fold increase, n = 6, for capsaicin-activated currents and 4.68 ± 1.04-fold increase, n = 7, for proton-activated currents, P < 0.05 vs. control). (D) Representative traces of increase of capsaicin-activated currents by direct activation of PKC with PMA (100 nM). Cells were perfused for 1 min with solution containing PMA before exposure to capsaicin. The third trace was obtained with a 2-min treatment of ATP after washing out of PMA in the same cell. (E) Effects of different ATP-related reagents, P2Y receptor agonist/antagonists, PKC activator/inhibitor on the capsaicin-activated VR1 currents in HEK293 cells. Currents were normalized to the currents evoked initially by capsaicin (20 nM) before application of the additives. Normalized currents in the presence of 100 μM ATP, ADP, AMP, UTP, or 2MeSATP was 5.25 ± 0.83 (n = 25), 3.27 ± 1.29 (n = 6), 1.13 ± 0.25 (n = 6), 1.36 ± 0.21 (n = 8), or 2.94 ± 0.85 (n = 11), respectively. Normalized currents in the presence of 100 μM ATP with 100 μM pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) or RB2 was 2.05 ± 0.26 (n = 13) or 1.36 ± 0.13 (n = 8), respectively. Normalized currents in the presence of 100 μM ATP with 1 μM calphostin C (CalpC) or 100 nM PMA were 1.13 ± 0.13 (n = 7) or 8.09 ± 2.81 (n = 10), respectively. *, P < 0.05; **, P < 0.01 vs. control (cont); #, P < 0.05 vs. ATP alone; two-tailed unpaired t test.
To distinguish between the subtypes of P2Y receptor that might be involved in this process, we examined the effect of several ATP-related reagents (each 100 μM) on the VR1 response (Fig. 3E). Among the reagents tested, ATP was the most effective (5.25 ± 0.83-fold, n = 25, P < 0.01 vs. control), followed by ADP (3.27 ± 1.29-fold, n = 6, P < 0.05). UTP exhibited a small but significant effect (1.36 ± 0.21-fold, n = 8, P < 0.05), whereas AMP failed to significantly potentiate capsaicin-evoked currents (1.13 ± 0.25-fold, n = 6, P > 0.05). This rank order of potency (ATP > ADP >> UTP > AMP) is most consistent with the involvement of P2Y1 receptors (11, 12). Further evidence for P2Y1 receptor involvement comes from our finding that 2-methylthio ATP (2MeSATP), an agonist that activates P2Y1 receptors much more effectively than other P2Y subtypes (12), produced a significant potentiation of capsaicin-evoked currents (2.94 ± 0.85-fold, n = 11, P < 0.05 vs. control) (Fig. 3 B and E).
One major consequence of P2Y1 receptor stimulation is activation of phospholipase C through the G protein Gq/11, leading to the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (12, 35). If Gq/11 activation underlies the potentiation of VR1 by extracellular ATP, stimulation of other Gq/11-coupled receptors might produce similar effects. When VR1 was coexpressed with the Gq/11-coupled M1 muscarinic acetylcholine receptor in HEK293 cells (36), extracellular application of acetylcholine significantly increased the magnitude of currents evoked by either capsaicin or protons (5.30 ± 0.96- and 4.68 ± 1.04-fold for capsaicin- and proton-activated currents, respectively, P < 0.05 vs. control) (Fig. 3C), supporting the involvement of Gq/11-coupled signaling in VR1 sensitization. Ca2+ mobilization by IP3 is not a likely mechanism for the capsaicin-evoked current increase observed in our experiments because cytosolic-free Ca2+ is tightly chelated with the 5 mM EGTA included in the pipette solution. Therefore, activation of PKC by DAG remains a more likely mechanism for ATP-induced potentiation (37). To test this possibility, we examined the effect of a highly potent and selective PKC inhibitor, calphostin C (38). When 1 μM calphostin C was added to the pipette solution, the ATP effect was almost completely abolished (1.13 ± 0.13-fold, n = 7, P < 0.05 vs. ATP alone) (Fig. 3E). Acetylcholine effects observed in the cells expressing both VR1 and M1 muscarinic acetylcholine receptor were abolished by calphostin C, too (1.29 ± 0.18-fold for capsaicin-activated currents, n = 5; 1.22 ± 0.19-fold for proton-activated currents, n = 4, P < 0.05 vs. acetylcholine alone) (data not shown). Furthermore, direct activation of PKC by 100 nM phorbol 12-myristate 13-acetate (PMA) caused a robust increase in the magnitude of capsaicin-evoked currents (8.09 ± 2.81, n = 10, P < 0.05 vs. control) (Fig. 3 D and E). The maximal response to capsaicin was not changed after treatment of PMA, suggesting a further leftward shift of the capsaicin dose-response curve. When the cells were exposed to ATP after PMA treatment, no further potentiation of the capsaicin-activated currents was observed (Fig. 3D), indicating that the same pathway is involved in the potentiation by ATP and PMA. These data clearly indicate the involvement of a PKC-dependent pathway in VR1 potentiation by ATP and are consistent with the report that PKC-ɛ is specifically involved in sensitization of heat-activated channels by bradykinin in DRG neurons (39).
