Abstract
The aim of this study was to compare the sensitivity of the serological level of anti-p53 antibodies in breast cancer patients and to correlate its expression level with patient age, histological stage and grade of tumor differentiation. Total p53 protein expression (mutant and wild-type) was also determined in the breast cancer tissues using immunohistochemistry (IHC). The serological levels of mutant p53 expression were found to be age-dependent, reaching the highest level at 50 years of age. Faint or low detection was observed in patients ≤30 years of age. Anti-p53-antibodies were detected in patients ≤40 and ≥61 years of age. The serological levels of mutant p53 protein were highly detected in all stages of breast cancer, including the early stages. However, anti-p53 antibodies reached a high level of detection only in stage III breast carcinomas. No expression was found in patients with benign breast disease. The detection of p53 mutations was dependent on the grade of tumor differentiation, achieving the highest level in the poorly differentiated breast carcinomas. Results from IHC were highly correlated with serological p53 mutational analysis. Our findings indicate that mutant p53 in serum is a promising novel parameter for the evaluation of cellular biology and the prognosis of breast cancer from its early stages using blood samples. Anti-p53 antibodies were demonstrated to be less sensitive in this study. It is also possible to use the expression of mutant p53 protein as a molecular marker to differentiate benign breast disease from breast carcinoma prior to surgery.
Keywords: breast cancer, p53 mutational analysis, serological detection of p53 mutations
Introduction
Among women, breast cancer is the leading cause of cancer-related mortality and the most common type of cancer worldwide (1–3). Breast cancer is the second most common cancer in the world, and its incidence is increasing, with a total of 1,050,100 cases in 2002 compared to 572,100 in 1980. Worldwide, the ratio of mortality to incidence is approximately 36% (1–3). Breast cancer causes 370,000 annual deaths, representing 13.9% of cancer-related deaths in women. It is the most prevalent cancer in the world today, with incidence rates highest in industrialized countries. While researchers are rigorously searching for the etiology of the disease, a more sensitive and early detection employing novel biomarkers is required for breast cancer patients. Presently, there is a growing enthusiasm for applying proteomic approaches to the identification of serum biomarkers for the early non-invasive diagnosis of cancer and the monitoring of tumor progression. In this study, we compared the sensitivity of the serological level of mutated p53 protein and the level of anti-p53 antibodies with p53 protein expression in breast cancer patients.
p53 is a multifunctional transcription factor that promotes tumor cell death by regulating the expression of genes involved in cell cycle control and apoptosis, DNA repair and angiogenesis (4). Mutations or down-regulation of p53 contribute to cancer development and progression (4). p53 mutations in cancer patients are often associated with poor prognosis (4). Mutations in the p53 gene, including amino acid substitutions, are present in more than 50% of patients with malignant tumors. These mutations change the conformational structure of p53, which triggers the inhibition of the DNA repair mechanisms and induces programmed cell death by apoptosis (5–7). The accumulation of inactive p53 protein in cells significantly increases the expression of mutant p53 protein with a longer half-life (several hours compared to 20 min for wild-type p53). Therefore, p53 accumulation in tumor tissues is directly related to the presence of mutation in p53 protein (8,9).
Antibodies to p53 in the sera of cancer patients have been reported since 1982 (10). However, the reported antibody frequency in cancer has varied widely, ranging from 2.7 to 31% in different types of cancers. This is in part due to variations in the assay systems used (11). Although the sensitivity of p53 antibodies in diagnosis is not high, these antibodies are rare in healthy people (12), making anti-p53 antibodies a dependable marker for cancer.
We previously reported the presence of p53 antibodies in less than 17% of breast cancer patients (13). In the present study, serological expression of mutant p53 protein was analyzed in 55 cases of human breast tumors at different stages of progression. The results revealed that the level of mutant p53 protein in the serum was directly correlated with p53 protein accumulation in breast tumor tissues. Serological levels of mutant p53 protein in breast cancer patients were higher in early-stage and poorly differentiated tumors. This finding may be important in the detection of breast cancer at early stages, and may be used as a diagnostic tool for differentiating benign and malignant breast disease prior to breast surgery.
