Abstract
To facilitate the development of a peptide-based cancer vaccine for prostate cancer patients, we examined whether any of the 13 peptides previously reported to induce HLA-class I-restricted cytotoxic T lymphocyte (CTL) activity in HLA-A3 supertype (-A3, -A11, -A31 and -A33)-positive prostate cancer patients are also capable of inducing CTLs restricted to HLA-A2, HLA-A24 or HLA-A26 alleles. Among the 13 peptides tested, a peptide at positions 309 to 318 of β-tubulin 5 exhibited binding activity to the HLA-A*2402 molecule and induced HLA-A24-restricted CTL activity against prostate cancer cells derived from peripheral blood mononuclear cells of prostate cancer patients. The CTL activity was determined to be specific to this peptide and was mediated by CD8+ T cells in an HLA-class I-restricted manner. These results suggest that this peptide could be applicable as a peptide vaccine, not only for HLA-A3 supertype-positive, but also for HLA-A24-positive prostate cancer patients.
Keywords: β-tubulin 5, peptide, cytotoxic T lymphocyte, HLA-A24, cancer vaccine, peripheral blood mononuclear cell, prostate cancer
Introduction
A peptide-based cancer vaccine is one of the new treatment modalities for cancer. We recently reported that it has a clinical benefit for advanced prostate cancer patients in a randomized clinical trial (1). However, peptide-based immunotherapy for cancer patients is highly restricted by HLA-A alleles, which in turn is hampering the development of peptide-based cancer vaccines at the commercial level. Therefore, the identification of candidate peptides widely applicable for patients with different HLA-A alleles is required. We previously found and reported (2–5) such epitope peptides, which bind to more than one HLA-class IA allele. Therefore, in the present study we examined whether or not the 13 different peptides that have been reported to induce HLA-A3 supertype-restricted cytotoxic T lymphocyte (CTL) activity (2,3,6–10) also induce CTL activity restricted to the HLA-A2, HLA-A24 and HLA-A26 alleles in the peripheral blood mononuclear cells (PBMCs) of prostate cancer patients.
Materials and methods
Peptide-HLA stabilization assay
To assess the binding and stabilizing activity of peptides to HLA-A*0201, -A*0206, -A*0207, -A*2402 and -A*2601 molecules, a previously reported method was employed, with several modifications (2,3,6–10). Briefly, RMA-S-A*0201, -A*0206, -A*0207, -A*2402 and -A*2601 (5×105 cells/well in a 24-well plate) were incubated in 500 μl RPMI-1640 (Invitrogen) supplemented with 20% fetal bovine serum (FBS) (MP Biomedicals Inc., Eschwege, Germany) for 20 h at 26°C in 5% CO2. Then, the cells were incubated in 500 μl Opti-MEM (Invitrogen, Carlsbad, CA, USA) containing 0.1–100 μM peptides and human β2 microglobulin (2 μg/ml) at 26°C for 2 h, and then for 3 h at 37°C in 5% CO2. Cells were washed and incubated for 30 min on ice with an appropriate dilution of anti-HLA-A24 or BB7.2 supernatant (anti-HLA-A2). After being washed with phosphate-buffered saline (PBS), the cells were stained by Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) for 30 min on ice. The mean fluorescence intensity (MFI) was measured by flow cytometry, and peptides which exhibited a >25% increase in the MFI were defined as positive binding peptides.
Cell lines
The cell lines used as target cells for cytotoxicity were the PC3 (HLA-A*2402) and LNCaP (HLA-A*0201) prostate cancer cell lines, and LNCap transfected with the HLA-A*2402 gene (LNCaP-A24) as previously reported (11). PC3 and LNCaP-A24 tumor cells were used as relevant tumor cells for the measurement of HLA-A24-restricted CTL activity, whereas LNCaP cells were used as irrelevant target cells. The cell cultures were maintained in RPMI-1640 medium supplemented with 10% FBS. For the pulsing of peptides to the assessed induction of peptide-specific CTLs from PBMCs as reported previously (1,8,11), we used C1R-A*2402 cells (an HLA-A*2402 gene-stable transfectant of the B lymphoblastoid cell line, kindly provided by Dr M. Takiguchi, Kumamoto University, Japan), as reported previously (11,12). RMA-S cells were derived from a mouse mutant cell line deficient in antigen processing, which showed decreased cell surface expression of MHC class I molecules. The HLA-A*0201, -A*0206, -A*0207, -A*2402 and -A*2601 genes were also individually transfected into RMA-S cells using the FuGENE transfection reagent (Roche, Mannheim, Germany). Clones of stably HLA gene-transfected cells were established from a separate well in the presence of geneticin (0.5 mg/ml). The detailed methods for establishing these transfectants have been reported previously (7).
