Abstract
We previously reported that prostaglandin D2 (PGD2) stimulates heat shock protein 27 (HSP27) induction through p44/p42 mitogen-activated protein (MAP) kinase, p38 MAP kinase and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in osteoblast-like MC3T3-E1 cells. In addition, we recently showed that PGD2 activates Rho-kinase, resulting in the regulation of interleukin-6 synthesis via activation of p38 MAP kinase but not p44/p42 MAP kinase in these cells. In the present study, in order to investigate whether Rho-kinase is involved in the PGD2-stimulated HSP27 induction in MC3T3-E1 cells, we examined the effects of Rho-kinase inhibitors on HSP27 induction. Y27632 and fasudil, Rho-kinase inhibitors, markedly suppressed the HSP27 induction stimulated by PGD2 in a dose-dependent manner without affecting levels of HSP70 in the presence of PGD2. Immunofluorescence microscopy studies also revealed that Y27632 and fasudil markedly suppressed the induction of HSP27. Y27632 and fasudil attenuated the PGD2-induced phosphorylation levels of SAPK/JNK. In conclusion, Rho-kinase inhibitors regulate PGD2-stimulated HSP27 induction via activation of both SAPK/JNK and p38 MAP kinase in osteoblasts.
Keywords: Rho-kinase, prostaglandin D2, heat shock protein 27, osteoblast
Introduction
Heat shock proteins (HSPs) are induced when cells are exposed to biological stress such as heat stress and chemical stress (1). It is generally recognized that high-molecular-weight HSPs such as HSP70, HSP90 and HSP110 act as molecular chaperones in protein folding, oligomerization and translocation in cells (2). On the other hand, functions of the low-molecular-weight HSPs with molecular masses from 10 to 30 kDa, such as HSP27, αB-crystallin and HSP20 are less understood than those of the high-molecular-weight HSPs (2). The low-molecular-weight HSPs share high homology in amino acid sequences ‘α-crystallin domain’ (2). It has been shown that HSP27 activity is regulated by post-translational modification such as phosphorylation (3). Under unstimulated conditions, HSP27 exists as a high-molecular-weight aggregated form and is rapidly dissociated as a result of phosphorylation (4,5). Although the phosphorylation-elicited dissociation from the aggregated form reportedly correlates with the loss of molecular chaperone activity (4,5), the physiological significance of phosphorylated HSP27 remains controversial.
Bone metabolism is regulated by two functional cells, osteoblasts and osteoclasts, responsible for bone formation and bone resorption, respectively (6). The maintenance of bone structures and bone remodeling results from the coupling process; bone resorption by activated osteoclasts with subsequent deposition of new matrix by osteoblasts. In osteoblasts, it has been shown that the down-regulation of proliferation is accompanied by a transient increase in HSP27 mRNA expression (7). In addition, heat-stimulated induction of HSP27 is reportedly facilitated by estrogen (8). These findings lead us to speculate that HSP27 plays an important role in osteoblast cell functions. However, the exact role of HSP27 in osteoblasts has not yet been clarified.
Prostaglandins (PGs) act as autacoids in osteoblasts (9,10). Among PGs, PGD2 has been implicated in the control of osteoblast cell function and bone anabolism (10). It has been shown that PGD2 stimulates collagen synthesis during calcification of osteoblasts (11). In addition, PGD2 produced by osteoblasts reportedly modulates expression of osteoprotegerin and RANKL in osteoblasts (12). In our previous study (13), we reported that PGD2 stimulates interleukin (IL)-6 synthesis via Ca2+ mobilization in osteoblast-like MC3T3-E1 cells. In addition, we showed that PGD2 stimulates the induction of HSP27 via p44/p42 mitogen-activated protein (MAP) kinase, p38 MAP kinase and SAPK/JNK among the MAP kinase superfamily (14) in MC3T3-E1 cells (15,16). However, the exact mechanism behind PGD2-stimulated HSP27 induction in osteoblasts remains to be elucidated.
