Abstract
Objective
Human metapneumovirus (hMPV) is a respiratory pathogen responsible for disease and subsequent hospitalizations in young children around the world. The disease pathology, including how viral load correlates with respiratory disease severity, remains unclear. This study investigated the correlation between viral load and clinical characteristics of hMPV infections.
Methods
Nasopharyngeal aspirate (NPA) samples collected from 18 infants hospitalized for lower respiratory tract infections (LRTIs) in winter were tested for hMPV by reverse transcriptase polymerase chain reaction (RT-PCR) and real-time RT-PCR. Their NPA samples were collected every-other-day to monitor changes in hMPV viral load during hospitalization. Also all these 18 patients were monitored to characterize clinically their illness.
Findings
hMPV load was not correlated with infection severity (P=0.5, 0.9, 0.5). In contrast, the log10 of hMPV viral load was significantly different between those lasted for 6–11 days and those for less than 5 days (P=0.01), also the significant difference was shown between those of 6–11 days duration and those of more than 11 days (P=0.006), but there was no significant difference between those lasted for less than 5 days and those for more than 11 days (P=0.4). Additionally, high hMPV viral shedding occured between 6 and 11days.
Conclusion
hMPV load was significantly correlated with the course of illness. The association between hMPV viral load and the course of disease suggested that hMPV is an important pathogen in lower respiratory tract infection in children. But hMPV did not always lead to more severe respiratory illness.
Keywords: Human metapneumovirus, Lower respiratory tract infection, Real-time RT-PCR, Nasopharyngeal aspirate, Viral load
Introduction
Acute respiratory tract infections (ARTIs) are a leading cause of morbidity and mortality in children worldwide[1, 2]. Many viruses are associated with respiratory syndromes in all age groups[3, 4]. However, inadequate diagnostic methods and unknown viral pathogens limit current understanding of ARTI etiology.
One known viral pathogen, human metapneumovirus (hMPV), is a globally distributed pathogen associated with respiratory infection in children[5–15]. After the pathogen respiratory syncytial virus (RSV), hMPV is the leading cause of respiratory infection in children in influenza off-seasons. One study suggested that, in China, almost all children are exposed to hMPV by 6 years of age[16]. In Chongqing, China, hMPV was found in children with ARTIs[17]. These studies suggest that hMPV has significant epidemiological and pathological impacts as a significant respiratory pathogen in children.
Previous hMPV studies focused on its epidemiological characteristics and compared its viral genetics to those of RSV. However, whether the hMPV viral load is correlated with the severity of respiratory infection remains unknown.
Compared to conventional methods, such as virus culture and indirect immunofluorescence, real-time reverse transcriptase polymerase chain reaction (real-time PCR) is more sensitive and efficient at quantifying viral load[18–20]. With real-time RT-PCR, we evaluated the changes in hMPV viral load every-other-day from 18 hospitalized children with lower respiratory tract infections to elucidate the association of hMPV viral load in airway with the disease course and severity.
Subjects and Methods
Patients'clinical data and NPAs collection: Eighteen children hospitalized in the respiratory medicine division of Children's Hospital of Chongqing Medical University from December 2007 to January 2008 with LRTIs were enrolled in the study. Parents of all 18 patients gave the consent for intervention. Besides, all the protocols have been approved by Institutional Review Board, Chongqing Medical University. For each patient following clinical data were collected: age at sample collection, gender, diagnosis, days since onset of illness, hospitalization duration, co-infection, and outcome of illness (Table 1). The scoring system used for respiratory disease severity assessment in children has been reported by Wang et al[21], Nasr et al[22], Mandelberg[23] and others.
Table 1.
