Abstract
Objective
Constipation is a common problem in children. There is some clinical evidence for the role of probiotics and prebiotics in the treatment of constipated children. This is the first study on the therapeutic effect of synbiotics (combination of probiotics and prebiotic) in treatment of childhood constipation.
Methods
In a double-blind randomized placebo controlled study 102 children aged 4–12 years with functional constipation were assessed according to Rome III criteria for 4 weeks. They were divided into 3 groups: Group A, received 1.5 ml/kg/day oral liquid paraffin plus placebo, group B, 1 sachet synbiotic per day plus placebo and group C, 1.5 ml/kg/day oral liquid paraffin plus 1 sachet synbiotic per day. Frequency of bowel movements (BMs), stool consistency, number of fecal incontinence episodes, abdominal pain, painful defecation per week, success of treatment and side effects were determined in each group before and after treatment.
Findings
The frequency of BMs per week increased in all groups (P<0.001), but it differed between groups and was higher in group C (P=0.03). Stool consistency increased and number of fecal incontinence episodes, abdominal pain and painful defecation per week decreased in all groups similarly and there was statistically no difference between them. No side effects were reported in group B; the main side effect in group A and C was seepage of oil (P<0.001). Treatment success was similar in all groups without any significant difference between them (P=0.6).
Conclusion
This study showed that synbiotics have positive effects on symptoms of childhood constipation without any side effects.
Keywords: Constipation, Synbiotic, Probiotics, Prebiotics, Paraffin oil
Introduction
Childhood constipation is a common complaint accounting for 3% of visits to pediatricians and 10-25% of visits to pediatric gastroenterologist[1–3]. Commonly there is no underlying organic cause and the constipation is indentified as functional or idiopathic[4, 5].
Functional constipation is identified by Rome III criteria in children over 4 years old and adolescents having two months of at least 2 or more of the following conditions: ≤2 defecations in the toilet per week, ≥1 episode of fecal incontinence per week, history of retentive posturing or excessive volitional stool retention, history of painful or hard bowel movements, presence of a large fecal mass in the rectum, history of large diameter stools that may obstruct the toilet[4]. Children usually are treated with a combination of toilet training, running a bowel diary, and oral laxatives such as paraffin oil. In many patients this does not provide effective improvement so newer treatment is required[6, 7].
There are many articles on the efficacy of probiotics in organic and functional gastrointestinal disorders. Probiotics are live microbial food ingredients which are reported to be effective in the treatment of many forms of gastrointestinal disorders[8–12]. Colonic micro-flora influences the motility of the colon[12, 13]. Prebiotic is a selectively fermented ingredient that changes the composition and/or activity of the intestinal microflora and has shown beneficial effects in host's health[14]. Synbiotic is a product that contains both probiotic and prebiotic.
In this study, we aimed to determine the therapeutic effect of synbiotics (combination of probiotics containing strains of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, and fructooligo-saccharide as prebiotic) on childhood constipation.
Subjects and Methods
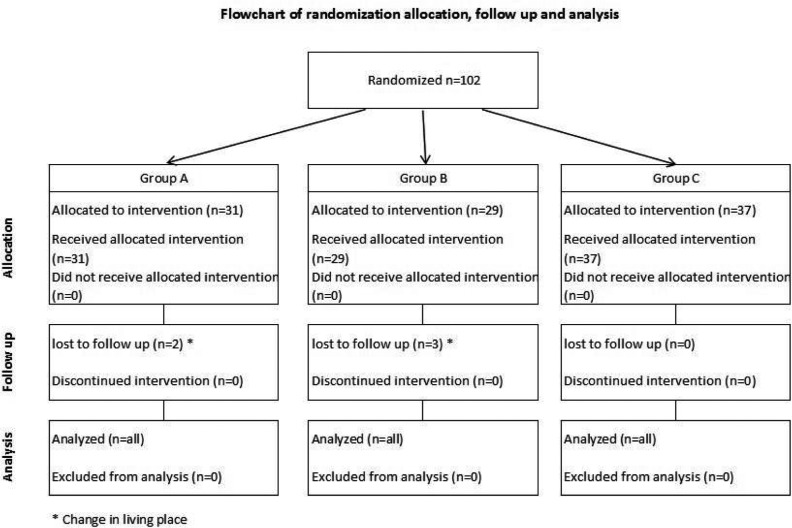
The study was performed at Children's Medical Center in Tehran, Iran, from January to December 2009. Children with constipation were referred to the pediatric gastroenterology clinic for evaluation and treatment of constipation. One-hundred two children aged 4–12 years with chronic functional constipation enrolled in this study, were divided into 3 groups by simple randomization method.