Another critical clinical question is whether anti-p53 antibodies might be used as early markers of incipient tumors in high risk populations. In the present study, we demonstrated that the level of anti-p53 antibodies is less sensitive than mutated p53 protein in serum. Further experiments are necessary in order to determine whether serological mutant p53 protein may be used as an early marker in breast cancer.
Materials and methods
Patients
Fifty-five patients from Bahía Blanca, Argentina with mammary pathology were evaluated at local hospitals (Sanatorio Privado del Sur, Interzonal Dr Jose Penna, Regional Español, Dr Leonidas Lucero, Hospital de la Asociación Médica). Informed consent was obtained from each patient prior to enrollment in the study. Serum and breast tissue from all breast cancer patients were available for analysis. Serum samples were collected pre-operatively and stored at −20°C until processing. The median age of the patients was 55 years (range 28–91 years).
Mutant p53 protein: Serological analysis
The presence of p53 mutant protein in serum was quantified employing the p53 ELISA Kit (mutant-selective) from Oncogene Research Products (Cambridge, MA, USA). Results were expressed in O.D. units and were categorized as negative or positive. The ELISA assay was deemed suitable for the quantitative determination of mutant p53 protein. The specific antibodies utilized in this assay react with an epitope exclusively expressed in the recombinant human p53 protein expressed in Escherichia coli, and are exposed only in human mutant p53 proteins, not in wild-type p53 forms, making the assay mutant-selective. Eight serum samples from healthy women without breast disease or a family history of breast cancer were utilized in the serologic assays as negative controls.
Anti-p53 antibodies using the ELISA assay
p53 auto-antibodies were quantified in serum employing the p53 ELISAPlus (Autoantibody) kit (Oncogene Research Products). The kit was designed to measure circulating antibodies to p53 in human serum samples. Control serum provided by the manufacturer was employed. The results were expressed in O.D. units and were categorized as negative or positive.
Histology
Human breast tumor sections (5 μm) were cut from formalin-fixed paraffin-embedded tissues and stained with H&E for histological evaluation. Nuclear grade was defined as grades I–III according to previously established criteria (14,15). Histological classification and the nuclear grade were determined by a medical pathologist. A duplicate of each tissue was cut in order to analyze the total expression of p53 protein using immunohistochemistry (IHC).
Immunohistochemistry
Tumor cell staining for p53 protein was performed using mouse monoclonal DO-1 antibody (Oncogene Research Products). All sections were de-paraffinized in xylene, dehydrated through a graded series of alcohols and washed in phosphate-buffered saline. This buffer was used for all subsequent washes. IHC using the streptavidin-biotin-peroxidase method was performed on paraffin-embedded tissues using the anti-p53 mouse monoclonal antibody DO-1 (diluted 1:100), which recognizes the N-terminus of the human p53 protein (amino acids 21–23). In addition, the antibody reacted with wild-type p53 and with numerous mutant p53 proteins. In order to perform a semi-quantitative assessment, the IHC results were scored. Nuclear staining in >10% of the tumor cells was interpreted as positive: +, 10–25%; ++, 25–50%; and +++, 50–100% of nuclear staining.
Statistical analysis
The frequency of p53 values at the cut-off and the frequency of p53 values below the cut-off was compared to the different parameters by the χ2 test (t-test).
Results
Age-dependent mutated p53 protein expression
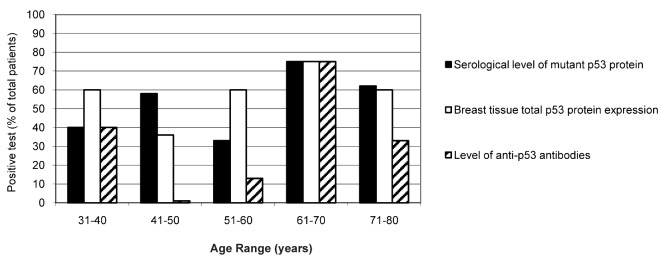
Fig. 1 shows the ELISA results illustrating the age-dependent correlation of the serological level of mutant p53 protein (black columns) and anti-p53 antibodies (hatched columns) in 55 patients with breast malignancies. The total p53 protein expression in breast tissue (white columns) determined using IHC is also shown. Mutated p53 protein levels were detected in patients of all ages, but were highly detected in patients ≥61 years of age (75% positivity at an age range of 61–70 and 62% at an age range of 71–80 years). A similar sensitivity (75% at an age range of 61–70 years and 60% at ≥71 years of age) was observed for the total p53 protein expression in breast tissue by IHC. In addition, the levels of anti-p53 antibodies were higher in patients in the age range of 61–70 years (75% of positivity). This sensitivity decreased (33%) in patients ≥71 years of age, probably due to immune system depression and the lower level of antibody synthesis at this age.
