Abstract
Objective
Human milk (HM) is the ideal food for all newborns and infants. Apart from various bioactive compounds, including cytokines, antibodies, hormones, vitamines, it also contains polyamines, such as spermine (Sp), spermidine (Spd) and putrescine (Put).
Aim
The present study investigated polyamine metabolism in colostrum and mature human milk by measuring the polyamine oxidase (PAO) and diamine oxidase (DAO) enzyme activities, which are necessary for polyamine catabolism, as well as by determining the malondialdehyde (MDA) levels, the final product of polyamine biodegradation.
Methods
The PAO, DAO activity and MDA levels were quantified in colostrum (1st and 2nd day) as well as in mature human milk, 30th day of lactation.
Findings
We found the steady increase of PAO activity and steady decrease of DAO activity and MDA levels during first month of lactation.
Conclusion
Since the products of PAO activity such as, amino aldehydes and hydrogen peroxide (H2O2) might have potential antimicrobial effects, promoting the oxidative stress, it is likely that human milk PAO throughout the lactation period, contributes to the protective effects of human milk.
Keywords: Human Colostrum, Mature Milk, Polyamine Oxidase, Diamine Oxidase, Malondialdehyde
Introduction
Human milk (HM) is the ideal food for all newborns and infants. It contains various enzymes of which some are involved in milk biosynthesis pathway in the mammary gland while the others are specific for the digestion of proteins, fats or carbohydrates, facilitating the infant's ability to exploit these HM major constituents [1]. HM also contains biologically active compounds, hormones, vitamins, cytokines and antibodies, as well as polyamines such as spermine (Sp), spermidine (Spd) and putrescine (PUT) [2–5]. Among the other things, it has been shown that polyamines have important role in gastrointestinal tract functional maturation during the neonatal period preventing the food allergies in sucking babies by decreasing mucosal permeability to antigenic proteins [6–8].
Polyamine oxidase (PAO; EC 1.5.3.11) enzyme, which is present in all vertebrate tissues and biological fluids represent one of the key enzymes in catabolic pathways of polyamines. PAO catalyzes the oxidative deamination of Sp or Spd, producing spermidine or PUT, depending on the substrate nature [9–11]. Diamine oxidase (DAO), histaminase (EC, 1.4.3.6), a copper-containing enzyme, catalyses the biodegradation of PUT, producing γ-Aminobutyric acid (GABA) or malondialdehyde (MDA) [12, 13]. The study sought to elucidate polyamine metabolism in colostrum (1st and 2nd day) and mature human milk, 30th day of lactation, through the investigation of PAO and DAO activities, as well as by measuring the levels of MDA, the end product of polyamine biodegradation.
Subjects and Methods
The study group consisted of 60 healthy nursing mothers, admitted for delivery at a Clinic of Obstetrics and Gynecology in Clinical Center, Faculty of Medicine Nis, Serbia, between May 2010 and July 2010. All the mothers recruited belonged to middle/higher social classes, had to have termed delivery, practiced exclusive breastfeeding, had no treatment during pregnancy except folic acid, and had no history of infection since the beginning of parturition. Exclusion criteria were the presence of any clinical sign of lactating mastitis in nursing mothers or failure to meet the inclusion criteria as outlined.
The study was approved by the Ethical Committee for Medical Research, Clinical Center, Faculty of Medicine, University of Nis (protocol number 01-3565-1). Informed consent was obtained from every nursing mother.
The morning samples (between 8 a.m. and 10 a.m.) of human milk had been collected with a manual breast pump (Ginevri, Milan, Italy) on the 1st and 2nd day of lactation (colostrum) as well as 30th day of lactation (mature milk) being stored at −20°C, until analyzed.
To measure the PAO activity, spermine-tetrahydrochloride “Sigma” and to measure DAO activity, PUT-dihydrochloride “Sigma” was used as a substrate. The measurements were done by a spectrophotometric method of Bacharach and Reaches [14], modified by Quash et al [14, 15]. One unit of the enzyme activity was defined as an increase in optical density of 0.100 at 660 nm.
The amount of MDA, reflecting the level of lipid peroxidation in human milk, was determined by spectrophotometric method, using thiobarbituric acid (TBA) purchased from “Sigma“. The MDA standard was prepared with 1,1,3,1 tetramethoxypropan (Sigma) [16]. All milk samples for enzymatic analysis had been dissolved (1:10) in bidistilled water. Amount of MDA was measured in the samples dissolved (1:4).
