Abstract
Objective
Sickle cell disease (SCD) is a common hereditary disease in Iran. In developed countries, newborn screening programs have been established to ensure early diagnosis, but in most developing countries, screening is not performed and the diagnosis is often delayed. The aim of the present work was to investigate the clinical presentation of SCD in Iran and comparison of its hematologic indices with normal children.
Methods
The study included 44 pediatric patients (26 boys and 18 girls) with sickle cell anemia (SS), 27 sickle /β°-thalassemia (Sβ°), and 21 sickle /β+-thalassemia (Sβ+). Fifty seven healthy individuals matched with the patients were randomly selected as controls.
Findings
Mean age at diagnosis in SS group was 4.3 years. At the time of diagnosis all patients were anemic, 89% complained of painful crises. Hemoglobin(Hb) concentration, red blood cell (RBC) count and Hb×RBC product in SS group was significantly lower than in control group (P<0.001), mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) showed no significant differences. Hb×RBC product below 45 and MCH/RBC above 7 have the best sensitivity and specificity for differenting SS group and the control normal group (91 and 98% for Hb×RBC and 89 and 100% for MCH/RBC respectively). Mean age at diagnosis in Sβ+ group was higher than in SS and Sβ° groups (7.45 year vs 4.26 and 4.25 year) (P<0.001). In addition, Sβ° and Sβ+ groups had significantly lower MCV, MCH, and Hb×RBC indices compared with control group.
Conclusion
We suggest that in an anemic patient with history of pain crises, normochrome normocytic anemia, Hb×RBC <45 and MCH/RBC ≥7, SCD should be considered and the patient evaluated accordingly to confirm the diagnosis.
Keywords: Sickle cell disease, Sickle Cell Anemia, Hemoglobin SC Disease, Thalassemia, Iran, Children
Introduction
Sickle cell disease (SCD) is an autosomal recessive genetic disease that results from the substitution of valine for glutamic acid at position 6 of the β-globin gene, leading to production of a defective form of hemoglobin, hemoglobin S (Hb S)[1]. SCD is a common reason urging patients of African descent to seek emergency medical care. In Iran β-thalassemia and sickle cell disorders are among common genetic disorders affecting red blood cells[2, 3].
High prevalence of β-thalassemia (around 10%) is found in Northern provinces neighboring Caspian Sea and Southern provinces neighboring Persian Gulf. The prevalence of β-thalassemia alleles has been estimated to be 4–8% in other parts of Iran[2, 4]. In South Iran the prevalence of sickle cell trait has been estimated to be around 1.43% and sickle cell anemia 0.1%[3]. Although knowledge of the pathophysiological basis for sickle cell anemia has led to advances in its treatment, emergency physicians remain challenged by its varied clinical presentations, including vasoocclusive, hematologic, and infectious crises.
Patients who are heterozygous for the Hb S gene are carriers of the condition. Under stressful conditions, carriers may display some clinical manifestations (eg, severe hypoxia).
Homozygotes for βS (α2β2 Glu→Val) have a serious illness that generally shortens life span[5, 6]. The severity of combined heterozygote condition known as Hb S/β-thalassemia is variable depending on the amount of Hb A production. Patients with S/β0-thalassemia are almost similar to patients with sickle cell anemia, but can be identified by the presence of microcytosis, elevated Hb A2 concentration and family study[7]. The clinical manifestations of these various forms of SCD are diverse and often diagnosed delayed in pediatric patients. The aim of the present work was to investigate the clinical presentation of SCD in Iranian patients and to compare hematologic indices with those of normal children.
Subjects and Methods
Patients and controls: The study included 44 sickle cell anemia (SS), 27 sickle/β°-thalassemia (Sβ°), and 21 sickle/β+-thalassemia (Sβ+) pediatric patients. Diagnosis based on hemoglobin electrophoresis of the patients and their parents. Patients were aged below 17 years, with an average of 8.01±6.51 years. We excluded iron deficiency anemia and recently (under three months) transfused patients. Fifty seven age and sex matched healthy individuals with normal hematological indices, ferritin level and Hb electrophoresis were randomly selected as controls. All of them originated from South Iran. This study was approved by ethics committee of Jundishapour University of Ahvaz Medical Sciences. Informed consent was obtained from parents of the subjects.