Figure 1.
Age-dependent p53 analysis in breast cancer patients. The graph represents age-dependent p53 expression in the different assays performed. Black columns indicate the percent of patients expressing mutant p53 protein in serum with respect to the total number of patients. White columns represent the percent of positive cases expressing p53 protein in breast tissue by IHC. The hatched columns represent the percent of positive cases demonstrating the presence of anti-p53 antibodies in serum samples.
Notably, at the age range of 41–50 years, the sensitivity of mutant protein detection in serum was higher compared to the total p53 protein expression in breast tissues (58 and 36%, respectively). However, anti-p53 antibodies were practically not detected at that age range (only 1% of the total number of patients). It is now important to ascertain why the immune system did not respond to the increased level of serological mutant p53 protein. There are several explanations for this discrepancy, including one which suggests that mutant p53 protein conformational structures are ‘hidden’ to the immune system, which does not recognize the foreign epitopes in the new p53 mutated protein. Further molecular and genetic analysis is necessary to prove this hypothesis.
In the age range of 51–60 years, the sensitivity of serological mutant p53 detection was lower in comparison with the total p53 protein expression in breast tissues (33 and 60%, respectively). Anti-p53 antibody sensitivity was again low (13%) in this age range. The ELISA kit for the detection of mutant p53 protein in serum is designed specifically against an epitope present in the mutant p53 protein, and did not recognize the wild-type form. However, the antibody employed in the IHC recognized both forms of p53 protein, mutated and wild-type. This is likely the reason for the observed higher levels of p53 protein in breast tissues compared to the level of mutated p53 protein in serum. The wild-type p53 form has a shorter half-life compared to the mutated form. It is believed that menopausal breast cancer patients (51–60 years) have accumulated a higher level of the wild-type p53 form. In order to demonstrate this hypothesis, mutational analysis by sequencing of the whole p53 gene and a correlation of the presence of point mutations with hormonal changes during the menopausal period must be carried out. Similar results were observed in patients <40 years of age. In the age range of 31–40 years, IHC was the most sensitive technique for the detection of the accumulation of p53 protein, followed by mutant p53 protein in serum (60 and 40%, respectively). Notably, the levels of anti-p53 antibodies were high (40%) in this age range. One possible explanation for this observation is possibly that the immunological system of patients <40 years of age is more sensitive to the presence of foreign or mutated proteins, thus stimulating antibody synthesis, compared to patients >70 years of age, who are less susceptible.
Histology-dependent curve and p53 protein expression
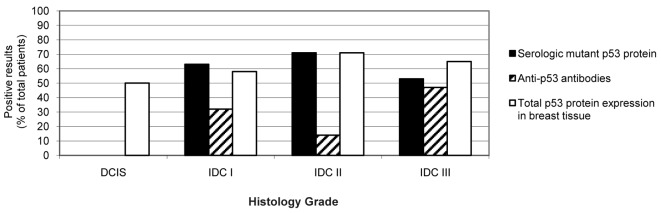
Fig. 2 shows the number of cases (% with respect to the total number of patients) positive for the presence of mutant p53 protein in serum (black column) or for total p53 protein expression by IHC (white column) as well as anti-p53 antibody levels (hatched column) in samples from 55 patients with different stages of breast carcinoma and in 8 serum samples from healthy women, used as negative controls. In this study, we analyzed 6 patients with benign disease, 2 patients with ductal carcinoma in situ (DCIS), 19 patients with invasive ductal carcinoma (IDC) stage I, 7 patients with IDC stage II, 17 patients with IDC stage III, 1 patient with phylloides tumor and 3 patients still undiagnosed at the time of publishing.