Descriptive data were presented as a mean values (± standard deviation). One way Anova test was used for further statistical analyses. The value P<0.05 was considered statistically significant. All statistics were done using the SPSS computer package version 13 (SPSS, Chicago, IL, USA).
Findings
A total of 60 healthy nursing mothers of term infants, mean age 24±6,3 years (age range 18-36 years) were included in the study. 120 colostrum and 60 mature milk samples were analyzed for enzymes activity.
We have demonstrated the significant increase of PAO activity as well as marked decrease of DAO activity, throughout the first lactation month. We also found persistent drop of MDA levels during the same period.
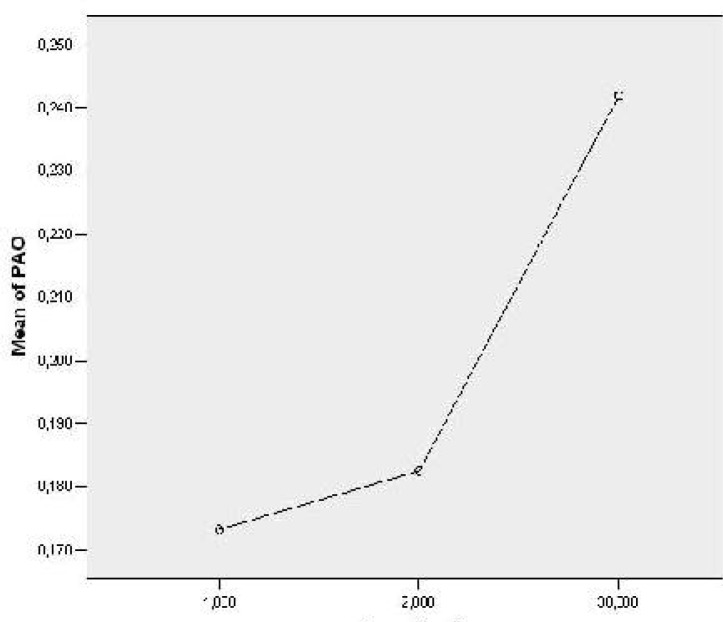
The PAO activity was significantly lower on the 1st (0.17±0.02 U/ml) as well as 2nd lactation day (0.18±0.03 U/ml) compared to PAO activity in mature milk, on the 30th lactation day (0.24±0.06 U/ml, P<0.01) (Fig. 1).
Fig. 1.
Measured values of PAO activity (Units/ml) in human colostrum and mature milk on the 1st, 2nd and 30th lactation day; 1-1st lactation day, 2-2nd lactation day and 3-30th lactation day.
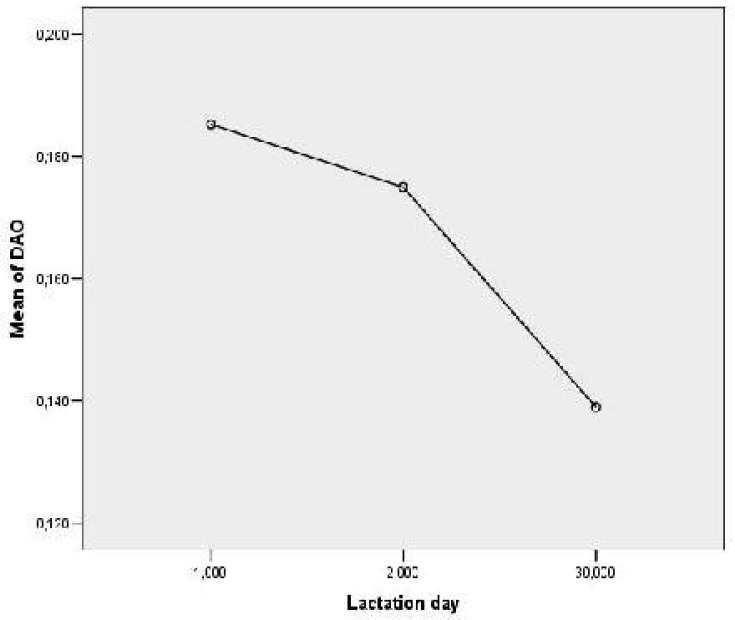
DAO activities were found to be significantly higher on the 1st (0.186±0.02 Units/L) as well as 2nd lactation day (0.175±0.02) compared to DAO activity measured on the 30th lactation day (0.139±0.026 Units/ml, P<0.01) (Fig 2).
Fig. 2.