It is well known that Rho and the downstream effector, Rho-associated kinase (Rho-kinase), play important roles in a variety of cellular functions such as smooth muscle contraction and cell motility (17–19). With regard to osteoblasts, it has been reported that Rho and p38 MAP kinase are involved in the endothelin-1-induced expression of prostaglandin endoperoxide G/H synthase mRNA in osteoblasts (20). In addition, it has been shown that the Rho/Rho-kinase pathway stimulates osteoblast proliferation whereas it has an inhibitory role in osteoblast differentiation (21). In a recent study (22), we demonstrated that Rho-kinase functions at the point of p38 MAP kinase but not p44/p42 MAP kinase activation in the PGD2-stimulated synthesis of IL-6 in osteoblast-like MC3T3-E1 cells. In the present study, we investigated the involvement of Rho-kinase in the PGD2-stimulated HSP27 induction in these cells.
Materials and methods
Materials
PGD2 and HSP70 antibodies were obtained from R&D Systems, Inc. (Minneapolis, MN). Y27632 was obtained from Calbiochem-Novabiochem Co. (La Jolla, CA). Hydroxyfasudil (fasudil) was purchased from Sigma (St. Louis, MO). Phospho-specific SAPK/JNK antibodies and SAPK/JNK antibodies were purchased from Cell Signaling, Inc. (Beverly, MA). Glyceraldehyde 3 phosphate dehydrogenase (GAPDH) antibodies and HSP27 antibodies for Western blot analysis, and HSP27 antibodies for immunofluorescence microscopy studies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Alexa Fluor 488®-conjugated calf anti-goat antibodies and Alexa Fluor 555® phalloidin were obtained from Invitrogen Corporation, Inc. (Carlsbad, CA). DAPI was obtained from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). The ECL Western blot detection system was purchased from GE Healthcare UK Ltd. (Buckinghamshire, UK). Other materials and chemicals were obtained from commercial sources. Y27632 was dissolved in dimethyl sulfoxide. The maximum concentration of dimethyl sulfoxide was 0.1%, which did not affect the assay for Western blot analysis or the immunofluorescence microscopy study.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from newborn mouse calvaria (23) were maintained as previously described (24). Briefly, the cells were cultured in α-minimum essential medium (α-MEM) containing 10% fetal calf serum (FCS) at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were seeded into 90-mm diameter dishes (20×104/dish) for Western blot analysis or 35-mm diameter glass-bottom dishes (3×104/dish) for immunofluorescence microscopy study. After 5 days, the medium was exchanged with α-MEM containing 0.3% FCS. The cells were then used for experiments after 48 h.
Western blot analysis
Western blot analysis was performed as described previously (25). In brief, the cultured cells were pretreated with various doses of Y27632 or fasudil for 60 min and then stimulated by PGD2 in the presence of the inhibitors in α-MEM containing 0.3% FCS for the indicated periods. The cells were washed twice with phosphate-buffered saline and then lysed, homogenized and sonicated in a lysis buffer containing 62.5 mM Tris/HCl (pH 6.8), 3% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10% glycerol. SDS-polyacrylamide gel electrophoresis (PAGE) was performed according to Laemmli in 10% polyacrylamide gel (26). The protein was fractionated and transferred onto an Immun-Blot PVDF membrane (Bio-Rad, Hercules, CA). Membranes were blocked with 5% fat-free dry milk in Tris-buffered saline-Tween-20 (TBS-T; 20 mM Tris/HCl, pH 7.6, 137 mM NaCl, 0.1% Tween-20) for 2 h before incubation with the primary antibodies. Western blot analysis was performed using HSP27, HSP70 and GAPDH antibodies with peroxidase-labeled antibodies raised in goat anti-rabbit IgG which were used as second antibodies. Peroxidase activity on PVDF membranes was visualized on X-ray film by means of the ECL Western blot detection system. All of the Western blot analyses were repeated at least three times in independent experiments.
Immunofluorescence microscopy study
The cultured cells were pretreated with various doses of Y27632 or fasudil at the indicated concentrations for 1 h and then exposed to PGD2 (10 μM) or vehicle for 12 h. They were then fixed with 3% paraformaldehyde for 10 min on ice and exposed to 0.1% Triton X-100 for 10 min to permeabilize the cell membrane. They were then exposed to anti-HSP27 antibodies (1:100 dilution) in the presence of 1% BSA for 1 h, followed by exposure to Alexa Fluor 488-conjugated calf anti-goat IgG antibodies (1:500) for 1 h. Finally, they were exposed to Alexa Fluor 555 phalloidin and DAPI for 20 min. The cells were examined by fluorescence microscopy (Biorevo, BZ-9000; Keyence, Tokyo, Japan) according to the manufacturer’s protocol.