Clinical characteristics and descriptors for the 18 hMPV-positive cases
| Case | Sex | Age (month) | RSV by PCR | Sputum culture | Onset days | Diagnosis | Length of stay | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | male | 6.6 | + | Moraxella catarrhalis | 4 | bronchiolitis | 6 | cured |
| 2 | male | 3.6 | ++ | - | 5 | bronchiolitis | 7 | cured |
| 3 | female | 1.7 | + | - | 1 | pneumonia | 10 | cured |
| 4 | male | 2.7 | − | Hemophilus parainfluenzae | 7 | bronchiolitis/diarrhea | 7 | cured |
| 5 | female | 3.5 | + | Hemophilus parainfluenzae/ Moraxella catarrhalis | 6 | pneumonia | 6 | cured |
| 6 | male | 3.4 | - | - | 27 | pneumonia | 11 | cured |
| 7 | male | 3 | − | Hemophilus parainfluenzae | 9 | bronchiolitis/diarrhea | 8 | cured |
| 8 | female | 2 | + | - | 2 | bronchiolitis | 8 | cured |
| 9 | male | 5 | - | - | 15 | pneumonia/diarrhea | 3 | improved |
| 10 | female | 2 | − | Klebsiella pneumonia | 15 | bronchiolitis | 5 | cured |
| 11 | male | 5 | + | Streptococcus pneumonia | 4 | bronchiolitis | 6 | cured |
| 12 | male | 14 | − | - | 6 | asthma/pneumonia/anemia | 7 | cured |
| 13 | male | 17 | + | - | 20 | pneumonia/congenital heart disease/diarrhea | 7 | cured |
| 14 | male | 11.5 | - | - | 7 | bronchiolitis | 3 | improved |
| 15 | female | 29 | + | - | 7 | pneumonia | 6 | cured |
| 16 | male | 4.7 | + | - | 6 | bronchiolitis | 8 | cured |
| 17 | male | 16 | ++ | MP+/ Streptococcus pneumonia | 9 | pneumonia/anemia | 9 | cured |
| 18 | male | 6 | + | Staphylococcus aureus | 14 | pneumonia/laryngitis/otitis media/diarrhea | 2 | improved |
A severity score was assigned for each of four categories (respiratory rate, retractions, wheeze, and general appearance). A single point was given to patients with a respiratory rate of 31 to 45 breaths/min, or wheezing at terminal expiration or audible only with stethoscope, or intercostal retraction, and normal general condition. Two points were given to patients with a respiratory rate of 45 to 60 breaths/min, or wheezing during the entire expiration or audible on expiration without stethoscope, or tracheosternal retractions, and stable general condition. Three points were given to patients with a respiratory rate over 60 breaths/min, or inspiratory and expiratory wheezing without stethoscope, or severe retraction with nasal flaring, or disturbance of general condition including irritability, lethargy, and poor feeding. For each patient, the total severity score was calculated by summing the score for each category. Oxygen saturation and the need for supplemental oxygen were also recorded. Oxygen saturation was measured using Nellcor Oximeter (HEMENS, SC6002XL).
From each patient, about 0.5 ml NPA sample was collected every time. NPAs were sampled every-other-day during hospitalization. Overall, 68 NPAs were collected from the 18 hMPV-positive children. Each sample was centrifuged twice at 1500 rpm for 10 min. Between the two centrifugations, 1 ml of PBS was added to each sample. After centrifugation, supernatants of NPAs were labeled and stored at −70°C for future batch detection.
RT-PCR for hMPV and RSV detection: The supernatants of NPAs were used as templates for real-time RT-PCR of the fusion glycoprotein (F) gene from hMPV and for the G glycoprotein (G) gene from RSV. Total RNA was extracted from each NPA sample with the QIAamp Viral RNA Kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol. Approximately 6.5 µl of each RNA sample was incubated in a solution containing 100 µM of random 6-mers and 50 µM of Oligo-dT Primer (PrimeScriptTM RT reagent Kit, TaKaRa) in a final volume of 10 µl at 37°C for 15 min followed by 5 s at 85°C to synthesize cDNA. The F gene from hMPV was amplified using virus specific primers (10 µM), (Table 2) in a 25 µl reaction mixture containing 2 µl cDNA, 8.5 µl ddH2O, 12.5 µl 2×Master Mix, and 1 µl forward and 1 µl reverse primer for hMPV virus; and 25 µl reaction for amplification of G gene from RSV containing 3 µl cDNA, and 22 µl Master Mixture including 200 µM dNTPs, 1.5 mM MgCl2, 1.5 U Taq DNA polymerase, and 50 pM of each of the forward and reverse primers for RSV virus. The PCR cycling conditions for hMPV included initial denaturation at 94°C for 4 min, followed by 40 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. At the end, 72°C for 10 min again; the PCR cycling conditions for RSV included initial denaturation at 94°C for 3 min, followed by 33 cycles of 94°C for 1 min, 50°C for 1min and 72°C for 1.5 min. Finally, again 72°C for 10 min. Products were visualized on agarose gels.