Hematological indices: Fasting blood samples were collected in EDTA tubes and used for determination of hematological indices including Hb concentration, mean corpuscular volume (MCV), mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC) and red blood cell (RBC) count using a cell counter. Red blood cells were washed 3 times with cold saline and hemolyzed by addition of cold distilled water. Hemolysate was applied onto a cellulose acetate membrane and electrophoresis was performed at alkaline pH using Tris/EDTA/ borate buffer (pH 8.6). Citrate agar gel electrophoresis was done using agar gel plates and citrate buffer at pH 6.0. Sickle cell phenotype was diagnosed using cellulose acetate electrophoresis at alkaline and citrate agar gel electrophoresis at acid pH and also by solubility test[8]. Hb A2 was determined by microcolumn chromatography elution using the anion exchange resin diethyaminoethyl (DEAE) cellulose (Whatman DE-52 microgranular per-swollen) with glycin-KCN developer[9].
Statistical analysis: Data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). All the variables were compared using Student's t-test, ANOVA analysis, and chi-squared test (for the quantitative and qualitative variables, respectively). Fisher's exact test served to evaluate the relation between two binary variables. Quantitative variables are provided as mean (±SD). In addition, the risk is expressed as OR with 95% CI and P-values <0.05 are regarded as being statistically significant
Findings
Mean age at diagnosis in SS group (26 boys and 18 girls) was 4.3 years. At the time of diagnosis all of them had anemia. History of painful crises was found in 39 cases (89%), splenomegaly in 70% (six of them were splenectomized). Hb concentration, RBC count and Hb×RBC product in SS group were significantly lower than in control group [(8.75g/dl vs 13.09 g/dl), (3.05×1012/L vs. 4.76 ×1012/L) and (27.99 vs 62.63), respectively] (P<0.001); MCV and MCH had no significant differences (Table 1).
Table 1.
Hematological indices for patients and control groups
| Group | No | Hb (g/dl) | RBC (×1012/l) | MCV (fl) | MCH (pg) | MCHC (g/dl) | Hb×RBC | MCH/RBC |
|---|---|---|---|---|---|---|---|---|
| SS | 44 | 8.75±2.12a | 3.05±0.68a b | 88.56±7.79b | 29.34±2.82b | 33.14±2.89 | 27.99±11.60a c | 10.33±3.77a b |
| Sβ° | 27 | 8.75±1.80a | 4.17±0.71 | 65.30±5.86a | 21.22±1.47a | 33.29±1.71 | 36.96±12.99a | 5.30±0.90 |
| Sβ+ | 21 | 8.79±1.85a | 4.22±0.82 | 63.95±6.36a | 21.28±2.23a | 32.42±3.29 | 38.51±14.22a | 5.30±1.54 |
| NL | 57 | 13.09±1.15 | 4.76±0.36 | 84.56±4.37 | 27.43±1.80 | 32.53±1.45 | 62.63±9.51 | 5.79±0.62 |
Statistically significant at P<0.001 compared with NL group.
Statistically significant at P<0.001 compared with Sβ° and Sβ+ groups.
Statistically significant at P<0.05 compared with Sβ° and Sβ+ groups.
Hb: hemoglobin/ RBC: Red blood cell/ MCV: Mean corpuscular volume/ MCH; Mean corpuscular Hb/ MCHC: Mean corpuscular Hb concentration
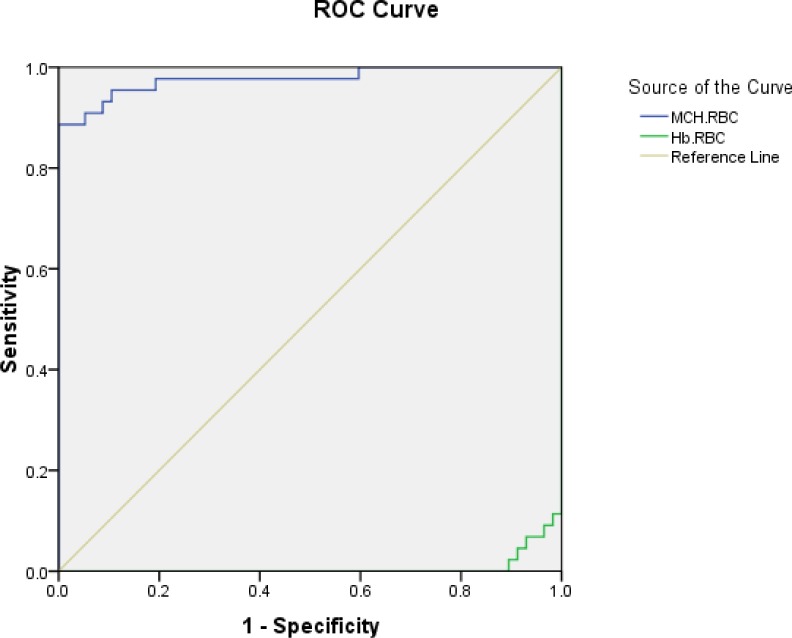
We found that Hb×RBC product below 45 and MCH/RBC above 7 have the best sensitivity and specificity to differentiae SS group and the control group (Fig 1 and Table 2).