Statistical analysis
All data are presented as the mean ± SEM of triplicate determinations. The data were analyzed by ANOVA followed by Bonferroni method for multiple comparisons between pairs, and p<0.05 was considered significant.
Results
Effects of Y27632 or fasudil on HSP27 induction stimulated by PGD2 in MC3T3-E1 cells
We previously reported that PGD2 stimulated HSP27 induction in osteoblast-like MC3T3-E1 cells (15). In addition, we showed that Rho kinase was activated by PGD2 in these cells (22). In the present study, we examined the effect of Y27632, a specific inhibitor of Rho-kinase (19), on HSP27 induction stimulated by PGD2. Y27632 significantly suppressed the PGD2-stimulated HSP27 induction in a dose-dependent manner in the range between 1 and 10 μM (Fig. 1). As well, we also found that fasudil dose dependently reduced the PGD2-stimulated HSP27 induction (Fig. 2). We previously demonstrated that PGD2 did not affect the levels of HSP70, a high-molecular-weight HSP, in MC3T3-E1 cells (15,16). In the present study, we found that Y27632 or fasudil had little effect on the levels of HSP70 in the presence of PGD2 (Figs. 1 and 2).
Figure 1.
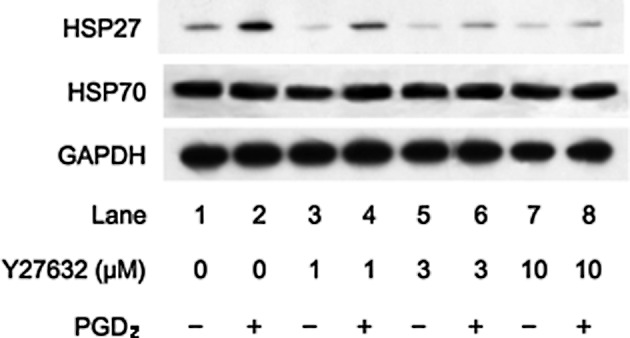
Effect of Y27632 on PGD2-stimulated HSP27 induction in MC3T3-E1 cells. The cultured cells were pretreated with various doses of Y27632 for 60 min and were then stimulated with 10 μM PGD2 or vehicle for 12 h. Extracts of cells were subjected to SDS-PAGE with subsequent Western blot analysis with antibodies against HSP27, HSP70 and GAPDH.
Figure 2.
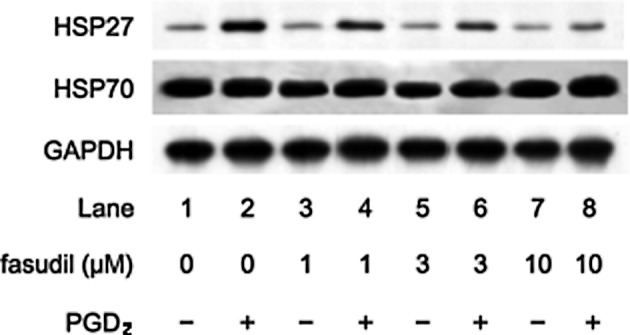
Effect of fasudil on PGD2-stimulated HSP27 induction in MC3T3-E1 cells. The cultured cells were pretreated with various doses of fasudil for 60 min, and were then stimulated with 10 μM PGD2 or vehicle for 12 h. Extracts of cells were subjected to SDS-PAGE with subsequent Western blot analysis with antibodies against HSP27, HSP70 and GAPDH.
Effects of Y27632 and fasudil on HSP27 induction stimulated by PGD2 in MC3T3-E1 cells by immunofluorescence microscopy study
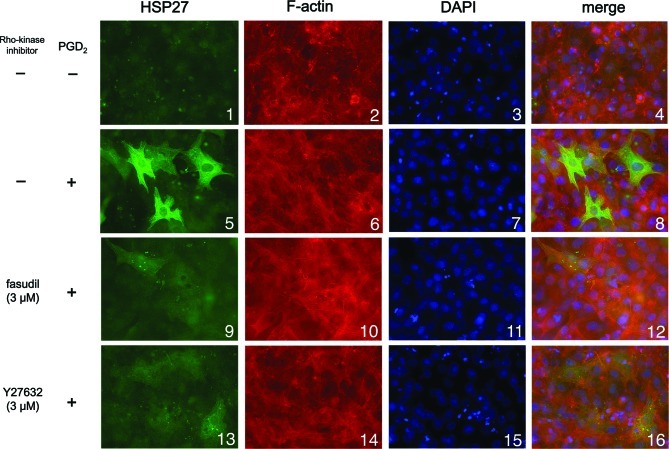
We next examined the effect of Y27632 or fasudil on HSP27 induction stimulated by PGD2 using immunofluorescence microscopy. We found that PGD2 markedly stimulated HSP27 induction (green signal) in the cytosol of the osteoblast-like MC3T3-E1 cells (Fig. 3; panel 5 in comparison with panel 1), consistent with the results shown in Figs. 1 and 2. Either Y27632 (3 μM) or fasudil (3 μM) markedly suppressed HSP27 induction stimulated by PGD2 (Fig. 3; panels 9 and 13 in comparison with panel 5), also consistent with the results shown in Figs. 1 and 2.