Inclusion criterion was childhood functional constipation defined by Rome III criteria. Exclusion criteria were organic causes for constipation such as Hirschsprung's disease, spina bifida occulta, hypothyroidism, cystic fibrosis, neurologic abnormalities and intestinal pseudo-obstruction. These patients were excluded from the study by history and physical examination and Rome III criteria of childhood constipation.
The research protocol was approved by the medical ethics committee of Tehran University of Medical Sciences. Informed consent was obtained from parents of all patients before any intervention. The randomization and allocation sequence was generated before study began by our biostatistics consultant. Patients assigned to each group were selected randomly. Label of drugs was replaced by a new label indicating drug A or B. Contents of sachets or bottles were not known to the physicians or nurses involved in the study.
Group A received 1.5 ml/kg/day oral liquid paraffin plus placebo, group B received 1 sachet synbiotic per day (restore* 1x109 CFU/1 sachet, Protexin Co, UK). Synbiotic combination consisted of probiotic strains containing L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, fructooligosaccharide as prebiotic, and placebo. Group C received 1.5 ml/kg/day oral liquid paraffin and 1 sachet synbiotic per day. All patients in the 3 groups received drugs in bottles and sachets with similar shape, taste and color.
Groups were studied for 4 weeks. Frequency of BMs, stool consistency, number of fecal incontinence, episodes of abdominal pain, painful defecation per week and side effects were determined in each patient before and after treatment. Success treatment was defined as ≥3 BMs per week, ≤2 incontinence per month and no abdominal pain[15].
Study design: From seven days prior to study and during the treatment period all children were requested to record frequency of BMs, number of fecal incontinence episodes, stool consistency, abdominal pain, painful defecation and effects such as vomiting, diarrhea and oil seepage in a bowel diary.
Before starting treatment, all children were examined and patients with fecal impaction received rectal enema (paraffin oil 15–30ml/year) once daily for three days in order to accomplish rectal disimpaction. Dietary and toilet training advice was given to all patients similarly. Toilet training consisted of sitting on the toilet 3 times per day for 5 minutes after each meal. Patients were not allowed to use any other kind of medication for constipation during the study period. Clinical efficacy was recorded, patients were seen at the end of 4 weeks, and their charts were reviewed.
Outcome measures: Primary outcome measures were frequency of BMs per week, stool consistency, fecal incontinence episodes per week, presence of abdominal pain, and painful defecation; secondary outcomes were success treatment and incidence of adverse effects such as vomiting, diarrhea and seepage. Stool consistency was rated by the patients as hard, normal or watery.
Statistical analysis: Data of baseline information were analyzed with the SPSS (Ver. 17). Continuous variables were expressed as mean (SD), and categorical data were shown as frequency and percent. Number of BMs and fecal incontinence episodes in baseline information were analyzed by Kruskal-Wallis test. The contingency table (Chi square) was used.
Change of frequency of BMs and fecal incontinence episodes were analyzed by non-parametric paired Wilcoxon test. For assessment changes of stool consistency and abdominal pain between baseline and after treatment, the McNemar test was used. P values below 0.05 were considered significant.