Measured values of DAO activity (Units/ml) in human colostrum and mature milk on the 1st, 2nd and 30th lactation day; 1- st lactation day, 2-2nd lactation day and 3-30th lactation day.
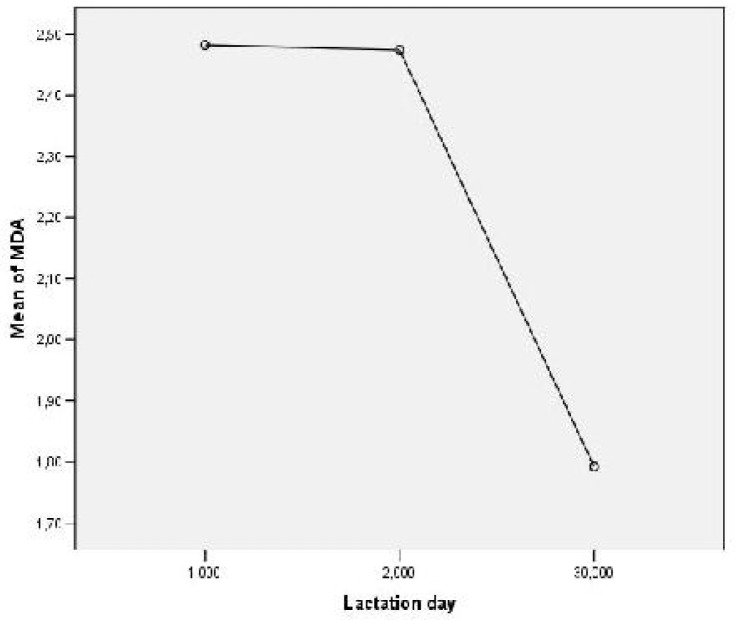
MDA levels, were higher in colostrum 1st and 2nd days of lactation (2.48±0.4 µmol/ml; 2.47±0.38 µmol/ml respectively), vs MDA level measured on the 30th day of lactation (1.7±0.26 µmol/ml, P<0.001) (Fig. 3).
Fig. 3.
Measured values of MDA levels (µmol/ml) in human colostrum and mature milk on the 1st, 2nd and 30th lactation day; 1-1st lactation day, 2-2nd lactation day and 3-30th lactation day.
Discussion
We have found the significant increase of human milk PAO activity during first month of lactation. On the other hand we have demonstrated marked decrease of DAO activity and MDA levels during the same period.
To investigate the effect of stage of lactation on the concentration of Sp, Spd, and PUT in sow colostrum and milk, Cheng et al 1997, observed the highest concentration of Sp in sow milk on day 7, with levels gradually declining to day 28 of lactation [17]. The decreasing levels of Sp was explained as a consequence of increased PAO activity, the same was observed in our investigation.
As an integral component of the polyamine inter-conversion pathway, PAO, has an important place in regulating cellular polyamine levels [7, 11, 18].
Our results could be explained as a reflection of PAO metabolic activity of lactating mammary gland on polyamines synthesis rate.
In spite of the fact that the mechanism of polyamines action is not completely elucidated, their role in regulating intestinal maturation and remodeling was recently emphasized [19–22]. It was shown that polyamines, mainly Sp, Spd and much less PUT have essential role in proliferation, differentiation and migration of mammalian cells. Recent data also suggest their important regulatory role on intestinal epithelial cell membrane structure, by means of nucleic acid and protein synthesis promotion, thus improving gut maturation by decreasing mucosal permeability to antigenic proteins. From the clinical viewpoint this could prevent the development of food allergies in suckling babies [23, 24], Considering our results, demonstrating significant increase in the activity of human milk PAO during first month of lactation, it could be possible that PAO via its metabolites (Spd or PUT) plays important role in gut maturation, enhancing the maternal milk polyamine supply in the first days of lactation.
Polyamine oxidase catalyzes the oxidative deamination of Sp and Spd, producing PUT as well as corresponding aminoaldehydes, amonia (NH3), and H2O2. These amino aldehydes and H2O2. are potentially toxic agents which are capable to induce oxidative stress. Thus, the increasing polyamine oxidase activity during the first month of lactation, observed in our investigation, may be interpreted as a body tendency to raise the levels of aminoaldehide as a potent antimicrobial agent [25, 26].
The naturally substrate for DAO is PUT. PUT levels in human milk remained very low and varied little during the first week postpartum [26].