Table 2.
Sequences of primers and probes for detection of hMPV and RSV
| Target | Primer/probe name | Position at the 5end of strain | Primer/probe sequence (5–3) |
|---|---|---|---|
| hMPV | hMPVF(real-time) | 38 | CGTTTCTTACATGCCGACATCTG |
| hMPV | hMPVR(real-time) | 143 | GCTCCCGTAGACCCCTATCAG |
| hMPV | hMPVP | 89 | (FAM)CCCTTTCTTCGCACCATCGCACGG(Eclipse) |
| hMPV | hMPVF | 3705 | TTTGGACTTAATGACAGATG |
| hMPV | hMPVR | 4154 | TCTTCCTGTGCTAACTTTG |
| RSV | RSVF | 170 | TGGGACACTCTTAATCAT |
| RSV | RSVR | 419 | TGATTCCAAGCTGAGGAT |
Real-time RT-PCR for hMPV viral load quantification: Recombinant plasmids containing hMPV F gene were created as standards with a Topo TA Cloning Kit (Invitrogen, USA). After plasmid purification with the Plasmid Mini Kit I (OMEGA, USA), the concentration of the purified plasmid was calculated by absorption spectrophotometry with a Thermo NANODROP ND1000 (Biometra, Germany). Ten-fold serial dilutions (100–107 copies/µL) were used to construct a real-time PCR standard quantitative curve that showed an ideal linear relationship between the log values of initial template concentration and cycle threshold values. The hMPV F gene primers (4 µM) and probe sequences (Table 2) were designed based on the prototype strain from China (GenBank accession number DQ336144). The PCR mixture consisted of 12.5 µL of 2× Probe Premix Ex Taq, 0.5 µl of each hMPV primer, 1 µl hMPV probe, and 2 µl of cDNA in a volume of 25 µl. The cDNA was amplified with Premix Ex Taq Kit (Perfect Real Time, TaKaRa) according to the manufacturer's protocol in a LightCycler instrument (CFX96 real-time system, Bio-Rad). The PCR had the following cycling conditions: an initial rapid increase to 95°C for 10 s, followed by 40 cycles of 5 s at 95°C, and 34 s at 60°C, with continuous fluorescence reading. The cycle threshold (Ct) was ≤36.
Statistical analyses: Viral load was calculated as the initial copy number per RT-PCR reaction. The correlation between course of illness or severity of illness and log10-transformed quantitative viral load was assessed with a general linear model of repeated measurement data with the log viral load as a continuous linear variable. The correlation of age or gender with the log viral load was evaluated by a multivariate stepwise regression analysis. SAS version 6.12 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Findings
Clinical data analysis: Each hMPV-patient's clinical state was evaluated for respiratory rate, retractions, wheeze, and general appearance. The severity scores were divided into three categories: mild (0–4.9 points, state of illness=1), moderate (5-8.9 points, state of illness=2), and severe (9–12 points, state of illness=3). Among the 18 hMPV-positive children, 6 cases were mild, 8 cases moderate, and 4 cases severe. When SLE=0.1 and SLS=0.05, multivariate stepwise regression analyses revealed no significant correlation between age or gender (P=0.1, P=0.07).
The hMPV-positive patients ranged in age from 1 month to 29 months (median=7.5 months).