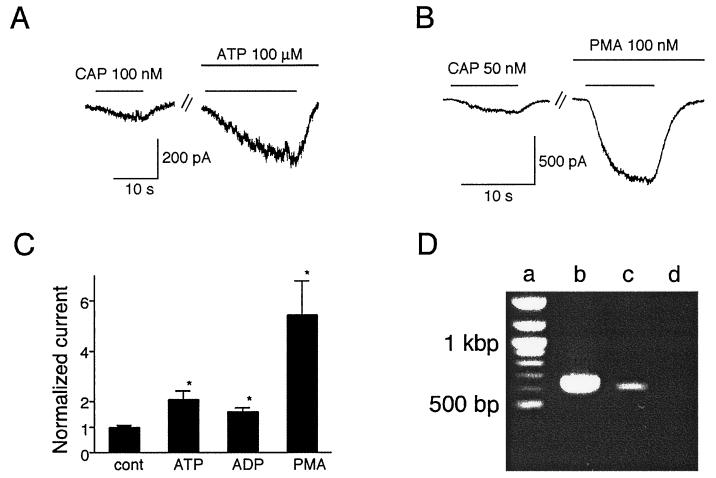
To examine whether the VR1 potentiation by ATP occurs in native neurons, we performed voltage-clamp experiments on rat DRG neurons cultured in the presence of nerve growth factor. Because the sensitivity of these cells to capsaicin appears to be slightly lower than that of VR1-transfected HEK293 cells (data not shown and ref. 40), we applied capsaicin to these cells at 50 nM instead of 20 nM. Nevertheless, in capsaicin-responsive neurons, we found that ATP did, indeed, potentiate capsaicin-evoked currents (2.04 ± 0.34-fold, n = 7, P < 0.05 vs. control) (Fig. 4 A and C). In some DRG neurons, application of ATP alone caused transient current responses, most likely mediated by ionotropic P2X receptors. Within these latter neurons, the magnitude of ATP-induced potentiation of capsaicin-evoked currents was indistinguishable from that seen in neurons lacking P2X responses (data not shown). ADP also potentiated capsaicin-evoked currents in DRG neurons (1.56 ± 0.15-fold, n = 4, P < 0.05 vs. control), although less effectively than ATP (Fig. 4C). Direct activation of PKC by 100 nM PMA greatly increased capsaicin-evoked currents (5.38 ± 1.33-fold, n = 5, P < 0.05 vs. control) (Fig. 4 B and C), supporting the involvement of a PKC-dependent pathway in DRG neurons. Moreover, expression of the P2Y1 receptor gene in HEK293 cells and rat DRG neurons was confirmed by reverse transcription–PCR (Fig. 4D), consistent with previously reported findings (41). Still, a contribution from other metabotropic ATP receptor subtypes to the potentiation of VR1 responses cannot be excluded.
Figure 4.
Extracellular ATP potentiates capsaicin-evoked currents in rat DRG neurons. (A) Representative traces of increase of the capsaicin (CAP)-activated currents by extracellular ATP (100 μM). Similar results were observed in 10 different cells tested. Holding potential was −60 mV. (B) Representative traces of increase of the capsaicin-activated currents by PMA (100 nM). Similar results were observed in five different cells tested. (C) Effects of ATP, ADP, or PMA on the capsaicin-activated currents. Currents were normalized to the currents evoked initially by capsaicin (50 nM) in the absence of the additives. Normalized currents in the presence of ATP, ADP, or PMA were 2.04 ± 0.34 (n = 7), 1.56 ± 0.15 (n = 4), or 5.38 ± 1.33 (n = 5), respectively. *, P < 0.05 vs. control (cont); two-tailed unpaired t test. (D) PCR amplification of P2Y1 cDNA fragments from the RNAs of rat DRG neurons and HEK293 cells. The expected sizes of the DNA fragments for rat and human P2Y1 are 652 and 628 bp, respectively. Lanes: a, size marker; b, rat DRG neurons; c, HEK293 cells; d, negative control.