Figure 3.
Immunofluorescence microscopy studies of the expression of HSP27. Cultured cells were pretreated with 3 μM Y27632 (panels 13–16), 3 μM fasudil (panels 9–12) or vehicle (panels 1–8) for 60 min, followed by stimulation with 10 μM PGD2 (panel 5–16) or vehicle (panel 1–4) for 12 h. The extracts of cells were fixed with paraformaldehyde. After permeabilization of the cells with 0.1% Triton X-100, the cells were exposed to HSP27 antibodies (1:100 dilution) and were then treated with Alexa Fluor 488-conjugated anti-goat secondary antibodies (green signal). They were then exposed to Alexa Fluor 555 phalloidin for F-actin (red signal) and DAPI for the nucleus (blue signal) for 20 min and examined by confocal microscopy.
Effects of Y27632 or fasudil on the FGF-2-induced phosphorylation of SAPK/JNK in MC3T3-E1 cells
We previously reported that PGD2 stimulates HSP27 induction via p44/p42 MAP kinase, p38 MAP kinase and SAPK/JNK in osteoblast-like MC3T3-E1 cells (15,16). We investigated whether the effect of Rho-kinase on the PGD2-stimulated HSP27 induction is dependent on the activation of these three MAP kinases in these cells. In our previous study (22), we showed that Rho-kinase activated by PGD2 inhibits p38 MAP kinase phosphorylation but not p44/p42 MAP kinase in MC3T3-E1 cells. Therefore, we next examined the effects of Rho-kinase inhibitors on the PGD2-induced phosphorylation of SAPK/JNK. Y27632 markedly attenuated the PGD2-induced phosphorylation levels of SAPK/JNK (Fig. 4). In addition, fasudil also reduced the phosphorylation levels of SAPK/JNK by PGD2 (Fig. 5).
Figure 4.
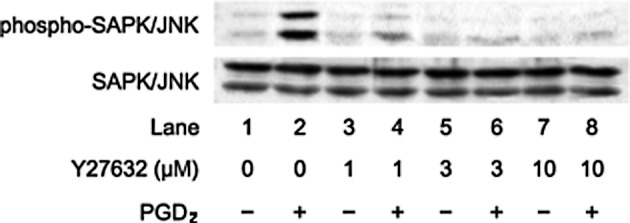
Effect of Y27632 on the PGD2-induced phosphorylation of SAPK/JNK in MC3T3-E1 cells. The cultured cells were pretreated with various doses of Y27632 for 60 min and were then stimulated with 10 μM PGD2 or vehicle for 20 min. Extracts of cells were subjected to SDS-PAGE with subsequent Western blot analysis with antibodies against phosphorylated SAPK/JNK and SAPK/JNK. Similar results were obtained with two additional and different cell preparations.
Figure 5.
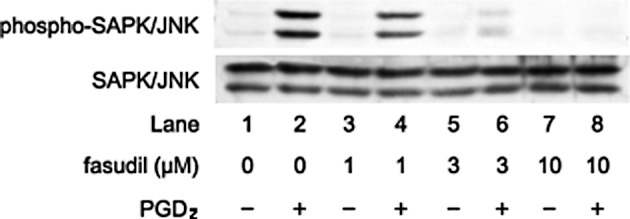
Effect of fasudil on the PGD2-induced phosphorylation of SAPK/JNK in MC3T3-E1 cells. The cultured cells were pretreated with various doses of fasudil for 60 min and were then stimulated with 10 μM PGD2 or vehicle for 20 min. Extracts of cells were subjected to SDS-PAGE with subsequent Western blot analysis with antibodies against phosphorylated SAPK/JNK and SAPK/JNK. Similar results were obtained with two additional and different cell preparations.