Figure 2.
Tumor grade-dependent p53 protein expression. The graph represents the number of cases positive for p53 mutated protein expression using ELISA (black columns), anti-p53 antibodies (hatched columns) and total p53 protein expression in breast tissue by IHC (white columns) in healthy, benign carcinoma in situ (DCIS) patients and in patients with invasive ductal carcinoma (IDC) stages I, II and III.
In patients with benign disease, mutant p53 protein was not detected in serum (Fig. 2). Detection of anti-p53 antibodies and p53 protein expression in breast tissue was also negative in these patients. Total p53 protein expression was detected in 1 of 2 patients with DCIS (Fig. 2), indicating that 50% of DCIS patients showed positive expression of p53 by IHC, but none were positive for the presence of mutant p53 protein in their serum (Fig. 2). Further analysis employing a higher number of patients with DCIS is needed to reach a conclusion concerning the expression of p53 mutations.
Among the breast cancer patients with IDC stage I, 12 of 19 patients (63%) were positive for the mutant p53 protein in serum. Similar results were obtained from the IHC (11/19, 58% of patients). However, the presence of anti-p53 antibodies was lower (6/19, 32% of patients) (Fig. 2). Among the breast cancer patients with IDC stage II, 5 of 7 patients (71%) were positive for mutant p53 in serum. The same results were obtained using IHC (5/7, 71% of patients). Nonetheless, a very low sensitivity was found for the detection of anti-p53 antibodies (1/7, 14% of patients). Among breast cancer patients with IDC stage III, 9 of 17 patients (53%) were positive for mutant p53 protein in serum, 11 of 17 (65%) were positive for total p53 protein expression in breast tissues by IHC, and 8 of 17 (47%) exhibited high levels of anti-p53 antibodies in their serum samples.
The present data clearly demonstrate that mutant p53 protein expression in breast cancer is stage-dependent. Agreement was found in the results upon comparing the three molecular biology techniques employed in this study. All techniques detected mutations in p53 in IDC from stage I to III, but at a different sensitivity. Anti-p53 antibodies were highest in patients with IDC stage III; however they were also detected during the early stages of the disease. These results suggest that serological expression of mutant p53 protein is a more sensitive technique than anti-p53 antibodies for detecting p53 alterations in breast cancer, particularly during the early stages of the disease.
Grade of tumor differentiation and expression of p53 mutations in breast cancer patients
To determine whether the grade of tumor differentiation affects the detection of p53 mutations, we analyzed 55 patients with poor, semi- and well-differentiated breast carcinomas (Fig. 3). In patients with poorly differentiated breast carcinomas, we found mutated p53 protein in serum in 7 of 13 patients (63.64%), while 8 of 13 (72.73%) exhibited total p53 protein expression using IHC. At the semi-differentiated level, 8 of 24 patients (33.33%) showed the presence of p53-auto-antibodies, 13 of 24 (54.17%) showed mutant p53 protein, and 14 of 24 (58.33%) were positive for the presence of p53 protein by IHC (Fig. 3). In well-differentiated tumors, 5 of 8 patients (62.50%) showed mutant p53 protein expression in serum by ELISA and in breast tumor tissues by IHC (Fig. 3). These results indicate that poorly differentiated breast tumors can be identified by detecting p53 protein expression using IHC. No statistically significant differences were found in the detection of mutant p53 in serum among the poor, semi- or well-differentiated breast carcinomas. However, the sensitivity of anti-p53 antibody detection was lower than that of mutant p53 protein in serum.
Figure 3.