Peptides
Peptides with >90% purity were purchased from Hokkaido System Science (Sapporo, Japan) or Genenet (Fukuoka, Japan) and dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 μg/ml. Fourteen peptides that were previously shown to be capable of inducing HLA-A3 supertype (-A3, -A11, -A31 and -A33)-restricted CTLs (2,3,6–10) were used in this study. In addition, Epstein-Barr virus (EBV)-derived and human immunodeficient virus (HIV)-derived peptides were used as controls binding to the HLA-A2 and -A24 alleles, as reported previously (13–16). A peptide derived from positions 155 to 163 of prostate acid phosphatase (PAP) was used as a positive control for binding to the HLA-A*0201, -A*0206 and -A*2402 alleles, but not the other alleles, as reported previously (2). NS31582–1590 was also used as a positive-control peptide for HLA-A26 (2).
Patients
The Institutional Ethical Review Board of Kurume University approved the study protocol, which conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Informed written consent was obtained from all participants who donated PBMCs for this study. PBMCs were obtained from 9 prostate cancer patients and from 3 healthy donors (HDs) who were homozygous for the HLA-A24 allele. None of the participants were infected with HIV. The patient characteristics are presented in brief in Table I. PBMCs were isolated from blood samples by density centrifugation using Ficoll-Conray (density 1.077), and were cryopreserved until use. The expression of HLA-A24 molecules on PBMCs was discriminated by staining with anti-HLA-A24 mAb and analyzed by flow cytometry (2).
Table I.
Characteristics of the prostate cancer patients.
| Patient no. | Age | Gender | TMN category | GS | PSA (before surgery) | PSA (after surgery) |
|---|---|---|---|---|---|---|
| 1 | 74 | Male | cT2aN0M0 | 3+4=7 | 9.99 | 0.348 |
| 2 | 65 | Male | T2bN0M0 | 3+4=7 | 4.11 | 0.529 |
| 3 | 76 | Male | ||||
| 4 | 58 | Male | cT2aN0M0 | 4+4=8 | 5.05 | 0.028 |
| 5 | 65 | Male | cT1cN2M0 | 4+3=7 | 9.09 | <0.005 |
| 6 | 66 | Male | cT2aN0M0 | 3+4=7 | 14.70 | 0.006 |
| 7 | 75 | Male | cT3bN0M0 | 4+5=9 | 46.92 | <0.005 |
| 8 | 81 | Male | cT1cN0M0 | 3+3=6 | 4.70 | 0.144 |
| 9 | 70 | Male | cT1cN0M0 | 3+4=7 | 7.45 | 0.049 |
GS, Gleason score; PSA, prostate-specific antigen.
Induction of peptide-specific CTLs from PBMCs
The induction of peptide-specific CTLs and the detection of interferon (INF)-γ produced by CTLs were carried out according to a previously reported method with several modifications (6). Briefly, PBMCs (1×105 cells per well in a 96-well U-bottom-type plate) were incubated with 10 μg/ml of each peptide in culture medium. The culture medium consisted of 45% RPMI-1640, 45% AIM-V medium (Invitrogen, Gaithersburg, MD, USA), 10% FBS, 100 U/ml interleukin-2 and 0.1 mM MEM Non-Essential Amino Acids Solution (Life Technologies) at 37°C in 5% CO2. On Day 15 of culture, the cells were divided into four wells. Two of these wells were mixed with the corresponding peptide-pulsed C1R-A*2402 cells, while the other two were mixed with the irrelevant (HIV) peptide and incubated for 18 h at 37°C in 5% CO2. The IFN-γ production of CTLs was determined by an enzyme-linked immunosorbent assay. Discrimination of the induction of peptide-specific CTLs was considered to be successful when the P-value was <0.05 and when the difference in IFN-γ production compared to the control HIV peptide exceeded 50 pg/ml.
