Abstract
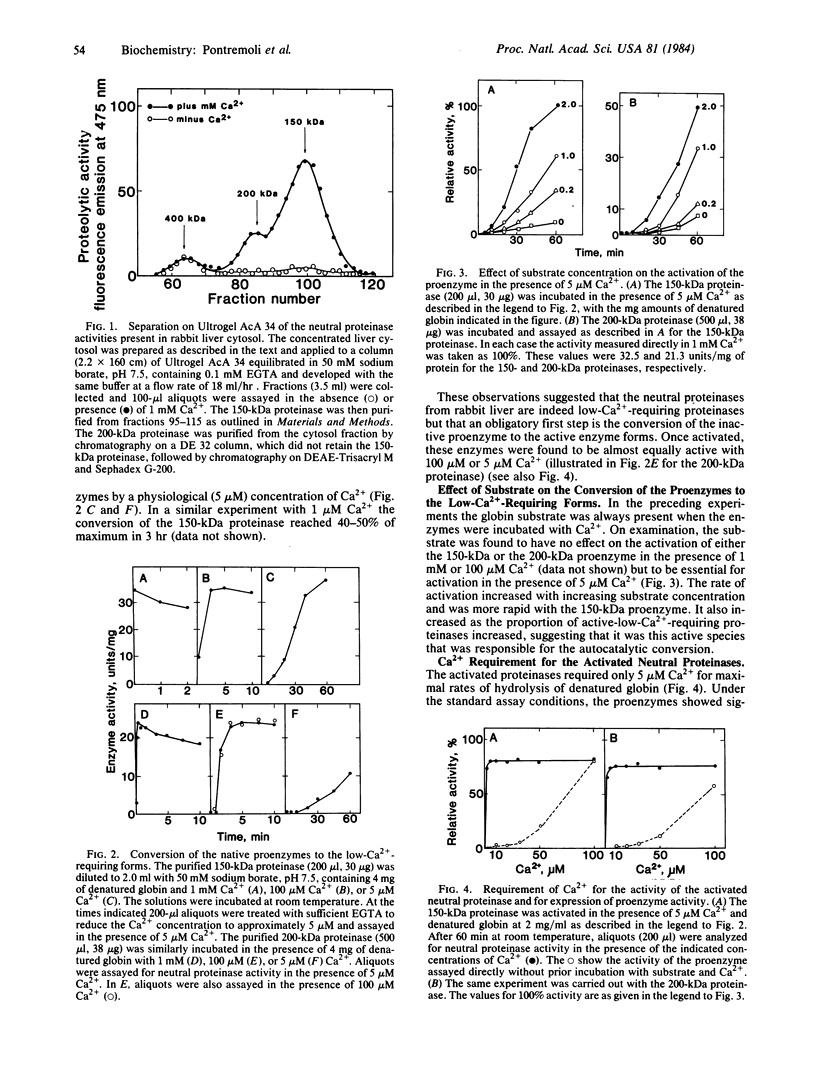
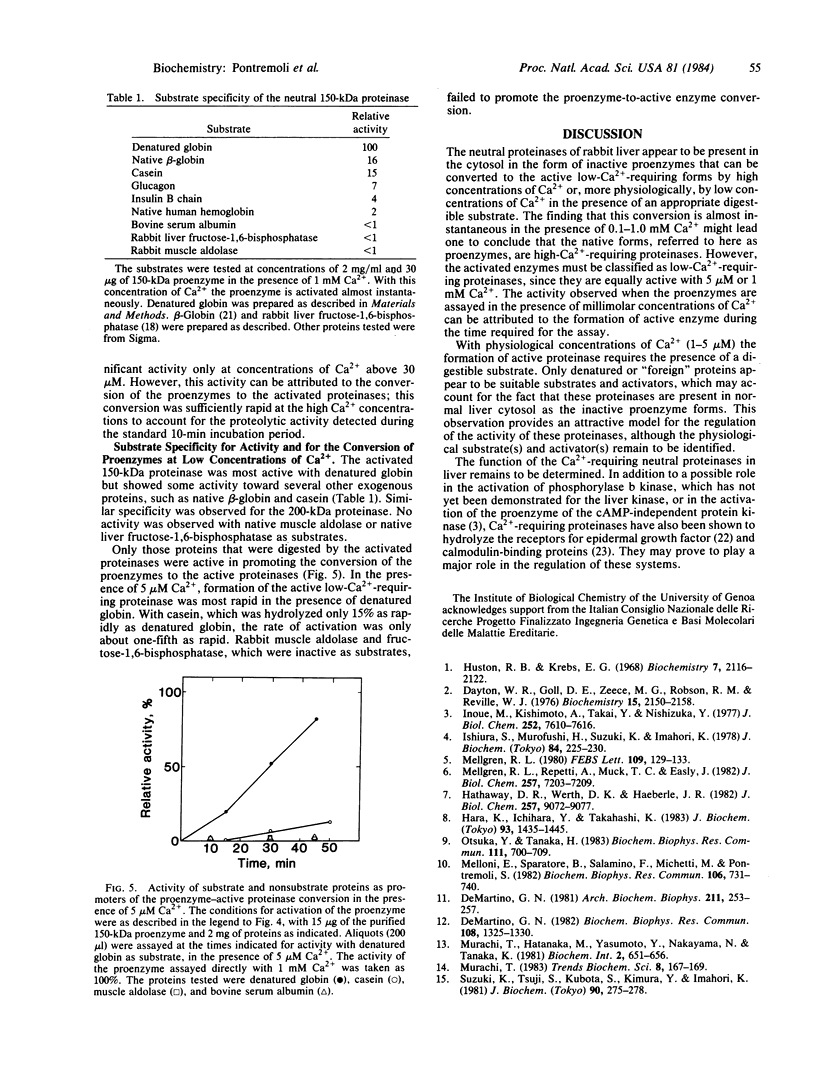
Two neutral Ca2+-dependent proteinases, differing in molecular size, have been isolated from rabbit liver. Both are recovered as inactive proenzymes that can be converted to the active forms by high (0.1-1.0 mM) concentrations of Ca2+ in the absence of substrate or, in the presence of a protein substrate, by low (1-5 microM) concentrations of Ca2+. The activated proteinases required only 1-5 microM Ca2+ for maximal activity. Substrates hydrolyzed were denatured globin, globin, casein, and to a lesser extent, several extracellular proteins; no digestion was observed with several intracellular cytosolic enzymes tested. Only those proteins that served as substrates were capable of promoting conversion of the proenzymes to the active low-Ca2+-requiring proteinases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dayton W. R., Goll D. E., Zeece M. G., Robson R. M., Reville W. J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976 May 18;15(10):2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- De Martino G. N. Calcium-dependent proteolytic activity in rat liver: identification of two proteases with different calcium requirements. Arch Biochem Biophys. 1981 Oct 1;211(1):253–257. doi: 10.1016/0003-9861(81)90452-5. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N. Cytoplasmic proteases of rat liver parenchymal cells. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1325–1330. doi: 10.1016/0006-291x(82)92145-3. [DOI] [PubMed] [Google Scholar]
- Gates R. E., King L. E., Jr Proteolysis of the epidermal growth factor receptor by endogenous calcium-activated neutral protease from rat liver. Biochem Biophys Res Commun. 1983 May 31;113(1):255–261. doi: 10.1016/0006-291x(83)90459-x. [DOI] [PubMed] [Google Scholar]
- HAYMAN S., ALBERTY R. A. The isolation and kinetics of two forms of fumarase from torula yeast. Ann N Y Acad Sci. 1961 Nov 2;94:812–824. doi: 10.1111/j.1749-6632.1961.tb35575.x. [DOI] [PubMed] [Google Scholar]