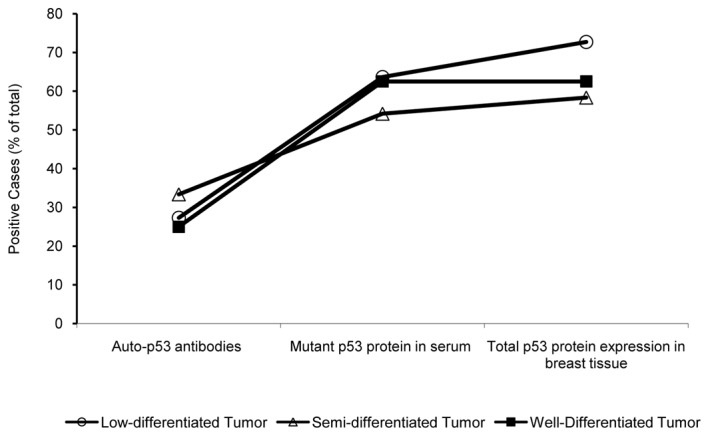
p53 expression and tumor differentiation-dependent curve in breast cancer patients. The graph shows the percent of positive cases detected in all the assays at different grades of tumor differentiation: poor, semi- and well-differentiated. The level of anti-p53 antibodies, mutant p53 protein in serum and expression of total p53 protein in breast tissues by IHC is indicated. Open circles, poor or low differentiated; black squares, semi-differentiated; open triangles, well-differentiated breast carcinomas.
Discussion
The development of molecular markers is required to improve the diagnosis and assessment of tumor progression in breast cancer patients. Mutations in the p53 tumor suppressor gene, as well as overexpression of serum p53 antibodies and p53 protein in tumor tissues, have been encountered in a variety of human malignancies (16). The p53 antibody was originally described in 1982 by Crawford et al (17) in the serum of 9% of breast cancer patients using Western blot analysis. More than 15 studies were performed by Soussi et al using ELISA in breast cancer (16). The frequency of the p53 antibody in breast cancer ranged from 15 to 20%. However, the majority of these studies were performed in European countries or in the US. No studies have been performed in South America or in Argentina, where the frequency of breast cancer is similar or slightly lower than that observed in the studies conducted.
Overexpression of mutant p53 protein in breast cancer patients has usually been evaluated in tumor tissue with immunohistochemical staining; however, a serum assay for p53 oncoproteins using ELISA can be performed easily and repeatedly due to its minimal invasiveness compared with assays using tissue materials (18,19). In the present study, the median serum level of mutant p53 protein in patients with IDC was significantly different (26 of 43, 60.45%) (p<0.001) compared to the controls.
Our results are well correlated with those of studies performed in cervical carcinomas by Sobti et al (20), who detected p53 mutant protein in serum from 61.5% patients with invasive cervical carcinoma (20). In addition, Oh et al (21) recently demonstrated similar results. However, there have been few results involving breast cancer. Micelli et al (22) demonstrated the presence of mutant p53 protein in serum in 23% of breast cancer patients, and showed a 100% mutant p53 specificity employing 20 healthy controls (22).
In the present study, mutant p53 protein was predominantly detected in serum from IDC patients with the early stages of the disease: 12/19 (63.15%) in stage I (p<0.0001), and 5/6 (83.33%) in stage II (p<0.0001). It was maintained at a high level in late stages: 8/16 (50%) of patients in stage III. The specificity of mutant p53 protein detection was 100%, since it was found to be negative in the serum of normal control patients, and was also negative in patients with benign diseases (0/6, 0%). In this study, we observed the expression of p53 protein using IHC in 1 of 2 patients with DCIS. Further analyses are needed to demonstrate the sensitivity of p53 mutation detection in the serum of patients with DCIS.
The presence of mutant p53 protein in serum and p53 accumulation in tissue was correlated with poorly differentiated tumors in patients with IDC (63.64 and 72.73%, respectively; p<0.005) compared to those with well-differentiated tumors. Several studies have demonstrated the presence of p53-accumulated protein in breast cancer by IHC. Al-Moundhri et al (23) found p53 overexpression in 41.7% of breast tumors. They also reported that the p53 accumulation was related to poor differentiation in human breast cancer (23).
Expression of conformational altered protein induces an immune response, thus leading to the presence of circulating anti-p53 antibodies in cancer patients (24). Trivers et al (25) used an anti-p53 antibody as a molecular marker and found a great level of anti-p53 antibody among five workers occupationally exposed to vinyl chloride, who later developed angiosarcoma of the liver (25).