Cytotoxicity assay
Peptide-stimulated PBMCs were tested for their cytotoxicity against PC3, LNCaP and LNCap-A24 prostate cancer cells by a standard 6-h 51Cr-release assay (2). Phytohemagglutinin (PHA)-activated T cells from HLA-A24-positive patients were used as a negative control. The PBMCs were also tested for their cytotoxicity against CIR-A*2401 cells that were pre-pulsed with either a corresponding peptide or the HIV peptide.
51Cr-labeled target cells (2,000 cells/well) were mixed with effector cells at the indicated effector-to-target (E/T) ratios in 96 round-well plates. Immediately before the cytotoxicity assay, CD8+ T cells were positively isolated using a CD8 Positive Isolation kit (Dynal, Oslo, Norway) according to the manufacturer's manual. After incubation for 20 h, the plates were centrifuged and the supernatant was collected to measure radioactive quantitation by a gamma counter. The specific 51Cr release was according to the formula (test cpm - spontaneous cpm). Spontaneous 51Cr release was calculated by measuring the radioactive quantitation of the 51Cr-labeled target cell supernatant alone, and the total 51Cr release was then calculated by measuring the radioactive quantitation of 51Cr-labeled target cell lysis by 1% Triton X-100 (Wako Pure Chemical Industries, Osaka, Japan). For the blocking assay, 10 μg/ml of either anti-HLA class I (W6/32: mouse IgG2a), anti-HLA-DR (L243: mouse IgG2a) or anti-HLA-B,C (B1-23, IgG2a; kindly donated by Dr Pierre G. Coulie, Catholique de Louvain University, Brussels, Belgium) was added to the medium at the initiation of the mixed culture.
The peptide-stimulated CTLs were confirmed by specific peptide recognition using a cold inhibition assay. In brief, 51Cr-labeled target cells (2×104 cells per well) were mixed with the effector cells (2×104 cells per well) in 96 round-well plates with 2×104 cold target cells and peptide-pulsed C1R-A*2402 cells.
Statistical analysis
The Student's t-test was used to test statistical significance, and P-values of <0.05 were considered significant.
Results
HLA stabilization assay
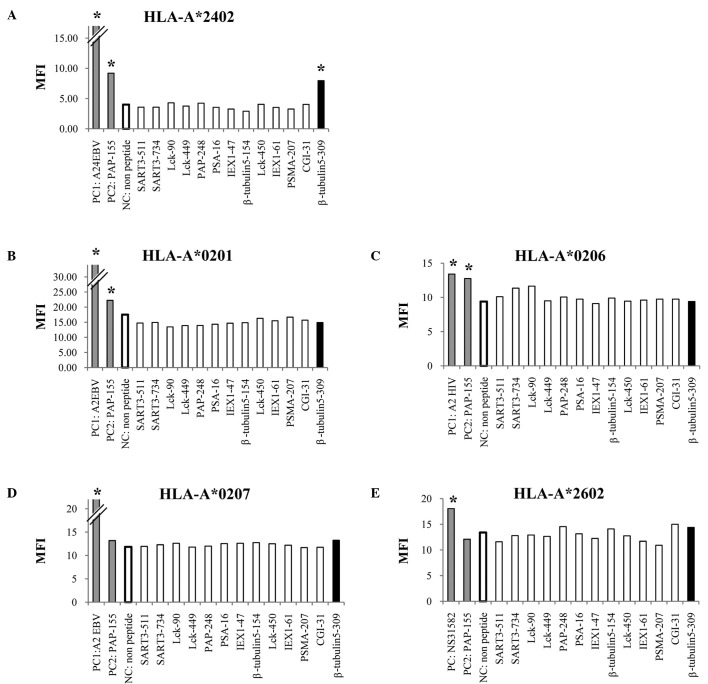
We first screened the binding activity of each of the 13 different HLA-A3 supertype peptides (100 μM) to the HLA-A*0201, -A*0206, -A*0207, -A*2402 and -A*2601 alleles by means of an HLA stabilization assay using RMA-S cells expressing each HLA molecule. A PAP-derived peptide consisting of the amino acid sequence from positions 155 to 163 was used as a positive control for binding to the HLA-A*0201, -A*0206 and -A*2402 alleles, but not the other alleles, as reported previously (4). As a result, one peptide from positions 309 to 318 of β-tublin 5 (β-tubulin 5309–318) showed binding activity to HLA-A*2402 molecules, but not to any of the other molecules tested (Fig. 1). The surface expression of the HLA-A*2402 molecules on RMA-S-A*2402 cells was stabilized in a dose-dependent manner when cells were cultured with either a positive control or the β-tubulin 5309–318 peptide (Fig. 2).