Findings
Totally, 102 children (aged 4–12 years) with chronic functional constipation enrolled in this study and were divided into 3 groups. Five patients were lost to follow-up and remaining 97 patients consisted of 47.4% males and 52.6% females with a mean age of 6.3±2.1 years.
As shown in Table 1, no significant differences were found with respect to demographic data and recorded baseline characteristics between the three treatment groups. Characteristics of the patients and the main outcomes are summarized in Table 1 and 2. As shown in Table 2, the frequency of BMs per week increased in all groups, the highest rise occurring in group C (P=0.03). Improvement in stool consistency and decrease in number of fecal incontinence episodes happened in all groups (P=0.2, P=0.3 respectively) without any statistically significant difference. Also, abdominal pain and painful defecation per week decreased (P=0.6, P=0.9 respectively) similarly and there was no statistically significant difference between groups. No side effects were reported in group B but in group A 18 patients and in group C 21 patients had seepage (P<0.001). Treatment success in group A was 24/29 (82.8%), in group B 22/31 (71.0%) and in group C 28/37 (75.7%) without statistically significant difference between them (P=0.6).
Table 1.
Baseline characteristics of three groups
| Characteristic | Liquid paraffin+Placebo | Synbiotics+Placebo | Liquid paraffin+Synbiotics | P value |
|---|---|---|---|---|
| No of patients at randomization | 29 | 31 | 37 | |
| Mean age (yr) (±SD) | 6.9 (±2.4) | 6.2 (±1.9) | 5.9 (±2.2) | 0.191 |
| Sex, males (%) | 13 (44.8) | 15 (48.4%) | 18 (48.6%) | 0.945 |
| No. of patients with history of defecation frequency ≤2/week | 19 (65.5) | 17 (54.8%) | 28 (75.7%) | 0.195 |
| No. of patients with history of encopresis ≥1 | 10 (34.5) | 13 (41.9%) | 9 (24.3%) | 0.300 |
| Painful defecation (%) | 21 (72.4) | 20 (64.5) | 28 (75.7) | 0.590 |
| Abdominal pain (%) | 17 (58.6) | 21 (67.7) | 24 (64.9) | 0.754 |
| Hard stool consistency (%) | 25 (86.2) | 27 (87.1) | 32 (86.5) | 0.911 |
| Patients lost to follow-up | 3 | 2 | 0 |
Table 2.
Comparison of characteristics between three groups before and after treatment
| Characteristic | Liquid paraffin+Placebo | Synbiotics+Placebo | Liquid paraffin+Synbiotics | P value |
|---|---|---|---|---|
| No of patients at randomization (%) | 29 (29.9) | 31 (32.0) | 37 (38.1) | – |
| No. of stool frequency per week pretreatment [median] (±SD) | 1.81 [1.55] (±0.9) | 2.19 [1.75] (±1.9) | 1.83 [1.40] (±1.5) | 0.665 |
| No. of stool frequency per week after treatment [median] (±SD) | 6.75 [7.00] (±2.6) | 5.22 [3.50] (±3.2) | 7.49 [7.00] (±4.4) | 0.030 |
| No. of patients with hard stool consistency pretreatment (%) | 25 (86.2) | 27 (87.1) | 32 (86.5) | 0.911 |
| No. of patients with hard stool consistency after treatment (%) | 2 (6.9) | 7 (22.6) | 4 (10.8) | 0.172 |
| No. of patients with painful defecation pretreatment (%) | 21 (72.4) | 20 (64.5) | 28 (75.7) | 0.590 |
| No. of patients with painful defecation after treatment (%) | 2 (6.9) | 3 (9.7) | 4 (10.8) | 0.859 |
| No. of encopresis per week pretreatment (±SD) | 2.34 (±4.9) | 2.68 (±4.7) | 0.92 (±2.9) | 0.208 |
| No. of Encopresis per week after treatment (±SD) | 0.24 (±1.3) | 0.06 (±0.25) | 0.0 (±0.0) | 0.317 |
| No. of patients with abdominal pain pretreatment (%) | 17 (58.6) | 21 (67.6) | 24 (64.9) | 0.754 |
| No. of patients with abdominal pain after treatment (%) | 4 (13.8) | 2 (6.5) | 5 (13.5) | 0.582 |
| No. of patients with side effects (seepage) (%) | 18 (62.1) | 0 (0) | 21 (56.8) | < 0.001 |
| No. of patients with successful treatment (%) | 24/29 (82.8) | 22/31 (71.0) | 28/37 (75.7) | 0.559 |
Discussion
New investigations have shown that gut bacterial colonization has an important role in health and disease. Probiotics are live microorganisms which in adequate amounts have beneficial effects in host[16].