Discussion
We previously demonstrated that PGD2 activates Rho-kinase in osteoblast-like MC3T3-E1 cells (22). In the present study, we showed that Y27632 and fasudil markedly reduced the PGD2-stimulated HSP27 induction, a low-molecular-weight HSP, in these cells. Additionally, we also found that Y27632 and fasudil markedly reduced PGD2-stimulated HSP27 induction in immunofluorescence microscopy studies. Based on our findings, it is probable that Rho-kinase is involved in PGD2-stimulated HSP27 induction in osteoblast-like MC3T3-E1 cells.
In our previous studies (15,16), PGD2 stimulated the induction of HSP27 via p44/p42 MAP kinase, p38 MAP kinase and SAPK/JNK among the MAP kinase superfamily (14) in osteoblast-like MC3T3-E1 cells. It is well known that three MAP kinases are the central elements used by mammalian cells to transduce diverse messages (14). Furthermore, we showed that Rho-kinase functions at a point upstream from p38 MAP kinase in PGD2-induced IL-6 synthesis in MC3T3-E1 cells and that Rho-kinase inhibitors failed to affect the PGD2-stimulated phosphorylation of p44/p42 MAP kinase (22). Therefore, it seems unlikely that Rho-kinase affects the PGD2-stimulated HSP27 induction through the modulation of p44/p42 MAP kinase in MC3T3-E1 cells. In the present study, we showed that Y27632 and fasudil markedly attenuated the PGD2-induced phosphorylated levels of SAPK/JNK. Taking our findings into account as a whole, it is most likely that Rho-kinase functions at a point upstream from SAPK/JNK and p38 MAP kinase among the MAP kinase superfamily in the PGD2-stimulated HSP27 induction in osteoblast-like MC3T3-E1 cells.
Rho-kinase is well known to play an important role in a variety of cellular functions, particularly vascular smooth muscle contraction (17–19). In bone metabolism, it has been reported that the activation of Rho-kinase suppresses the differentiation of osteoblasts and induces their proliferation (21). Our present results reveal that Rho-kinase stimulated by PGD2 acts as a positive regulator in HSP27 induction in osteoblasts. It is generally recognized that most HSPs including HSP27 function mainly as molecular chaperones in protein folding, oligomerization and translocation (2). Although the physiological significance of HSP27 in osteoblasts has not yet been clarified, our finding that SAPK/JNK and p38 MAP kinase, but not p44/p42 MAP kinase, are regulated by Rho-kinase suggests the importance of the fine tuning of MAP kinase-mediated HSP27 induction stimulated by PGD2, which is significantly correlated with IL-6 synthesis (22), in bone remodeling. Our present findings regarding the involvement of Rho-kinase in the induction of HSP27 in osteoblasts present novel aspects of Rho-kinase and/or HSP27 as therapeutic targets for metabolic or inflammatory bone diseases such as osteoporosis and rheumatic arthritis. However, the exact role of Rho-kinase in osteoblasts remains to be clarified. Further investigations are necessary to elucidate the exact roles of Rho-kinase in bone metabolism.
In conclusion, our results strongly suggest that Rho-kinase inhibitors suppress PGD2-stimulated HSP27 induction via the activation of both SAPK/JNK and p38 MAP kinase in osteoblasts.
Acknowledgments
We are grateful to Yoko Kawamura for her skillful technical assistance. This investigation was supported in part by a Grant-in-Aid for Scientific Research (19591042) from the Ministry of Education, Science, Sports and Culture of Japan, the Foundation for Growth Science, the Research Grants for Longevity Sciences (21A-4), Research on Proteomics and Research on Longevity Sciences from the Ministry of Health, Labour and Welfare of Japan.