Figure 1.
Stabilization assay of the β-tubulin 5309–318 peptide for various HLA alleles. The binding activities of the β-tubulin 5309–318 peptide to various HLA-A alleles were examined using the stable transfectant cell lines RMA-S-A*0201 (A), -A*0206 (B), -A*2402 (C), -A*0207 (D) and -A*2601 (E) with a positive-control peptide and negative control (DMSO). The positive-control peptides used for each HLA were HIV-A2 (A and B), HIV-A24 (C), EBV-A2 (D) and NS31582–1590 (E). A peptide derived from positions 155 to 163 of prostate acid phosphatase (PAP) was used as a positive control for binding to the HLA-A*0201, -A*0206 and -A*2402 alleles, but not the other alleles, as reported previously (4). The mean fluorescence intensity (MFI) was indicated at 100 μM of the peptide against HLA-A*2402 (A), HLA-A*0201 (B), HLA-*0206 (C), HLA-A*0207 (D) and HLA-A*2602 (E). Representative results from at least three separate experiments are shown. *Statistically significant at P<0.05.
Figure 2.
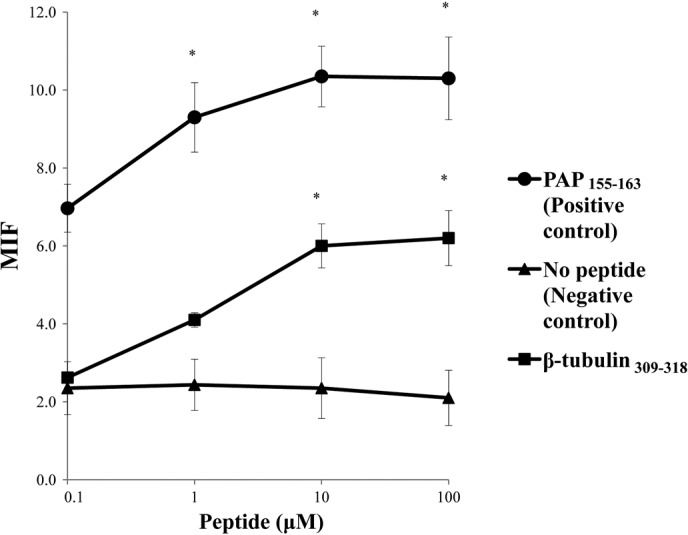
Dose-dependence in the stabilization assay of the β-tubulin 5309–318 peptide for the HLA-A*2402 allele. The mean fluorescence intensity (MFI) was recorded at 0.1, 1, 10 and 100 μM of the peptide or DMSO. The MFI increase induced by the β-tubulin 5309–318 peptide compared to DMSO was calculated. Representative results from at least three separate experiments are shown. A PAP-derived peptide at positions 155 to 163 was used as a positive control for binding to the HLA-A*0201, -A*0206 and -A*2402 alleles, but not the other alleles, as reported previously (2). *Statistically significant at P<0.05.
Induction of peptide-specific CTL activity
We attempted to determine by means of an IFN-γ production assay whether or not the β-tubulin 5309–318 peptide has the potential to generate peptide-specific CTLs from prostate cancer patients and HDs. PBMCs from HLA-A24/A24 homozygotes were stimulated in vitro with the β-tubulin 5309–318 peptide, a positive (EBV) control peptide or a negative (HIV) control peptide, followed by measurement of IFN-γ production in response to the appropriate peptide-pulsed cells. The results showed that this peptide induced peptide-specific CTL activity in the PBMCs from 5 of the 9 patients tested (Table II), but not in any of the 3 HDs tested (data not shown). Of the 9 patients, 6 showed CTL activity reactive to the EBV-derived peptide (a positive control), while none showed CTL activity reactive the HIV-derived peptide (a negative control) (Table II).
Table II.