- Hara K., Ichihara Y., Takahashi K. Purification and characterization of a calcium-activated neutral protease from monkey cardiac muscle. J Biochem. 1983 May;93(5):1435–1445. doi: 10.1093/oxfordjournals.jbchem.a134279. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Werth D. K., Haeberle J. R. Limited autolysis reduces the Ca2+ requirement of a smooth muscle Ca2+-activated protease. J Biol Chem. 1982 Aug 10;257(15):9072–9077. [PubMed] [Google Scholar]
- Huston R. B., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by Ca2+. II. Identification of the kinase activating factor as a proteolytic enzyme. Biochemistry. 1968 Jun;7(6):2116–2122. doi: 10.1021/bi00846a014. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Ishiura S., Murofushi H., Suzuki K., Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. I. Purification and characterization. J Biochem. 1978 Jul;84(1):225–230. doi: 10.1093/oxfordjournals.jbchem.a132111. [DOI] [PubMed] [Google Scholar]
- Kosaki G., Tsujinaka T., Kambayashi J., Morimoto K., Yamamoto K., Yamagami K., Sobue K., Kakiuchi S. Specific cleavage of calmodulin-binding proteins by low Ca2+-requiring form of Ca2+-activated neutral protease in human platelets. Biochem Int. 1983 Jun;6(6):767–775. [PubMed] [Google Scholar]
- Mellgren R. L. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980 Jan 1;109(1):129–133. doi: 10.1016/0014-5793(80)81326-3. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L., Repetti A., Muck T. C., Easly J. Rabbit skeletal muscle calcium-dependent protease requiring millimolar CA2+. Purification, subunit structure, and Ca2+-dependent autoproteolysis. J Biol Chem. 1982 Jun 25;257(12):7203–7209. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Salamino F., Sparatore B., Michetti M., Horecker B. L. Characterization of three rabbit liver lysosomal proteinases with fructose 1,6-bisphosphatase converting enzyme activity. Arch Biochem Biophys. 1981 Apr 15;208(1):175–183. doi: 10.1016/0003-9861(81)90137-5. [DOI] [PubMed] [Google Scholar]