Fig. 1.
Sensitivity and Specificity of Hb×RBC product and MCH/RBC in SS group
Table 2.
The value of Hb×RBC product and MCH/RBC indicesin all groups
| Groups | Sensitivity (%) | Specificity (%) | OR (95% CI) | |
|---|---|---|---|---|
| Hb×RBC<45 | SS | 91 | 98 | 4 (0.45-35.79) |
| Sβ° | 76 | 98 | 5 (0.58-42.80) | |
| Sβ + | 86 | 98 | 2 (0.18-22.08) | |
| MCH/RBC ≥ 7 | SS | 89 | 100 | Infinity |
Hb: hemoglobin/ RBC: Red blood cell/ MCH: Mean corpuscular/ SS: sickle cell anemia/
OR: Odds Ratio/ Sβ°: sickle/β°-thalassemia/ Sβ+: sickle/β+-thalassemia
We also compared the hematological indices between SS, Sβ°, and Sβ+ groups. Mean age at diagnosis in Sβ+ group was higher than in SS and Sβ° groups (7.45 years vs 4.26 and 4.25 years) (P<0.001). Age of diagnosis was not significantly different between SS and Sβ° groups (Table 3). Prevalence of splenomegaly showed also no significant difference in the three groups.
Table 3.
Diagnosis age and amount of hematological indices in patients
| Groups | Diagnosis age (yr) | Hb A (%) | Hb A2 (%) | Hb S (%) | Hb F (%) |
|---|---|---|---|---|---|
| SS | 4.26±3.91 | 0 | 2.23±1.10b | 81.44±13.05a | 17.15±13.57 |
| Sβ° | 4.25±3.74 | 0 | 3.37±1.12 | 74.78±9.36 a | 21.03±9.36 |
| Sβ+ | 7.45±4.93 c | 8.69±7.93 | 3.85±1.35 | 67.28±15.11a | 16.47±6.87 |
Statistically significant at P<0.05 compared with two other groups.
Statistically significant at P<0.001 compared with Sβ° and Sβ+ groups.
Statistically significant at P<0.001 compared with SS and Sβ° groups.
Hb: hemoglobin/ SS: sickle cell anemia/ Sβ°: sickle/β°-thalassemia/ Sβ+: sickle/β+-thalassemia
Hb S was significantly higher in SS and Sβ° groups than in Sβ+ group (81.44%, 74.78% and 67.28% respectively) and mean HbA2 in SS group was significantly lower than in Sβ° and Sβ+ groups (2.23% vs 3.37% and 3.38%), but mean Hb F value had no significant differences among the groups ( Table 3).
In SS group, RBC and Hb×RBC product were significantly lower than in Sβ° and Sβ+ groups [(3.05×1012/L vs 4.17 and 4.22×1012/L) and (27.99 vs 36.39 and 38.51) respectively]. In contrast, SS group had significantly higher MCV and MCH and MCH/RBC indexes compared with Sβ° and Sβ+ groups [(88.56 fl vs 65.30 and 63.95 fl), (29.34 pg vs 21.22 and 21.28 pg) and (10.33 vs 5.30 and 5.30) respectively] (Table 1). However, MCH/RBC index showed no significant difference between Sβ°, Sβ+, and control groups.
Sβ°, and Sβ+ groups had significantly lower MCV, MCH, and Hb×RBC indices compared with control group [(65.30 and 63.95 fl vs 84.56 fl), (21.22 and 21.28 pg vs 27.43 pg), and (36.69 and 38.51 vs 62.63), respectively]. Hb concentration, RBC count, MCV, MCH, MCHC, Hb×RBC, and MCH/RBC indices had no significant differences in Sβ° and Sβ+ groups. Also, changes of MCHC in patients and control groups were not significant.
Discussion
SCD is a major public health concern that has great impact on both individuals and society. The annual birth rate worldwide is over 200,000, with more than 90% of cases being in Africa[10].
Mortality associated with this disease is high despite knowledge of the pathophysiology[11, 12] and treatment of the various forms of crisis. Mortality from the disease is highest in the first 5 years of life, with approximately 50% of deaths occurring in the second 6 months of life[13–17].