Discussion
Inflammatory pain is initiated by tissue damage/inflammation and is characterized by hypersensitivity both at the site of damage and in adjacent tissue. Stimuli that normally would not produce pain do so (allodynia), whereas previously noxious stimuli evoke even greater pain responses (hyperalgesia). One mechanism underlying these phenomena is the modulation (sensitization) of ion channels, such as VR1, that detect noxious stimuli at the nociceptor terminal (1–4). Sensitization is triggered by extracellular inflammatory mediators that are released in vivo from surrounding damaged or inflamed tissue and from nociceptive neurons themselves (i.e., neurogenic inflammation). Mediators known to cause sensitization include prostaglandins, adenosine, serotonin, bradykinin, and ATP (1–5). In the present study, we demonstrated that extracellular ATP potentiates VR1 responses through metabotropic ATP (P2Y1) receptors in a PKC-dependent manner in both a heterologous expression system and rat DRG neurons. ATP enhances VR1 responses by lowering the VR1 activation threshold for capsaicin, proton, and heat stimulation. This effect of ATP might contribute to ATP-induced hypersensitivity. In addition to potentiating capsaicin- or proton-evoked currents, ATP also lowers the temperature threshold for heat activation of VR1, such that normally nonpainful thermal stimuli (i.e., normal body temperature) are capable of activating VR1, making ATP act as a direct activator of VR1. This represents a novel mechanism through which extracellular ATP might cause pain in a pathway distinct from the activation of P2X receptors. The existence of such a mechanism is consistent with the recent observation that ATP-evoked nociceptive behavior in mice is only partially reduced by disruption of the P2X3 gene (42, 43). In addition, the observation that ATP can be released from a subset of small, primary afferent nerves in response to capsaicin (9) suggests a possible autocrine mechanism for the exacerbation of pain. Activation of similar PKC-dependent events might underlie certain nociceptive effects of other Gq/11-coupled metabotropic receptors such as bradykinin receptors (3–5, 44) whose occupancy enhances heat-activated currents in sensory neurons (29). Indeed, it has been reported recently that activation of PKC can induce VR1 channel activity in the absence of any other agonist (45).
In our experiments, the involvement of Ca2+ in the potentiation of VR1 response could be ruled out because cytosolic Ca2+ was tightly chelated by EGTA. Kress and Guenther (28), however, reported a significant potentiation of the heat response in DRG neurons (likely mediated by VR1) when intracellular Ca2+ was raised. Both intracellular Ca2+-independent and Ca2+-dependent potentiation mechanisms might occur in native cells.
In nociceptors, short-term sensitization by inflammatory mediators is mediated by two distinct intracellular pathways, activation of protein kinase A by such agents as prostaglandins (46) and activation of PKC by such agents as bradykinin (47). In particular, electrophysiological, biochemical, and knockout mouse analyses (39, 48) have implicated PKC-ɛ in the sensitization of nociceptors whereas a protein kinase A-dependent pathway has been reported to be involved in the modulation of capsaicin-gated channel by prostaglandin E2 (49). We hypothesize that direct phosphorylation of VR1 or a closely associated protein changes the agonist sensitivity of this ion channel. Molecular and biochemical analysis of VR1 should allow this hypothesis to be tested.
It is well accepted that extracellular ATP plays an important role in nociception because ATP produces a sensation of pain in vivo (11–13, 31, 32) and because some P2 antagonists show analgesic activity (50, 51). Most attention in the pain field has focused on the role of ionotropic ATP receptors in ATP-evoked nociception. Our findings suggest that P2Y1 is also involved in this process and may represent a fruitful target for the development of drugs that blunt nociceptive signaling through capsaicin receptors.
Acknowledgments
We thank M. J. Caterina (Johns Hopkins University) and K. Inoue (National Institute of Health Sciences, Japan) for critical reading of the manuscript. This work was supported by grants from the Ministry of Culture, Education, Science and Sports in Japan (to M.T. and M.M.) and (to M.T.) by Novartis Foundation (Japan) for the Promotion of Science, The Mitsubishi Foundation, Yamazaki Spice and Herb Research Foundation, and Takeda Science Foundation.
Abbreviations
- VR1
capsaicin (vanilloid) receptor 1
- DRG
dorsal root ganglion
- PMA
phorbol 12-myristate 13-acetate
- PKC
protein kinase C
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 6537.