However, in the present study, the level of anti-p53 antibodies demonstrated low sensitivity in breast cancer patients. Its level was higher (47%) in advanced breast disease in patients with IDC stage III. No statistically significant differences have been found in the expression of p53 antibodies and the grade of tumor differentiation. We found a high reactivity in patients ≤40 and ≥61 years of age, reaching similar levels of serological mutant p53 protein in patients at those ages.
Recently, it was reported that TP53 and KRAS mutation detection in the plasma of healthy subjects was associated with environmental exposure to carcinogenic agents (26). These observations have implications for monitoring the early stages of bladder cancer development. In another report, analyses were performed to calculate the association between the prevalence of positivity for the p53 antibody or mutant-p53 antigen with accumulative vinyl chloride exposure in a population of healthy workers (27). The results from these studies demonstrate the utility of the TP53 mutation in a simple blood sample as a molecular marker to determine a minimum threshold for the effects of exposure to carcinogens.
In conclusion, mutant p53 protein from serum was elevated in invasive breast carcinomas, with a strong correlation with p53-accumulation detected by IHC. These data strongly indicate that the detection of mutant p53 in serum and p53 accumulation in breast tissue are well correlated, and both tests are sensitive and specific for invasive ductal breast carcinomas. A prospective study with a large sample size is warranted, as the presence of mutant p53 protein in serum is potentially useful as a biological marker of breast carcinoma, particularly for the prediction of prognosis and in follow-up after treatment.
Our findings indicate that mutant p53 in serum is a promising novel parameter for the evaluation of cellular biology and the prognosis of breast cancer using blood samples, thus avoiding surgery. The presence of mutant p53 protein in serum is potentially an important tool for discerning benign disease prior to performing breast surgery.
Acknowledgments
This work was supported by the Instituto de Análisis Clinicos Asociados (IACA Laboratory), Bahía Blanca, Argentina. Special thanks to all the doctors of gynecology from Sanatorio Privado del Sur, Interzonal Dr Jose Penna, Regional Español, Dr Leonidas Lucero, Hospital de la Asociación Médica from Bahía Blanca, Argentina, for their support.
References
- 1.Ries L, Eisner M, Kosary C, et al., editors. Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 2002. SEER; pp. 1973–1999. [Google Scholar]
- 2.Greenie RT, Murray T, Boldin S, Wingo P. Cancer statistics 2000. Cancer J Clin. 2000;50:7–23. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BW, Kleihues P, editors. World Cancer Report. IARC Press; France: 2003. [Google Scholar]
- 4.Oliveira AM, Ross JS, Fletcher JA. Tumor suppressor genes in breast cancer: the gatekeepers and the caretakers. Am J Clin Pathol. 2005;124:S16–S28. doi: 10.1309/5XW3L8LU445QWGQR. [DOI] [PubMed] [Google Scholar]
- 5.Bourdon JC, Laurenzi VD, Melino G, Lane D. p53: 25 years of research and more questions to answer. Cell Death Differ. 2003;10:397–399. doi: 10.1038/sj.cdd.4401243. [DOI] [PubMed] [Google Scholar]
- 6.Bourdon JC. p53 and its isoforms in cancer. Br J Cancer. 2007;97:277–282. doi: 10.1038/sj.bjc.6603886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soussi T. The p53 pathway and human cancer. Br J Surg. 2005;92:1331–1332. doi: 10.1002/bjs.5177. [DOI] [PubMed] [Google Scholar]
- 8.Casey G, Lopez ME, Ramos JC, Plummer SJ, Arboleda MJ, Shaughnessy M, Karlan B, Slamon DJ. DNA sequence analysis of exons 2 through 11 and immunohistochemical staining are required to detect all known p53 alterations in human malignancies. Oncogene. 1997;13:1971–1981. [PubMed] [Google Scholar]