DAO is also a histamine-degrading enzyme which is produced in high amounts in the placenta and has been supposed to act as a metabolic barrier to prevent excessive entry of bioactive histamine from the placenta into the maternal or fetal circulation [27]. The highest DAO activity in colostrum as well as decreased DAO activity in mature human milk (30th day of lactation), what we found, may be related in some way with the need of histamine degradation during early lactation [28, 29]
The importance of MDA in milk is even more difficult to evaluate. It was demonstrated that MDA from the blood might be excreted through the mammary gland to the colostrum before the parturition [30]. The question is whether it is formed or excreted from the mammary gland. The high concentrations at the beginning of lactation could support either theory. While investigating MDA levels in the first weeks of lactation, Smith et al 1976, found progressive MDA decrease during this period. The authors stated that dynamic changes of MDA level in their study, reflects the intensity of the metabolic changes, under endocrine regulation, that occur in the onset of lactation [31]. Our results are in agreement with this observation.
To summarize, mature human milk possesses a detectable activity of PAO and DAO, as well as considerable amount of MDA. PAO activity increases, meanwhile activity of DAO decreases during first month of lactation. The malondialdehyde (MDA) level, reflecting the level of lipid peroxidation and the final product of polyamine catabolism, also decreases.
It is most likely that the early changes in PAO and DAO activity as well as MDA level reflect merely the metabolic activity of the nursing mother mammary glands.
It is likely that human milk PAO throughout the lactation period, contributes to the protective effects of human milk via amino aldehydes and hydrogen peroxide (H2O2) which induce the oxidative stress. It is to be investigated however, whether these milk constituents have other possible health effects and benefits in various puerperal pathological clinical scenarios.
Our studies had limitations because the sample size was relatively small and the percentage of mothers with cesarean delivery was relatively high (13%), which may potentially influence the colostrum or mature milk composition [32].
Conclusion
Since the products of PAO activity such as, amino aldehydes and hydrogen peroxide (H2O2) might have potential antimicrobial effects, promoting the oxidative stress, it is likely that human milk PAO throughout the lactation period, contributes to the protective effects of human milk.
Acknowledgment
This work has been supported by the Serbian Ministry of Education and Science, grant No. TR31060
Conflict of Interest
None
References
- 1.Kelly S. Bovine colostrums: A review of clinical uses. Altern Med Rev. 2003;8(4):378–94. [PubMed] [Google Scholar]
- 2.Kulski K, Hartmann PE. Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci. 1981;59(1):101–4. doi: 10.1038/icb.1981.6. [DOI] [PubMed] [Google Scholar]
- 3.Blum W, Baumrucker R. Colostral and milk insulin-like growth factors and related substances: mammary gland and neonatal (intestinal and systemic) targets. Anim Endocrinol. 2002;23(1-2):101–10. doi: 10.1016/s0739-7240(02)00149-2. [DOI] [PubMed] [Google Scholar]
- 4.Sanguansermsri J, Georgy P, Zilliken F. Polyamines in human and cow's milk. Am J Clin Nutr. 1974;27(8):859–865. doi: 10.1093/ajcn/27.8.859. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Malo C, Buddington RK. Suckling induces rapid intestinal growth and changes in brush border function of newborn pigs. J Nutr. 1997;127(3):418–26. doi: 10.1093/jn/127.3.418. [DOI] [PubMed] [Google Scholar]
- 6.Moller K, Thymann T, Fink N, et al. Bovine colostrum is superior to enriched formulas in stimulating intestinal function and necrotising enterocolitis resistance in preterm pigs. Br J Nutr. 2011;105(1):44–53. doi: 10.1017/S0007114510003168. [DOI] [PubMed] [Google Scholar]
- 7.Tabor W, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–90. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 8.Zarban A, Taheri F, Chahkandi T, et al. Pattern of total antioxidant capacity in human milk during the course of lactation. Iran J Pediatr. 2007;17(Suppl 1):34–40. [Google Scholar]
- 9.Morgan L. Polyamine oxidase activity in human and bovine milk. Biochemical Society Transactions 610th meeting of the Biochemical Society; held on 6-7 Sept. 1984 ( Abstract) [Google Scholar]