References
- 1.Fields H L. Pain. New York: McGraw–Hill; 1987. [Google Scholar]
- 2.Wood J N, Perl E R. Curr Opin Genet Dev. 1999;9:328–322. doi: 10.1016/s0959-437x(99)80049-5. [DOI] [PubMed] [Google Scholar]
- 3.Mizumura K, Kumazawa T. Prog Brain Res. 1996;113:115–141. doi: 10.1016/s0079-6123(08)61084-5. [DOI] [PubMed] [Google Scholar]
- 4.Woolf C J, Salter M W. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 5.Cesare P, McNaughton P. Curr Opin Neurobiol. 1997;7:493–499. doi: 10.1016/s0959-4388(97)80028-1. [DOI] [PubMed] [Google Scholar]
- 6.McCleskey E W, Gold M S. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- 7.Caterina M J, Julius D. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 8.Holton P. J Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawynok J, Sweeney M I. Neuroscience. 1989;32:557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Perl E R. J Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North A N, Barnard E A. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 12.Ralevic V, Burnstock G. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 13.Burnstock G. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 14.Lewis C, Neldhart S, Holy C, North R A, Buell G, Surprenant A. Nature (London) 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen C-C, Akopian A N, Sivilotti L, Colquhoun D, Burnstock G, Wood J N. Nature (London) 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 16.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 17.Szallasi A, Blumberg P M. Pharmacol Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- 18.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 19.Tominaga M, Julius D. Jpn J Pharmacol. 2000;83:20–24. doi: 10.1254/jjp.83.20. [DOI] [PubMed] [Google Scholar]
- 20.Caterina M J, Leffler A, Malmberg A B, Martin W J, Trafton J, Petersen-Zeitz K R, Koltzenburg M, Basbaum A I, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 21.Davis J B, Gray J, Gunthorpe M J, Hatcher J P, Davey P T, Overend P, Harries M H, Latcham J, Clapham C, Atkinson K, et al. Nature (London) 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 22.Reichling D B, Levine J D. Proc Natl Acad Sci USA. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokuyama Y, Hara M, Jones E M C, Fan Z, Bell G I. Biochem Biophys Res Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- 24.Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems J-M. Biochem Biophys Res Commun. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- 25.Born G V R, Kratzer M A A. J Physiol. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caterina M J, Rosen T A, Tominaga M, Brake A J, Julius D. Nature (London) 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 27.Cesare P, Moriondo A, Vellani V, McNaughton P A. Proc Natl Acad Sci USA. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kress M, Guenther S. J Neurophysiol. 1999;81:2612–2619. doi: 10.1152/jn.1999.81.6.2612. [DOI] [PubMed] [Google Scholar]
- 29.Cesare P, McNaughton P. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz S, Greffrath W, Büsselberg D, Treede R-D. J Physiol. 2000;528:539–549. doi: 10.1111/j.1469-7793.2000.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleehen T, Keele C A. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 32.Bland-Ward P A, Humphrey P P A. Br J Pharmacol. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambrecht G, Friebe T, Grimm U, Windscheif U, Bungardt E, Hildebrandt C, Baumert H G, Spatz-Kumbel G, Mutschler E. Eur J Pharmacol. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- 34.Valera S, Hussy N, Evans R J, Adami N, North R A, Surprenant A, Buell G. Nature (London) 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 35.Schachter J B, Li Q, Boyer J L, Nicholas R A, Harden T K. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulme E C, Birdsall N J M, Buckley N J. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka C, Nishizuka Y. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 39.Cesare P, Dekker L V, Sardini A, Parker P J, McNaughton P A. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 40.Shin J S, Wang M-H, Hwang S W, Cho H, Cho S Y, Kwon M J, Lee S-Y, Oh U. Neurosci Lett. 2001;299:135–139. doi: 10.1016/s0304-3940(00)01777-8. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura F, Strittmatter S M. Proc Natl Acad Sci USA. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cockayne D A, Hamilton S G, Zhu Q M, Dunn P M, Zhong Y, Novakovic S, Malmberg A B, Cain G, Berson A, Kassotakis L, et al. Nature (London) 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 43.Souslova V, Cesare P, Ding Y, Akopian A N, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, et al. Nature (London) 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 44.Bhoola K D, Figueroa C D, Worthy K. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 45.Premkumar L S, Ahern G P. Nature (London) 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 46.Aley K O, Levine J D. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber L A, Vasko M R. J Neurochem. 1996;67:72–80. doi: 10.1046/j.1471-4159.1996.67010072.x. [DOI] [PubMed] [Google Scholar]
- 48.Khasar S G, Lin Y H, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley K O, Isenberg W, McCarter G, et al. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopshire J C, Nicol G D. J Neurosci. 1998;15:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho B T, Huo Y Y, Lu J G, Newman R A, Levin V A. Anticancer Drugs. 1992;3:91–94. doi: 10.1097/00001813-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Driessen B, Reimann W, Selve N, Friderchs E, Bültmann R. Brain Res. 1994;666:182–188. doi: 10.1016/0006-8993(94)90770-6. [DOI] [PubMed] [Google Scholar]