There are several hypotheses about the effectiveness of this microbial flora in treatment of constipation. According to some investigations gut microbial flora in chronic constipation is different from that in healthy persons[17, 18]. Short chain fatty acids produced by probiotics reduce colon pH and by this mechanism enhance colon motility and finally transit time will decrease.
There are a few RCTs about probiotics in the treatment of constipation in children[19]. In RCTs study by Banaszkiewicz and Szajewska it was concluded that L. rhamnosus GG was not an effective adjunct to lactulose in treating constipation in children[20]. Bu et al[11] evaluated the effect of L. casei rhamnosus Lcr35 compared to magnesium oxide(Mg0).Comparisons of the frequency of defecation, consistency of stool and the use of lactulose or enema during the period of treatment were made among the groups. The patients who received MgO or probiotics had a higher defecation frequency, percentage of treatment success (defined as ≥3 spontaneous defecations per week with no episodes of fecal soiling) and less hard stool. There is no statistically significant difference in efficacy between MgO and Lcr35, but less abdominal pain occurred when using Lcr35. In a study by Coccorullo et al[21] the beneficial effects of L. reuteri (DSM 17938) in infants with functional chronic constipation was evaluated. Administration of L. reuteri had a positive effect on bowel frequency, even when there was no improvement in stool consistency and episodes of inconsolable crying episodes. Evidence from non-RCTs suggests that at least some probiotics may be effective. For example, in children with constipation defined according to the Rome III criteria, administration of Bifidobacteria (B. bifidum, B. infantis, B. longum) and Lactobacilli (L. casei, L. plantarum, L. rhamnosus) to 20 children aged 4–16 years resulted in an increased frequency of bowel movements, a decreased number of fecal incontinence episodes, and reduced abdominal pain, although there was no change in stool consistency[12]. It seems that our study is the first RCT study which investigates the synbiotics effects in childhood constipation. According to our study, mixtures of different strains of probiotics and their specialized prebiotics are more effective than each of them alone and this combination augments their efficacy in all parameters of study, but in previous researches improvement had been seen only in some of symptoms and signs. Also adjunctive therapy of synbiotic and liquid paraffin could be more effective to improve BMs than any of them alone.
Conclusion
According to this RCT, synbiotic have got beneficial effects on symptoms of childhood constipation similar to liquid paraffin without any side effects and synbiotic is an effective adjunct to liquid paraffin to improve BMs.
Acknowledgment
This study was approved by Research committee of Faculty of Medicine in Tehran University of Medical Sciences and registration ID of this study in Iranian Registry of Clinical Trials was# IRCT138811193309N1.