- 9.Dowell SP, Wilson PO, Derias NW, Lane DP, Hall PA. Clinical utility of the immunocytochemical detection of p53 protein in cytological specimens. Cancer Res. 1994;54:2914–2918. [PubMed] [Google Scholar]
- 10.Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30:403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- 11.Soussi T. Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 12.Vogl FD, Frey M, Kreienberg R, Runnebaum IB. Autoimmunity against p53 predicts invasive cancer with poor survival in patients with an ovarian mass. Br J Cancer. 2000;83:1338–1343. doi: 10.1054/bjoc.2000.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balogh GA, Corte MM, Nardi H, et al. Mutant p53 protein in serum could be used as a molecular marker in human breast cancer. Int J Oncol. 2006;8:995–1002. doi: 10.3892/ijo.28.4.995. [DOI] [PubMed] [Google Scholar]
- 14.Lagios MD. Pathologic practice standards for breast carcinoma: tumor size, reliable data, or miscues? J Am Coll Surg. 2003;196:91–92. doi: 10.1016/s1072-7515(02)01540-5. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein MJ, Lagios MD. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17. Cancer. 2000;88:242–244. doi: 10.1002/(sici)1097-0142(20000101)88:1<242::aid-cncr35>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Soussi T. p53 antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 17.Crawford LV, Pimand DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30:403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- 18.Choi JH, Oh JY, Ryu SK, et al. Detection of epidermal growth factor receptor in the serum of gastric carcinoma patients. Cancer. 1997;79:1879–1883. [PubMed] [Google Scholar]
- 19.Oh MJ, Choi JH, Kim IH, et al. Detection of epidermal growth factor receptor in the serum of patients with cervical carcinoma. Clin Cancer Res. 2000;6:4760–4763. [PubMed] [Google Scholar]
- 20.Sobti RC, Parashar K, Kaurand R, Capalash N. Detection of human papillomavirus DNA, serum p53 and p53 antibodies in patients with cervical cancer. J Environ Pathol Toxicol Oncol. 2002;21:79–85. [PubMed] [Google Scholar]
- 21.Oh MJ, Choi JH, Lee YH, Lee JK, Hur JY, Park YK, Lee KW, Chough SY, Saw HS. Mutant p53 protein in the serum of patients with cervical carcinoma: correlation with the level of serum epidermal growth factor receptor and prognostic significance. Cancer Lett. 2004;203:107–112. doi: 10.1016/j.canlet.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Micelli G, Donadeo A, Quaranta M. The p53 tumor suppressor gene. A preliminary clinical study in breast cancer patients. Cell Biophys. 1992;21:25–31. doi: 10.1007/BF02789475. [DOI] [PubMed] [Google Scholar]
- 23.Al-Moundhri M, Nirmala V, Al-Mawaly K, Ganguly S, Burney I, Rizvi A, Grant C. Significance of p53, Bcl-2 and HER-2/neu protein expression in Omani Arab females with breast cancer. Pathol Oncol Res. 2003;9:226–231. doi: 10.1007/BF02893382. [DOI] [PubMed] [Google Scholar]
- 24.Labrecque S, Naor N, Thomson D, Matlashewski G. Analysis of the anti-p53 antibody response in cancer patients. Cancer Res. 1993;53:3468–3471. [PubMed] [Google Scholar]
- 25.Trivers GE, Cawley HL, De Benedetti VM, Hollstein M, Marion MJ, Bennett WP, Hoover ML, Prives CC, Tamburro CC, Harris CC. Anti-p53 antibodies in sera of workers occupationally exposed to vinyl chloride. J Natl Cancer Inst. 1995;87:1400–1407. doi: 10.1093/jnci/87.18.1400. [DOI] [PubMed] [Google Scholar]
- 26.Gormally E, Vineis P, Matullo G, et al. TP53 and KRAS2 mutations in plasma DNA of healthy subjects and subsequent cancer occurrence: a prospective study. Cancer Res. 2006;66:6871–6876. doi: 10.1158/0008-5472.CAN-05-4556. [DOI] [PubMed] [Google Scholar]
- 27.Mocci F, Nettuno M. Plasma mutant-p53 protein and anti-p53 antibody as a marker: an experience in vinyl chloride workers in Italy. J Occup Environ Med. 2006;48:158–164. doi: 10.1097/01.jom.0000183097.72738.a7. [DOI] [PubMed] [Google Scholar]