Acute infections and severe anemic sequestration are responsible for most of these deaths16, 18]. For most affected children, the parents are usually unaware of the presence of the disease; the diagnosis is sometimes made post mortem[13]. In developed countries, newborn screening programs have been established to ensure early diagnosis and thus early enrolment into a comprehensive healthcare program[19–24], but this screening is not performed in Iran and in most developing countries. The clinical manifestations of homozygote sickle cell anemia (SS) are diverse, and diagnosis is often delayed in pediatric patients. Acute painful episodes are the most common symptom and cause of hospital admission[10, 25]. More than 70% of individuals with SCD, especially patients with Hb SS, suffer from painful crisis[26]. Although all of our SS patients were anemic, the chief complaint was mostly painful crisis and in most of them sickle cell disease was diagnosed during workup of septic arthritis, juvenile rheumatoid arthritis, acute abdominal diseases or after abdominal surgery. This is more common than that in CSSCD (cooperative study of sickle cell disease) and Telfer studies[25, 27]; it could be due to lower prevalence of SCD in our population and its late diagnosis.
Mean age at diagnosis in our SS group was 4.3 years and some of them were diagnosed in workup for painful crisis. Gill et al found a mean age of 4.9[28] years and Miller et al 3.5 years[27] in their patients.
This age was similar to individuals with Sβ°, because of high similarity of its clinical manifestations to SCD. Mean age at diagnosis was significantly lower than in individuals with Hb Sβ+. This may be due to lower incidence of painful crises and sickling phenomena due to this small amount of Hb A. Also, other studies indicated that the outcome of Hb Sβ+ is better than that of Hb SS and Hb Sβ°[27, 29].
Sickle cell anemia causes a chronic form of anemia which can lead to fatigue. The sickled red blood cells are prone to breakage (membrane rupture) which causes a much shorter life span of these cells. Anemia and splenomegaly were common findings in our cases. Some of them were splenectomized.
Hematological indices revealed that individuals with Hb SS have low Hb, RBC count, and Hb×RBC, high MCH/RBC and equal to high MCV and MCH values compared with normal individuals. Thus, these patients had normochrome normocytic anemia. In an earlier study higher MCV and MCH levels among the Jamaican SS patients have been reported as compared to the normal (AA) controls[30]. In this study, we used the Hb×RBC and MCH/RBC for the first time and both of them revealed valuable in differentiating SCD patients and the normal group.
Conclusion
We suggest that if a patient has painful crisis with normochrome normocytic anemia, Hb×RBC <45, and MCH/RBC ≥7, the SCD should be considered and more comprehensive tests such as Hb electrophoresis or sickle preparation test should be performed to establish an exact diagnosis. Furthermore, Sβ° and Sβ+ patients have significantly lower MCV, MCH, and Hb×RBC indices; thus in patients with painful crisis and hypochrome microcytic anemia, these two diseases should not be forgotten.
The limitation of this study was simultaneous iron deficiency anemia and previous transfusion that were seen in numerous patients and we excluded about half of cases, so further investigations on larger number of individuals with sickle cell disease are helpful to support our findings.
Acknowledgment
This study was supported by the vice chanceller research of Jundishapour University of Medical Sciences. We would like to thank Mr. Cheraghy for helping us in statistical analysis and our colleagues in clinic and laboratory of Shafa and Abuzar hospitals for their precious help.
Conflict of Interest
None declared.