Among the patients, 13 were males and 5 females (male to female ratio=2.2:1). The clinical symptoms were cough (100%), wheezing (72.2%), diarrhea (33.3%), fever (11.1%), hoarseness (5.6%), poor feeding, shortness of breath, and retraction sign of three fossae (22.2%). The clinical diagnoses were bronchiolitis (9 cases), pneumonia (9 cases), and pneumonia combined with asthma (1 case). The complications of the illness were congenital heart disease (1 case), anemia (2 cases), otitis media and laryngitis (1 case), and diarrhea (5 cases). The course of illness in these patients was classified into 3 categories: 5 days or less (5 cases), 6–11 days (8 cases), and 12 days or more (5 cases) with an average of 7.33 days. Fifteen patients made a full recovery. Of the 18 hMPV-positive patients, 11 were co-infected with another virus and/or bacteria (Table 3). All co-infected patients were also RSV-positive.
Table 3.
Distribution of co-infection and single infection of hMPV in 18 hMPV-positive patients
| Pathogen | Total | Single infection | Coinfection with bacteria virus bacteria and virus | MP/CP-IgM | ||
|---|---|---|---|---|---|---|
| hMPV | 18 | 7 | 8 | 11 | 8 | 3 |
| S. pneumoniae | 2 | |||||
| H. parainfluenzae | 3 | |||||
| Klebsiella pneumoniae | 1 | |||||
| Moraxella catarrhalis | 2 | |||||
| Staphylococcus aureus | 1 | |||||
| RSV | 11 | |||||
This table shows that there were 7 cases infected with hMPV only, and the other 11 cases of hMPV-positive were co-infected with virus and/or bacteria. All 8 cases of bacteria infections were co-infected with virus, and one was dual-infected with bacteria in 8 bacterial infections.
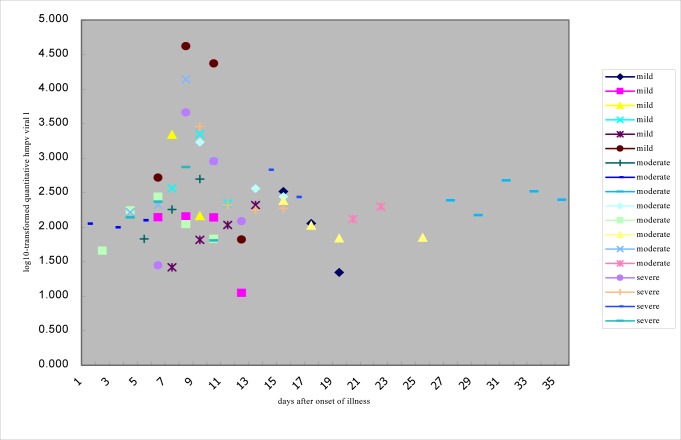
Viral load: Depending on the length of the illness, hMPV viral load was measured 2–6 times for each patient. The copies of viral load in case5, 7, 11, 14, 15, 16 and 17 were more than 1×105 copies/RT-PCR reaction, and those in other cases are less than 1×105 copies/RT-PCR reaction. Copies of viral load for each patient decreased with extension of course of disease and the hMPV almost could not be detected on discharge (Table 4). Viral load was not correlated with disease severity (P=0.5, 0.9, 0.5) but was correlated with days after onset of illness (Fig. 1). When the hMPV-positive patients were grouped by the length of the course of illness (Group 1:≤5 days; Group 2: 6–11 days; Group 3:>11 days), the viral load was significantly different between groups. Group 1 and 2 had significantly different log10 viral load values (P=0.01). Group 2 and 3 also had significantly different log10 viral load values (P=0.006). In contrast, Group 1 and 3 did not have significantly different log10 viral load values (P=0.4). Peak shedding time of hMPV was about 6–11 days post-infection.
Table 4.