Interferon-γ production in peptide-stimulated prostate cancer patient peripheral blood mononuclear cells.
| Patient no. | β-tubulin 5-309 | Positive (EBV) | Negative (HIV) |
|---|---|---|---|
| 1 | 86 | 51 | NS |
| 2 | ns | 242 | NS |
| 3 | ns | 539 | NS |
| 4 | 50 | ns | NS |
| 5 | 50 | 1,501 | NS |
| 6 | 272 | ns | NS |
| 7 | 178 | ns | NS |
| 8 | ns | 457 | NS |
| 9 | ns | 50 | NS |
EBV, Epstein-Barr virus; HIV, immunodeficient virus; NS, not significant.
Cytotoxicity assay
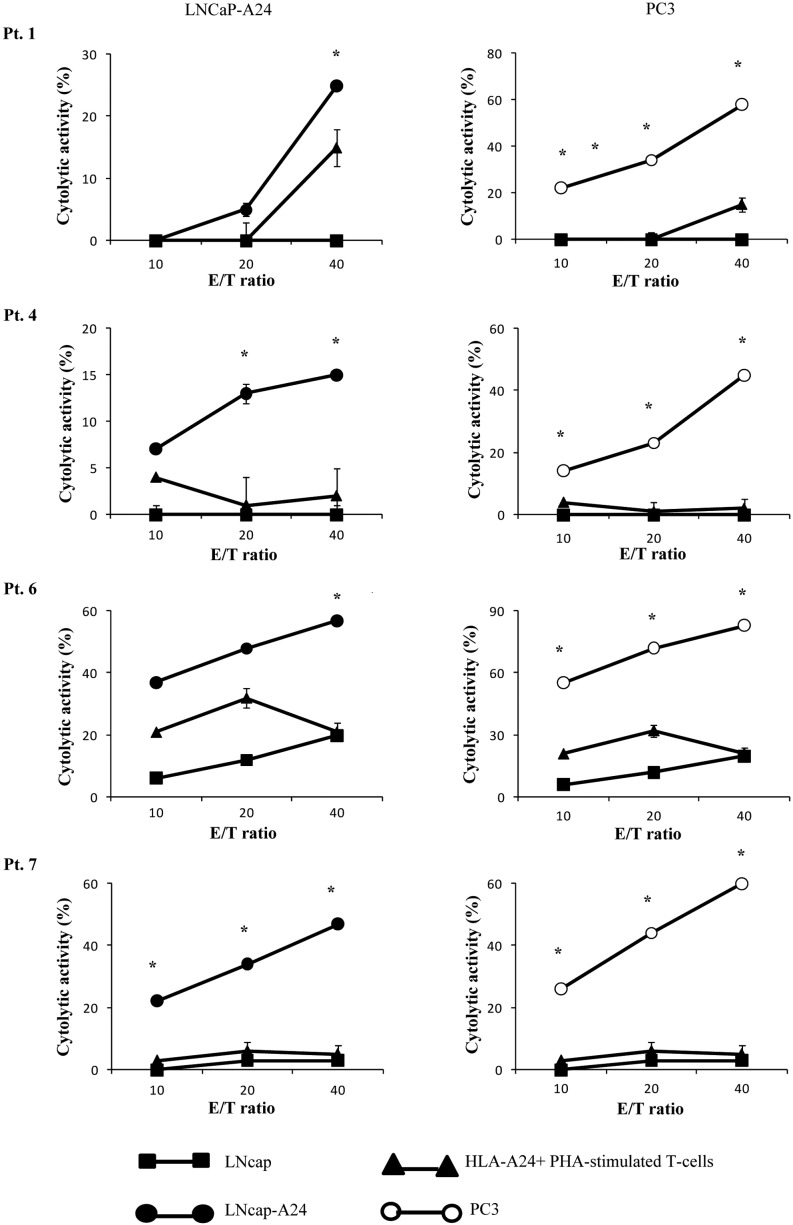
We then determined whether or not the CTLs induced by in vitro stimulation with the β-tubulin 5309–318 peptide showed cytotoxicity against prostate cancer cells in PBMCs from 4 of the 5 patients (pt. 1, 4, 6, 7) who exhibited a positive CTL response as indicated by the IFN-release assay (Table II). The peptide-stimulated PBMCs from all 4 of the patients exhibited significant levels of cytotoxicity against both PC3 and LNCaP-A24 cells, but not against LNCaP cells or HLA-A24+ PHA-stimulated T-cell blasts as indicated by the 51Cr release assay (Fig. 3). By contrast, as shown in Table II, PBMCs from none of the 3 patients (pt. 3, 8 and 9) whose samples responded negatively in the IFN-γ assay showed detectable levels of CTL activity with this assay (data not shown). The PBMCs from the remaining 3 patients were not eligible for the assay.