References
- 1.Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (Hb S) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151(9):839–45. doi: 10.1093/oxfordjournals.aje.a010288. [DOI] [PubMed] [Google Scholar]
- 2.Najmabadi H, Teimourian Sh, Khatibi T, et al. Amplification refractory mutation system (ARMS) and reverse hybridization in the detection of h-thalassemia mutations. Arch Irn Med. 2001;4(4):165–70. [Google Scholar]
- 3.Habibzadeh F, Yadollahie M, Ayatollahie M, Haghshenass M. The prevalence of sickle cell syndrome in south of Iran. Irn J Med Sci. 1999;24(1):32–4. [Google Scholar]
- 4.Merat A, Haghshenass M, Mostafavi pour Z, et al. thalassemia in Southwestern Iran. Hemoglobin. 1993;17(5):427–37. doi: 10.3109/03630269308997497. [DOI] [PubMed] [Google Scholar]
- 5.Nagel RL, Ranney HM. Genetic epidemiology of structural of the (-globin gene. Semin Hematol. 1990;27(4):342–359. [PubMed] [Google Scholar]
- 6.Weatherall DJ, Clegg JB, Higgs DR, Wood WG, Sly WS, Valle D. The hemoglobinopathies. In: Sriver CR, Beadet AL, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 1995. pp. 3417–84. [Google Scholar]
- 7.Kinney TR, Ware RE. Compound heterozygous states. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. Sickle Cell Disease: Basic Principles and Clinical Practice. New York: Raven Press; 1994. pp. 437–51. [Google Scholar]
- 8.Schneider RG. Differentiation of electrophoretically similar hemoglobins- such as S, D, G and P; or A2, C, E, and O by electrophoresis of the globin chains. Clin Chem. 1974;20(9):1111–5. [PubMed] [Google Scholar]
- 9.Fairbanks VF, Klee GG. Biochemical aspects of hematology. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. Philadelphia: Saunders; 1994. pp. 2041–2. [Google Scholar]
- 10.Davies SC. Haemoglobinopathies. Paediatrics and child health. 2007;17(8):311–6. [Google Scholar]
- 11.Lane PA. Sickle cell disease. Pediatr Clin North Am. 1996;43(3):639–64. doi: 10.1016/s0031-3955(05)70426-0. [DOI] [PubMed] [Google Scholar]
- 12.Bunn FH. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 13.Bainbridge R, Higgs DR, Maude GH, Sergeant GR. Clinical presentation of homozygous sickle cell disease. J Pediatr. 1985;106(6):881–5. doi: 10.1016/s0022-3476(85)80230-4. [DOI] [PubMed] [Google Scholar]
- 14.Williams S, Maude GH, Sergeant GR. Clinical presentation of sickle cell haemoglobin C disease. J Pediatr. 1986;109(4):586–9. doi: 10.1016/s0022-3476(86)80217-7. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien RT, McIntosh S, Aspnes GT, Pearson HA. Prospective study of sickle cell disease in infancy. J Pediatr. 1976;89(2):205–10. doi: 10.1016/s0022-3476(76)80449-0. [DOI] [PubMed] [Google Scholar]
- 16.Leikin SJ, Gallagher D, Kinney TR, et al. Mortality in children and adolescents with sickle cell disease. Pediatrics. 1989;84(3):500–8. [PubMed] [Google Scholar]
- 17.Thomas AN, Pattison C, Sergeant GR. Causes of death in sickle cell disease in Jamaica. Br Med J. 1982;285(6342):633–5. doi: 10.1136/bmj.285.6342.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers DW, Clark JM, Cupidore L, et al. Early deaths in Jamaican children with sickle cell disease. Br Med J. 1978;1(6126):1515–6. doi: 10.1136/bmj.1.6126.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vichinsky E, Hurt D, Earles A, et al. Newborn screening for sickle cell disease: effect on mortality. Pediatrics. 1988;81(6):749–55. [PubMed] [Google Scholar]
- 20.Githens JH, Lane PA, McCurdy RS, et al. Newborn screening for haemoglobino-pathies in Colorado: the first ten years. Am J Dis Child. 1990;144(4):466–70. doi: 10.1001/archpedi.1990.02150280088018. [DOI] [PubMed] [Google Scholar]
- 21.Vichinsky EP. Comprehensive health care in sickle cell disease: its impact on morbidity and mortality. Semin Haematol. 1991;28(3):220–6. [PubMed] [Google Scholar]
- 22.Pearson H. A neonatal program for sickle cell disease. Adv Pediatr. 1986;33:381–5. [PubMed] [Google Scholar]
- 23.Scott RB, Harrison DL. Screening of umbilical cord blood for sickle cell disease: utilization and implementation. Am J Pediatr Haematol Oncol. 1982;4(2):202–5. [PubMed] [Google Scholar]
- 24.Odunvbuna ME, Okoloa AA, Rahimy CM. Newborn screening for sickle cell disease in a Nigerian hospital. Public Health. 2008;122(10):1111–6. doi: 10.1016/j.puhe.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Telfer P, Coen P, Chakravorty S, et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica. 2007;92(7):905–12. doi: 10.3324/haematol.10937. [DOI] [PubMed] [Google Scholar]
- 26.Teinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340(13):1021–30. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 27.Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86(2):776–83. [PubMed] [Google Scholar]
- 28.Miller ST, Sleeper LA, Pegelow CH, et al. Predication of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–9. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 29.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004;103(11):4023–7. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serjeant GR. Sickle Cell Disease. 3rd ed. Oxford: Oxford University Press; 2001. The blood; pp. 113–5. [Google Scholar]