Every-other-day changes in hMPV viral load during the course of the disease. Copies/RT-PCR reaction×102
| Case | Onset days | State of illness | Admission-(day 1) | Day 3 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 11 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | severe | 137.896 | 233.235 | 740.814 | 63.836 | ||||
| 2 | 5 | moderate | 67.564 | 180.411 | 497.142 | 0 | ||||
| 3 | 1+ | moderate | 111.953 | 99.109 | 125.265 | 0 | ||||
| 4 | 7 | mild | 26.199 | 65.476 | 107.395 | 208.761 | ||||
| 5 | 6 | mild | 520.146 | 41748 | 23004 | 66.009 | ||||
| 6 | 27 | moderate | 243.885 | 148.504 | 474.351 | 329.724 | 249.21 | 0 | ||
| 7 | 9 | severe | 2871.24 | 206.631 | 176.151 | 187.589 | ||||
| 8 | 2 | moderate | 45.454 | 173.702 | 275.409 | 110.206 | 67.542 | |||
| 9 | 15 | mild | 328.659 | 113.401 | 22.067 | |||||
| 10 | 15 | moderate | 241.542 | 105.542 | 68.948 | 70.567 | ||||
| 11 | 4+ | moderate | 164.159 | 210.188 | 13845 | 901.842 | ||||
| 12 | 6 | mild | 138.557 | 142.667 | 137.705 | 11.148 | ||||
| 13 | 20 | moderate | 130.889 | 199.027 | ||||||
| 14 | 7 | mild | 2188.2 | 145.245 | ||||||
| 15 | 7 | mild | 364.35 | 2225.85 | 220.668 | |||||
| 16 | 6 | severe | 27.89 | 4568.85 | 897.369 | 121.431 | 0 | |||
| 17 | 9 | moderate | 1704 | 362.1 | 276.9 | 0 | ||||
| 18 | 14 | severe | 673.68 | 272.3 |
This shows the disease severity (mild, moderate or severe) and the length of the illness in 18 patients. Depending on the length of illness, hMPV viral load was measured 2-6 times for each patient. The copies of viral load in case 5, 7, 11, 14, 15, 16 and 17 are more than 1×105 copies/RT-PCR reaction and those in other cases are less than 1×105 copies/RT-PCR reaction. The copies of viral load for each patient decreased with extension of course of the disease.
Fig. 1.
Correlation of log10–transformed hMPV viral load and severity of disease or days after onset of the illness. The severity is divided into three groups (mild, moderate and severe). The days after onset of illness are from about one day to thirty-seven days.
Discussion
Similar to the most common respiratory viral pathogen RSV, the recently identified hMPV was a major cause of LRTIs and other wheezing diseases. However, none of the previous hMPV studies had investigated if differences in hMPV viral load were correlated with differences in duration of course or severity of the respiratory duration of course or severity of the respiratory disease.
Similar to RSV and influenza infections, reported hMPV infections were seasonal, although sporadic infections arose throughout the year[24, 25]. Because we previously reported[17] that hMPV infections were most common during winter and spring months in Chongqing area, we collected specimens in the winter. Among the 18 hMPV-positive cases, 11 patients were co-infected with RSV. Additionally, 8 of these 11 were also co-infected with bacteria. However, no significant difference was found in disease severity between patients with or without co-infection (P>0.05). These findings are consistent with previous studies which showed that RSV/hMPV dual infection was overrepresented in RSV patients requiring mechanical ventilation, indicating that co-infection of RSV and hMPV did not lead to a more severe RSV infection[26]. However, Bosis et al.[27] found that hMPV viral load was correlated with disease severity and associated with short or long clinical course. In an analysis of bacterial co-infection in patients with community-acquired pneumonia, 32.5% likely had single bacterial infections and 15.2% had co-infections with bacteria and viruses[28]. Further studies with this method should investigate whether bacterial and viral co-infection affects severity of other clinical diseases. Similar to what we found, mixed viral infections are common, especially for RSV and hmpv[29]. Currently, there is conflicting evidence of whether dual infections are related to the disease severity[30–33] or not[34–36].
Future studies should focus on whether viral load, viral genotype, co-infection, or combinations of these factors are related to the severity of illness.