- Melloni E., Salamino F., Sparatore B., Michetti M., Pontremoli S. Cooperation between soluble and membrane-bound proteinases in the degradation of beta-hemoglobin chains in intact human erythrocytes. Arch Biochem Biophys. 1982 Jul;216(2):495–502. doi: 10.1016/0003-9861(82)90238-7. [DOI] [PubMed] [Google Scholar]
- Melloni E., Sparatore B., Salamino F., Michetti M., Pontremoli S. Cytosolic calcium dependent proteinase of human erythrocytes: formation of an enzyme-natural inhibitor complex induced by Ca2+ ions. Biochem Biophys Res Commun. 1982 Jun 15;106(3):731–740. doi: 10.1016/0006-291x(82)91772-7. [DOI] [PubMed] [Google Scholar]
- Nakai N., Lai C. Y., Horecker B. L. Use of fluorescamine in the chromatographic analysis of peptides from proteins. Anal Biochem. 1974 Apr;58(2):563–570. doi: 10.1016/0003-2697(74)90225-5. [DOI] [PubMed] [Google Scholar]
- Otsuka Y., Tanaka H. Purification of new calcium activated protease (low calcium requiring form) and comparison to high calcium requiring form. Biochem Biophys Res Commun. 1983 Mar 16;111(2):700–709. doi: 10.1016/0006-291x(83)90362-5. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Ishiura S., Kimura Y., Kubota S., Imahori K. Autolysis of calcium-activated neutral protease of chicken skeletal muscle. J Biochem. 1981 Dec;90(6):1787–1793. doi: 10.1093/oxfordjournals.jbchem.a133656. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Kubota S., Kimura Y., Imahori K. Limited autolysis of Ca2+-activated neutral protease (CANP) changes its sensitivity to Ca2+ ions. J Biochem. 1981 Jul;90(1):275–278. doi: 10.1093/oxfordjournals.jbchem.a133463. [DOI] [PubMed] [Google Scholar]
- Traniello S., Melloni E., Pontremoli S., Sia C. L., Horecker R. L. Rabbit liver fructose 1,6-diphosphatase. Properties of the native enzyme and their modification by subtilisin. Arch Biochem Biophys. 1972 Mar;149(1):222–231. doi: 10.1016/0003-9861(72)90317-7. [DOI] [PubMed] [Google Scholar]