Footnotes
*A unique soluble probiotic and prebiotic specially formulated for babies and young children
Conflict of Interest
None
References
- 1.Candelli M, Nista EC, Zocco MA, Gasbarrini A. Idiopathic chronic constipation: pathophysiology, diagnosis and treatment. Hepatogastroenterology. 2001;48(40):1050–7. [PubMed] [Google Scholar]
- 2.Coffie J, Fitzgeral F. Pediatric Gastrointestinal Diseases. Baltimore: Mosby; 2000. Idiopathic constipation; pp. 830–41. [Google Scholar]
- 3.Farahmand F. A randomised trial of liquid paraffin versus lactulose in the treatment of chronic functional constipation in children. Acta Medica Iranica. 2007;45(3):183–8. [Google Scholar]
- 4.Rahhal R, Uc A. Motility disorders. In: Kleinman RE, Sanderson IR, Goulet O, editors. Walker's Pediatric Gastrointestinal Disease. 5th ed. BC Decker, Hamilton: 2008. pp. 675–82. [Google Scholar]
- 5.Karami H, Khademloo M, Niari P. Polyethylene glycol versus paraffin for the treatment of childhood functional constipation. Iran J Pediatr. 2009;19(3):255–61. [Google Scholar]
- 6.Voskuijl W, de Lorijn F, Verwijs W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut. 2004;53(11):1590–4. doi: 10.1136/gut.2004.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongers ME, Benninga MA, Maurice-Stam H, Grootenhuis MA. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual Life Outcomes. 2009;7:20. doi: 10.1186/1477-7525-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szymanski H, Pejcz J, Jawien M, et al. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains – a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23(2):247–53. doi: 10.1111/j.1365-2036.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 9.Doron S, Gorbach SL. Probiotics: their role in the treatment and prevention of disease. Expert Rev Anti Infect Ther. 2006;4(2):261–75. doi: 10.1586/14787210.4.2.261. [DOI] [PubMed] [Google Scholar]
- 10.Kruis W. Review article: antibiotics and probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(4):75–8. doi: 10.1111/j.1365-2036.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 11.Bu LN, Chang MH, Ni YH, et al. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr Int. 2007;49(4):485–90. doi: 10.1111/j.1442-200X.2007.02397.x. [DOI] [PubMed] [Google Scholar]
- 12.Bekkali NL, Bongers ME, Van den Berg MM, et al. The role of a probiotics mixture in the treatment of childhood constipation: a pilot study. Nutr J. 2007;6:17. doi: 10.1186/1475-2891-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picard C, Fioramonti J, Francois A, et al. Review article: bifidobacteria as probiotic agents, physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22(6):495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 14.Szajewska H. Prebiotics, synbiotics, and fermented products. In: Kleinman RE, Sanderson IR, Goulet O, editors. Walker's Pediatric Gastrointestinal Disease. 5th ed. BC Decker, Hamilton; 2008. pp. 399–409. [Google Scholar]
- 15.Loening-Baucke V. Constipation and encopresis. In: Wyllie R, Hyams JS, editors. Pediatric Gastrointestinal and Liver Disease. 3rd edn. Philadelphia: Saunders-Elsevier; 2006. pp. 177–91. [Google Scholar]
- 16.Joint FAO/WHO . London, Ontario, Canada: 2002. Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. [Google Scholar]
- 17.Zoppi G, Cinquetti M, Luciano A, et al. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998;87(8):836–41. doi: 10.1080/080352598750013590. [DOI] [PubMed] [Google Scholar]
- 18.Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand J Gastroenterol Suppl. 1997;222:45–8. doi: 10.1080/00365521.1997.11720717. [DOI] [PubMed] [Google Scholar]
- 19.Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: Probiotics for functional constipation. World J Gastroenterol. 2010;16(1):69–75. doi: 10.3748/wjg.v16.i1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr. 2005;146(3):364–9. doi: 10.1016/j.jpeds.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Coccorullo P, Strisciuglio C, Martinelli M, et al. Lactobacillus reuteri (DSM 17938) in Infants with Functional Chronic Constipation: A Double-Blind, Randomized, Placebo-Controlled Study. J Pediatr. 2010;157(4):598–602. doi: 10.1016/j.jpeds.2010.04.066. [DOI] [PubMed] [Google Scholar]