Previous studies divided hMPV patients into two categories: low viral load <1×105 copies and high viral load ≥1×105 copies[37, 38]. Here, patients in the middle of the course of infection (6–11 days after onset) had a high viral load while patients at the beginning or end of the course of the illness had a low viral load. The severe clinical symptoms of patients with a low viral load might be attributed to long disease course or co-infection with other viruses and bacteria. Regardless, viral load was correlated with the course of the disease, in agreement with previous studies[38]. Further studies should investigate whether clinical symptoms, severity, course of illness, and subsequent respiratory are associated with viral load of distinct hMPV subgroups.
It is possible that a correlation between viral load and disease severity exists, but it was not identified in our study. This missed association could stem from three major limitations for quantifying viral load. First, the NPAs were not always taken at the peak of acute LRTI, some were not collected until development of disease on the 15th day or after 1 month of onset. Second, patients often received therapy before hospitalization, especially in the form of steroids and bronchodilators. These treatments can interfere with clinical infection characteristics and/or virus replication. Third, it was not possible to perform a correct follow-up for all patients after discharge. Despite these limitations, we obtained hMPV viral loads in 18 hMPV-positive patients collected daily during the hospital visit. Our findings revealed that the hMPV load was significantly correlated with the course of illness.
The decrease of hMPV load indicated the beginning of recovery.
Conclusion
hMPV load was significantly correlated with the course of illness. The association between hMPV viral load and the course of disease suggested that hMPV is an important pathogen in lower respiratory tract infection in children. But hMPV did not always lead to more severe respiratory illness.
Acknowledgment
We thank the staff of P2 Laboratory, Children's Hospital, Chongqing Medical University, for their helpful instructions, and technicians of BIO-RAD Company, for their skillful technical assistance. We are also grateful to the staff of Department of Statistics, Chongqing Medical University, for the statistical analysis.
Conflict of Interest
None
References
- 1.Klig JE. Current challenges in lower respiratory infections in children. Curr Opin Pediatr. 2004;16(1):107–12. doi: 10.1097/00008480-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Klig JE, Shah NB. Office pediatrics: current issues in lower respiratory infections in children. Curr Opin Pediatr. 2005;17(1):111–8. doi: 10.1097/01.mop.0000150599.31091.f0. [DOI] [PubMed] [Google Scholar]
- 3.Kuiken T, Fouchier R, Rimmelzwaan G, Osterhaus A. Emerging viral infections in a rapidly changing world. Curr Opin Biotechnol. 2003;14(6):641–6. doi: 10.1016/j.copbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Principi N, Esposito S. Pediatric influenza prevention and control. Emerg Infect Dis. 2004;10(4):574–80. doi: 10.3201/eid1004.030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esper F, Martinello RA, Boucher D, et al. A 1-year experience with human metapneumo-virus in children aged <5 years. J Infect Dis. 2004;189(8):1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freymouth F, Vabret A, Legrand L, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22(1):92–4. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9(6):628–33. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nissen MD, Siebert DJ, Mackay IM, et al. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176(4):188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiano M, Videla C, Puch SS, et al. Evidence of human metapneumovirus in children in Argentina. J Med Virol. 2004;72(2):299–303. doi: 10.1002/jmv.10536. [DOI] [PubMed] [Google Scholar]
- 10.Mejías A, Chávez-Bueno S, Ramilo O. Human metapneumovirus: a not so new virus. Pediatr Infect Dis J. 2004;23(1):1–7. doi: 10.1097/01.inf.0000105103.60288.0e. [DOI] [PubMed] [Google Scholar]
- 11.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10(6):1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosis S, Esposito S, Niesters HG, et al. Impact of human metapneumovirus in childhood: comparison with respiratory syncytial virus and influenza viruses. J Med Viro. 2005;75(1):101–4. doi: 10.1002/jmv.20243. [DOI] [PubMed] [Google Scholar]