Figure 3.
The peptide-stimulated PBMCs exhibited significant levels of cytotoxicity against both PC3 and LNCaP-A24 cells. Peptide-stimulated PBMCs from the 4 patients (pt. 1, 4, 6 and 7 of Table II) were tested for their cytotoxicity towards three different targets by a 6-h 51Cr-release assay. Phytohemagglutinin (PHA)-stimulated T-cell blasts were derived from PBMCs of HLA-A24+ HDs. *Statistically significant at P<0.05.
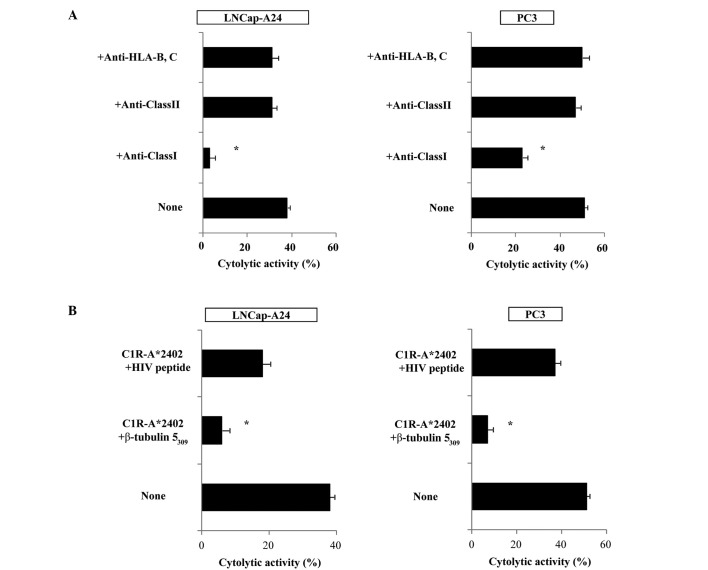
We then attempted to identify the cells responsible for the cytotoxicity of β-tubulin 5309–318 peptide-stimulated PBMCs. Purified CD8+ T cells were used in the following experiments. The levels of cytotoxicity by CD8+ T cells purified from the peptide-stimulated PBMCs against PC3, as well as LNCaP tumor cells, were significantly decreased by the addition of anti-HLA class I mAb (W6/32), but not by the addition of either anti-HLA class II (HLA-DR) or anti-HLA-B,C (B1–23, IgG2a) mAbs. Representative cases are shown in Fig. 4A. In addition, cytotoxicity was significantly inhibited by the addition of a corresponding peptide-pulsed unlabeled C1R-A*2402, but not by the addition of an HIV peptide-pulsed unlabeled C1R-A*2402. Representative cases are shown in Fig. 4B. These results indicate that CTL activity was determined to be specific to this peptide, and was mediated by CD8+ T cells in an HLA-class I-restricted manner.
Figure 4.
Inhibition assay of peptide-stimulated PBMCs with Abs. (A) Peptide-stimulated PBMCs from 4 patients (patient no. 1, 4, 6 and 7 of Table II) were tested for their cytotoxicity against LNCaP-A24 and PC3 cells in the presence of the indicated monoclonal antibodies. The results of patient no. 1 are presented, and similar results were obtained in the remaining 3 patients (data not shown). (B) Peptide-stimulated PBMCs from 4 patients (patient no. 1, 4, 6 and 7 of Table II) were tested for their cytotoxicity against LNCaP-A24 and PC3 cells in the presence of unlabeled C1R-A*2402 cells, which were pre-loaded with either the corresponding peptide or the HIV peptide. The results of patient no. 6 are presented, and similar results were obtained in the remaining 3 patients (data not shown). *Statistically significant at P<0.05.