References
- 1.Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 3.Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 4.Kato K, Hasegawa K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem. 1994;269:11274–11278. [PubMed] [Google Scholar]
- 5.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 6.Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66:855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- 7.Shakoori AR, Oberdorf AM, Owen TA, Weber LA, Hickey E, Stein JL, Lian JB, Stein GS. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1992;48:277–287. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- 8.Cooper LF, Uoshima K. Differential estrogenic regulation of small M(r) heat shock protein expression in osteoblasts. J Biol Chem. 1994;269:7869–7873. [PubMed] [Google Scholar]
- 9.Morgan EF, Barnes GL, Einhorn TA. The bone organ system: Form and function. In: Marcus R, Feldman D, Nelson D, Rosen CJ, editors. Osteoporosis. 3rd edition. Elsevier Press; Boston: 2008. pp. 3–25. [Google Scholar]
- 10.Hikiji H, Takato T, Shimizu T, Ishii S. The roles of prostanoids, leukotrienes, and platelet-activating factor in bone metabolism and disease. Prog Lipid Res. 2008;47:107–126. doi: 10.1016/j.plipres.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Tasaki Y, Takamori R, Koshihara Y. Prostaglandin D2 metabolite stimulates collagen synthesis by human osteoblasts during calcification. Prostaglandins. 1991;41:303–313. doi: 10.1016/0090-6980(91)90001-v. [DOI] [PubMed] [Google Scholar]
- 12.Gallant MA, Samadfam R, Hackett JA, Antoniou J, Parent JL, de Brum-Fernandes AJ. Production of prostaglandin D(2) by human osteoblasts and modulation of osteoprotegerin, RANKL, and cellular migration by DP and CRTH2 receptors. J Bone Miner Res. 2005;20:672–681. doi: 10.1359/JBMR.041211. [DOI] [PubMed] [Google Scholar]
- 13.Tokuda H, Kozawa O, Harada A, Uematsu T. Prostaglandin D2 induces interleukin-6 synthesis via Ca2+ mobilization in osteoblasts: regulation of protein kinase C. Prostaglandins Leukot Essent Fatty Acids. 1999;61:189–194. doi: 10.1054/plef.1999.0089. [DOI] [PubMed] [Google Scholar]
- 14.Widmann C, Gibson S, Jarpe MB, Johnson GJ. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 15.Kozawa O, Otsuka T, Hatakeyama D, Niwa M, Matsuno H, Ito H, Kato K, Matsui N, Uematsu T. Mechanism of prostaglandin D2-stimulated heat shock protein 27 induction in osteoblasts. Cell Signal. 2001;13:535–541. doi: 10.1016/s0898-6568(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Niwa M, Ishisaki A, Hirade K, Ito H, Shimizu K, Kato K, Kozawa O. Methotrexate enhances prostaglandin D2-stimulated heat shock protein 27 induction in osteoblasts. Prostaglandins Leukot Essent Fatty Acids. 2004;71:351–362. doi: 10.1016/j.plefa.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 18.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 19.Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Windischhofer W, Zach D, Fauler G, Raspotnig G, Kofeler H, Leis HJ. Involvement of Rho and p38 MAPK in endothelin-1-induced expression of PGHS-2 mRNA in osteoblast-like cells. J Bone Miner Res. 2002;17:1774–1784. doi: 10.1359/jbmr.2002.17.10.1774. [DOI] [PubMed] [Google Scholar]
- 21.Harmey D, Stenbeck G, Nobes CD, Lax AJ, Grigoriadis AE. Regulation of osteoblast differentiation by Pasteurella multocida toxin (PMT): a role for Rho GTPase in bone formation. J Bone Miner Res. 2004;19:661–670. doi: 10.1359/JBMR.040105. [DOI] [PubMed] [Google Scholar]
- 22.Tokuda H, Takai S, Matsushima-Nishiwaki R, Hanai Y, Adachi S, Minamitani C, Mizutani J, Otsuka T, Kozawa O. Function of Rho-kinase in prostaglandin D2-induced interleukin-6 synthesis in osteoblasts. Prostaglandins Leukot Essent Fatty Acids. 2008;79:41–46. doi: 10.1016/j.plefa.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Sudo H, Kodama H, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1993;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozawa O, Suzuki A, Tokuda H, Uematsu T. Prostaglandin F2α stimulates interleukin-6 synthesis via activation of PKC in osteoblast-like cells. Am J Physiol. 1997;272:E208–E211. doi: 10.1152/ajpendo.1997.272.2.E208. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Ito H, Hasegawa K, Inaguma Y, Kozawa O, Asano T. Modulation of the stress-induced synthesis of hsp27 and αB-crystallin by cyclic AMP in C6 rat glioma cells. J Neurochem. 1996;66:946–950. doi: 10.1046/j.1471-4159.1996.66030946.x. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]