- 14.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect. Dis. 2004;10(4):700–5. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerna G, Campanini G, Rovida F, et al. Changing circulation rate of human metapneumovirus strains and types among hospitalized pediatric patients during three consecutive winter-spring seasons. Arch Virol. 2005;150(11):2365–75. doi: 10.1007/s00705-005-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu RN, Qian Y, Deng J, et al. Human metapneumovirus may associate with acute respiratory infections in hospitalized pediatric patients in Beijing, China. Zhonghua Er Ke Za Zhi. 2003;41(6):441–4. (Chinese) [PubMed] [Google Scholar]
- 17.Zhang Q, Yang XQ, Zhao XD. Analysis of neutralizing activity of sera from 0 to 6 years old children in Chongqing area against human metapneumovirus. Zhonghua Er Ke Za Zhi. 2007;45(6):432–6. (Chinese) [PubMed] [Google Scholar]
- 18.Watzinger F, Suda M, Preuner S, et al. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J Clin Microbiol. 2004;42(11):5189–98. doi: 10.1128/JCM.42.11.5189-5198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44(7):2382–8. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Létant SE, Ortiz JI, Bentley Tammero LF, et al. Multiplexed reverse transcriptase PCR assay for identification of viral respiratory pathogens at the point of care. J Clin Microbiol. 2007;45(11):3498–505. doi: 10.1128/JCM.01712-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang EE, Milner RA, Navas L, et al. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145(1):106–9. doi: 10.1164/ajrccm/145.1.106. [DOI] [PubMed] [Google Scholar]
- 22.Nasr SZ, Strouse PJ, Soskolne E. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest. 2001;120(1):203–8. doi: 10.1378/chest.120.1.203. [DOI] [PubMed] [Google Scholar]
- 23.Mandelberg A, Tal G, Witzling M, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest. 2003;123(2):481–7. doi: 10.1378/chest.123.2.481. [DOI] [PubMed] [Google Scholar]
- 24.Schildgen O, Geikowski T, Glatzel T, et al. Frequency of human metapneumovirus in the upper respiratory tract of children with symptoms of an acute otitis media. Eur J Pediatr. 2005;164(6):400–1. doi: 10.1007/s00431-005-1655-6. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JL, Lee BE, Bastien N, Li Y. Seasonality and clinical features of human metapneumovirus infection in children in Northern Alberta. J Med Virol. 2005;76(1):98–105. doi: 10.1002/jmv.20329. [DOI] [PubMed] [Google Scholar]
- 26.van Woensel JB, Bos AP, Lutter R, et al. Absence of human metapneumovirus co-infection in cases of severe respiratory syncytial virus infection. Pediatr Pulmonol. 2006;41(9):872–4. doi: 10.1002/ppul.20459. [DOI] [PubMed] [Google Scholar]
- 27.Bosis S, Esposito S, Osterhaus AD, et al. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol. 2008;42(3):286–90. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamano-Hasegawa K, Morozumi M, et al. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14(6):424–32. doi: 10.1007/s10156-008-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greensill J, McNamara PS, Dove W, et al. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9(3):372–5. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard N, Komurian-Pradel F, Javouhey E, et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27(3):213–7. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 31.Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191(3):382–6. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulongne V, Guyon G, Rodière M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25(4):354–9. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 33.König B, König W, Arnold R, et al. Prospective study of human metapneumovirus infection in children less than 3 years of age. J Clin Microbiol. 2004;42(10):4632–5. doi: 10.1128/JCM.42.10.4632-4635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-García ML, Calvo C, Pérez-Brena P, et al. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in Spain. Pediatr Pulmonol. 2006;41(9):863–71. doi: 10.1002/ppul.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25(4):320–4. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 36.Wilkesmann A, Schildgen O, Eis-Hübinger AM, et al. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr. 2006;165(7):467–75. doi: 10.1007/s00431-006-0105-4. [DOI] [PubMed] [Google Scholar]
- 37.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62(4):382–8. doi: 10.1016/j.diagmicrobio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78(3):408–16. doi: 10.1002/jmv.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]