Discussion
The binding score of the β-tubulin 5309–318 peptide (RYLTVAAVFR) to HLA-A*2402 was lower than it was to HLA-A*3101 and -A*3302, but higher than it was to HLA-A3 or -A*1101, based on information from the BioInformatics and Molecular Analysis Section (BIMAS) website (2). In this study, we showed that the β-tubulin 5309–318 peptide, a previously reported peptide capable of inducing HLA-A3 supertype-restricted CTLs (7), did bind to HLA-A*2402, one of the dominant HLA-A types in Asian populations, including the Japanese. HLA-A24 binding peptides are characterized by the presence of Y or F residues at amino acid position 2; and of L, F, I or W residues at position 9 (12,17). These findings suggest that the peptide binds to HLA-A*2402 molecules. On the other hand, no binding of this peptide to HLA-A0201-transfected cells was expected, since its binding score to HLA-A*0201 on BIMAS is zero. Indeed, β-tubulin 5309–318 showed no binding activity to the HLA-A*0201, -A*0206 or -A*0207 molecules.
We previously reported that the use of the CTL assay with a 14-day incubation period and with stimulation administered five times did not detect CTL precursors by de novo sensitization to an epitope peptide (18). The sensitivity of this employed CTL assay was 1 out of 3,000 to 1 out of 5,000 CTL precursors. Thus, it is likely that the immune response against the β-tubulin 5309–318 peptide is relatively restricted in cancer patients whose tumors overexpress β-tubulin 5 antigen (19–21). This is primarily because naïve T cells from HDs do not induce CTL activity as readily as those from prostate cancer patients. Indeed, PBMCs from any of the three HDs homozygous for the HLA-A24 allele showed CTL activity (data not shown). By contrast, it was relatively easy to induce β-tubulin 5309–318-specific CTLs in prostate cancer patients, and such CTLs were detectable in 5 of the 9 patients tested. CTL precursors were not detectable in the remaining 4 patients, which may have been due, in part, to the immune suppression associated with prostate cancer. Alternatively, the employed CTL assays may not have been sufficiently sensitive, based on the finding that the CTL precursors to the EBV-derived peptide, which were used as a positive control, were also detectable in some of the patients tested.
Significantly higher fractions of β-tubulin class II and V mRNA were reported as compared to the other isotypes in lung tumor samples (22). In regard to biological function, β-tubulin 5, which is located in the cytoplasm and one of the structural subunit of microtubules, is important for cell proliferation (19). Tubulin is one of the major target molecules of anticancer drugs such as docetaxel, based on the fact that the expression of tubulin is reported more often in cancer cells than in normal cells (19–21).
Together with the results presented herein, these findings suggest that the β-tubulin 5 peptide has potential utility as a cancer vaccine, both in prostate and other types of cancer.
Acknowledgments
This study was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (no. 12213134 to K.I., no. 21591652 to S.S. and no. 18591449 to M.N.), and by the Toshi-Area Program (to K.I., S.S. and M.N.).
Abbreviations:
- CTL,
cytotoxic T lymphocyte;
- DMSO,
dimethyl sulfoxide;
- EBV,
Epstein-Barr virus;
- FBS,
fetal bovine serum;
- HDs,
healthy donors;
- HIV,
immunodeficient virus;
- IFN-γ,
interferon-γ;
- PAP,
prostate acid phosphatase;
- PBMCs,
peripheral blood mononuclear cells;
- PHA,
phytohemagglutinin
References
- 1.Noguchi N, Kakuma T, Uemura H, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010;59:1001–1009. doi: 10.1007/s00262-010-0822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terasaki Y, Shichijo S, Nui Y, et al. An HLA-A3-binding prostate acid phosphatase-derived peptide can induce CTLs restricted to HLA-A2 and -A24 alleles. Cancer Immunol Immunother. 2009;58:1877–1885. doi: 10.1007/s00262-009-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohamed RE, Naito M, Terasaki Y, et al. Capability of SART3109–118 peptide to induce cytotoxic T-lymphocytes from prostate cancer patients with HLA class I-A11, -A31 and -A33 alleles. Int J Oncol. 2009;34:529–536. [PubMed] [Google Scholar]