- 10.Morgan L. Polyamine oxidases. Biochem Soc Trans. 1985;13:322–6. doi: 10.1042/bst0130322. [DOI] [PubMed] [Google Scholar]
- 11.Morgan L, Illei G. Polyamine-polyamine oxidase interaction: part of maternal protective mechanism against fetal rejection. Br Med J. 1980;280(6227):1295–7. doi: 10.1136/bmj.280.6227.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holinka F, Gurpide E. Diamine oxidase activity in human decidua and endometrium. Am J Obstet Gynecol. 1984;150(4):359–63. doi: 10.1016/s0002-9378(84)80138-6. [DOI] [PubMed] [Google Scholar]
- 13.Southern L, Kobayashi Y, Brenner P, Weingold B. Diamine oxidase activity in human maternal and fetal plasma and tissues at parturition. J Appl Physiol. 1965;20(5):1048–51. doi: 10.1152/jappl.1965.20.5.1048. [DOI] [PubMed] [Google Scholar]
- 14.Bachrach U, Reches B. Enzymic assay for spermine and spermidine. Anal Biochem. 1966;17(1):38–48. doi: 10.1016/0003-2697(66)90005-4. [DOI] [PubMed] [Google Scholar]
- 15.Quash G, Keolouangkhot T, Gazzolo L, et al. Diamine oxidase and polyamine oxidase activities in normal and transformed cells. Biochem J. 1979;177(1):275–82. doi: 10.1042/bj1770275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quash G, Ripoll H, Gazzolo L, et al. Malondialdehyde production from spermine by homogenates of normal and transformed cells. Biochimie. 1987;69(2):101–8. doi: 10.1016/0300-9084(87)90241-0. [DOI] [PubMed] [Google Scholar]
- 17.Cheng B, Li F, Ge R, Xing J. Polyamines in sow colostrum and milk at different stages of lactation. Animal Sci. 2006;82(5):95–9. doi: 10.2527/2004.8251339x. [DOI] [PubMed] [Google Scholar]
- 18.Holtta E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase? Biochemistry. 1977;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- 19.Fox F, Kelly L. Indigenous enzymes in milk: Overview and historical aspects-Part 1. Int Dairy J. 2006;16:500–16. [Google Scholar]
- 20.Janne J, Alhonen L, Pietila M, et al. Genetic approaches to the cellular functions of polyamines in mammals. Eur J Biochem. 2004;271(5):877–94. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 21.Harrison A. Milk xanthine oxidase: hazard or benefit? J Nutr Env Med. 2002;12:231–8. [Google Scholar]
- 22.Vujic S, Diegelman P, Bacchi J, et al. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367(Pt 3):665–75. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vujic S, Liang P, Diegelman P, et al. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370(Pt 1):19–28. doi: 10.1042/BJ20021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashiro Y, Sato M, Shimizu T, et al. Possible biological growth factors in breast milk and postnatal development of the gastrointestinal tract. Pediatr Int. 2007;31(4):417–23. doi: 10.1111/j.1442-200x.1989.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 25.Caron C, Cote J, Kremzner T. Putrescine, a source of gamma-aminobutyric acid in the adrenal gland of the rat. Biochem J. 1988;251(2):559–62. doi: 10.1042/bj2510559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi H, Nakajama T, Saano I. Metabolism of putrescine in the central nervous system. J Biochem. 1977;81(2):355–60. doi: 10.1093/oxfordjournals.jbchem.a131466. [DOI] [PubMed] [Google Scholar]
- 27.Maintz L, Schwarzer V, Bieber T, et al. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Human reproduction Update. 2008;14(5):485–95. doi: 10.1093/humupd/dmn014. [DOI] [PubMed] [Google Scholar]
- 28.Donley A, Ilagan B, Rim H, et al. Copper transport to mammary gland and milk during lactation in rats. Am J Physiol Endocrinol Metab. 2002;283(4):E667–75. doi: 10.1152/ajpendo.00115.2002. [DOI] [PubMed] [Google Scholar]
- 29.Torok E, Brewer I, Dolkart E. Serum diamine oxidase in pregnancy and in trophoblastic diseases. J Clin End Met. 1970;30(1):59–65. doi: 10.1210/jcem-30-1-59. [DOI] [PubMed] [Google Scholar]
- 30.Olayaki A, Ajao M, Jimoh A, et al. Effect of vitamin C on malondialdehyde in pregnant Nigerian women. J Bas Appl Sc. 2008;4:105–10. [Google Scholar]
- 31.Smith B, Ingerman M, Silver J. Malondialdehyde formation as an indicator of prostaglandin production by human platelets. J Lab Clin Med. 1976;88(1):167–72. [PubMed] [Google Scholar]
- 32.Jin YY, Zhao W, Cao RM. Cells in human milk of Han Chinese. J Hum Lact. 27(2):155–62. doi: 10.1177/0890334410392041. [DOI] [PubMed] [Google Scholar]