- 4.Niu Y, Komatsu N, Komohara K, et al. A peptide derived from hepatitis C virus (HCV) core protein inducing cellular responses in patients with HCV with various HLA class IA alleles. J Med Virol. 2009;81:1232–1240. doi: 10.1002/jmv.21518. [DOI] [PubMed] [Google Scholar]
- 5.Niu Y, Terasaki Y, Komatsu N, Noguchi M, Shichijo S, Itoh K. Identification of peptides applicable as vaccines for HLA-A26-positive cancer patients. Cancer Sci. 2009;100:2167–2174. doi: 10.1111/j.1349-7006.2009.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsueda S, Takedatsu H, Yao A, et al. Identification of peptide vaccine candidates for prostate cancer patients with HLA-A3 supertype alleles. Clin Cancer Res. 2005;11:6933–6943. doi: 10.1158/1078-0432.CCR-05-0682. [DOI] [PubMed] [Google Scholar]
- 7.Takedatsu H, Shichijo S, Katagiri K, Sawamizu H, Sata M, Itoh K. Identification of peptide vaccine candidates sharing among HLA-A3+, -A11+, -A31+, and -A33+ cancer patients. Clin Cancer Res. 2004;10:1112–1120. doi: 10.1158/1078-0432.ccr-0797-3. [DOI] [PubMed] [Google Scholar]
- 8.Minami T, Matsueda S, Takedatsu H, et al. Identification of SART3-derived peptides having the potential to induce cancer-reactive cytotoxic T lymphocytes from prostate cancer patients with HLA-A3 supertype alleles. Cancer Immunol Immunother. 2007;56:689–698. doi: 10.1007/s00262-006-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsueda S, Takedatsu H, Sasada T, et al. New peptide vaccine candidates for epithelial cancer patients with HLA-A3 supertype alleles. J Immunother. 2007;30:274–281. doi: 10.1097/01.cji.0000211340.88835.e7. [DOI] [PubMed] [Google Scholar]
- 10.Naito M, Komohara Y, Ishihara Y, et al. Identification of Lck-derived peptides applicable to anti-cancer vaccine for patients with human leukocyte antigen-A3 supertype alleles. Br J Cancer. 2007;97:1648–1654. doi: 10.1038/sj.bjc.6604071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao A, Harada M, Matsueda S, et al. Identification of parathyroid hormone-related protein-derived peptides immunogenic in human histocompatibility leukocyte antigen-A24+ prostate cancer patients. Br J Cancer. 2004;91:287–296. doi: 10.1038/sj.bjc.6601960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torikai H, Akatsuka Y, Miyauchi H, et al. The HLA-A*0201-restricted minor histocompatibility antigen HA-1H peptide can also be presented by another HLA-A2 subtype, A*0206. Bone Marrow Transplant. 2007;40:165–174. doi: 10.1038/sj.bmt.1705689. [DOI] [PubMed] [Google Scholar]
- 13.Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–1607. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SP, Tierney RJ, Thomas WA, Brooks JM, Rickinson AB. Conserved CTL epitopes within EBV latent membrane protein 2. A potential target for CTL-based tumor therapy. J Immunol. 1997;158:3325–3334. [PubMed] [Google Scholar]
- 15.Parker KC, Bednarek MA, Hull LK, et al. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 16.Ikeda-Moore Y, Tomiyama H, Miwa K, et al. Identification and characterization of multiple HLA-A24-restricted HIV-1 CTL epitopes: strong epitopes are derived from V regions of HIV-1. J Immunol. 1997;159:6242–6252. [PubMed] [Google Scholar]
- 17.Rammensee HG, Flak K, Rotzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 18.Hida N, Maeda Y, Katagiri K, Takasu H, Harada M, Itoh K. A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother. 2002;51:219–228. doi: 10.1007/s00262-002-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Tajima O, Hashiba H, Nose K, Kuroki T. Elevated expression of secondary, but not early, responding genes to phorbol ester tumor promoters in papillomas and carcinomas of mouse skin. Mol Carcinog. 1990;3:302–308. doi: 10.1002/mc.2940030511. [DOI] [PubMed] [Google Scholar]
- 21.Kato K, Ito H, Inaguma Y, Okamoto K, Saga S. Synthesis and accumulation of αB crystallin in C6 glioma cells is induced by agents that promote the disassembly of microtubules. J Biol Chem. 1996;271:26989–26994. doi: 10.1074/jbc.271.43.26989. [DOI] [PubMed] [Google Scholar]
- 22.Cucchiarelli V, Hiser L, Smith H, et al. Beta-tubulin isotype classes II and V expression patterns in non-small cell lung carcinomas. Cell Motil Cytoskeleton. 2008;65:675–685. doi: 10.1002/cm.20297. [DOI] [PubMed] [Google Scholar]