Abstract
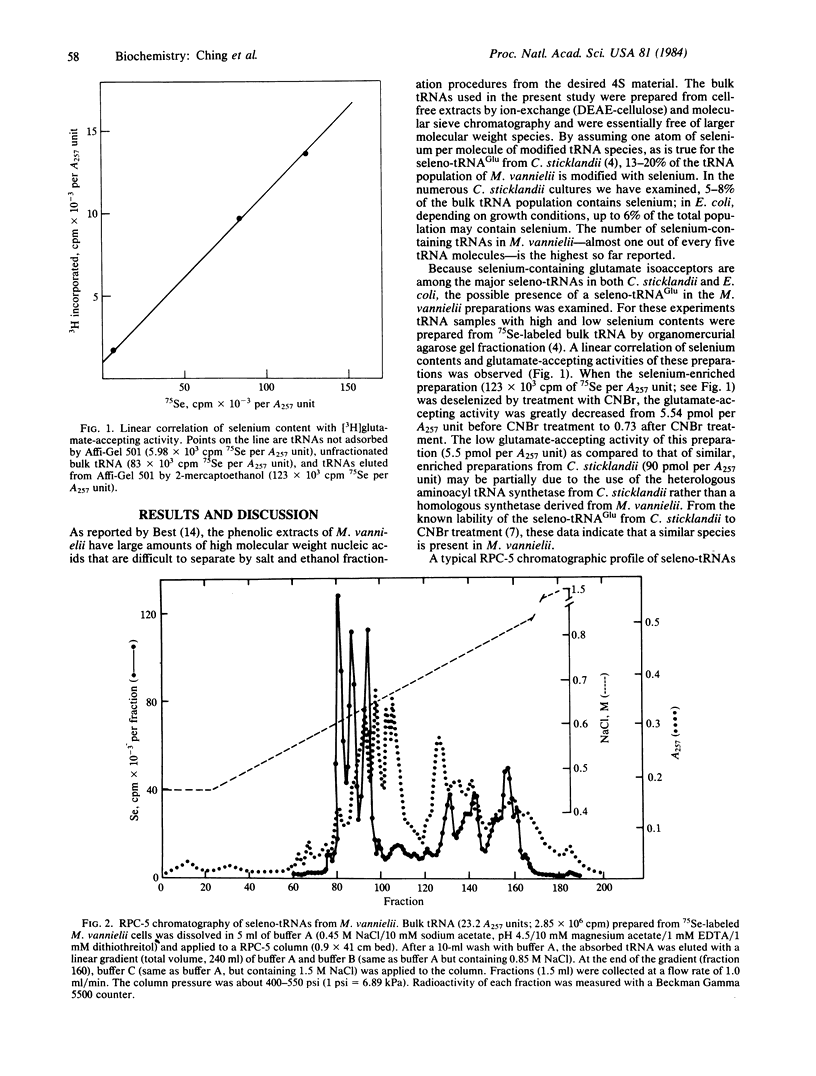
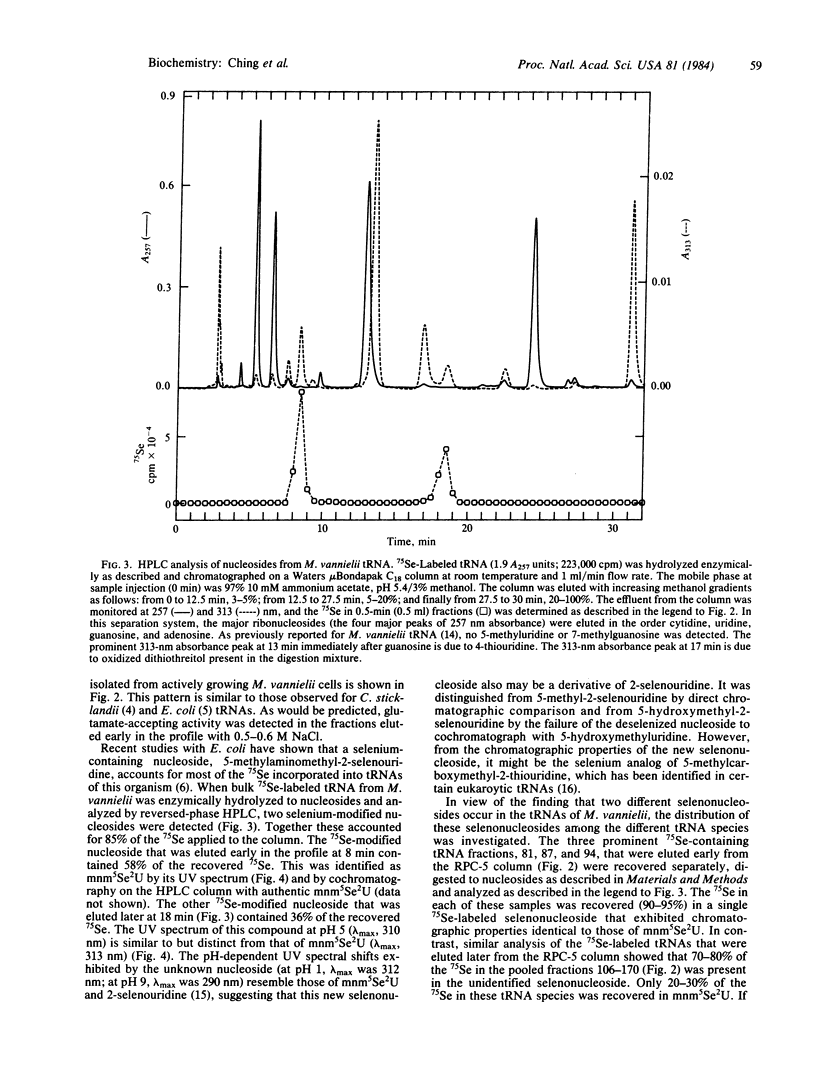
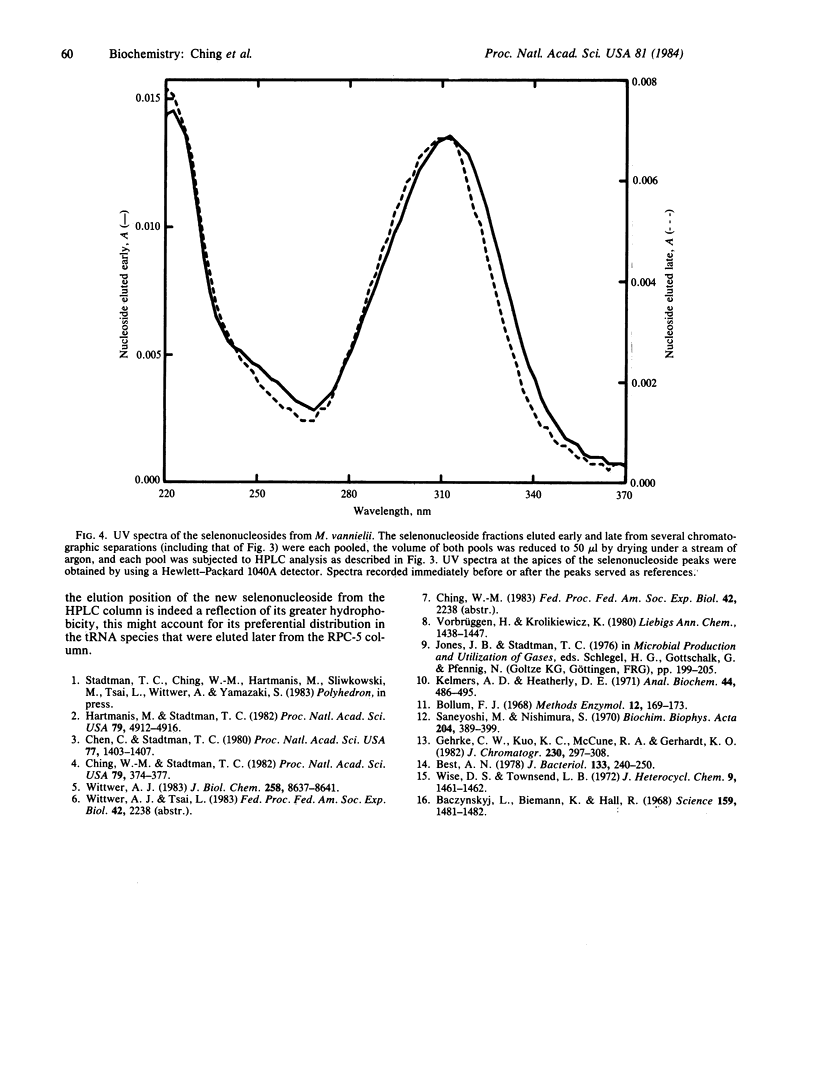
Naturally occurring seleno-tRNAs from Methanococcus vannielii account for 13-20% of the total tRNA population. Two different selenium-modified nucleosides were detected in these seleno-tRNAs. Of the total selenium incorporated, about 60% is present in 5-methylaminomethyl-2-selenouridine, and the other 40% occurs in a second selenonucleoside with spectral characteristics typical of a derivative of 2-selenouridine. The 5-methylaminomethyl-2-selenouridine was found in the seleno-tRNA species present in the early fractions of a linear salt gradient elution profile from a reversed-phase chromatographic system 5(RPC-5) column, whereas the second selenonucleoside occurred in the tRNA species eluted late in the profile.
Keywords: seleno-tRNAs, 5-methylaminomethyl-2-selenouridine, methane-producing bacteria, HPLC of nucleosides
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczynskyj L., Biemann K., Hall R. H. Sulfur-containing nucleoside from yeast transfer ribonucleic acid: 2-thio-5(or 6)-uridine acetic acid methyl ester. Science. 1968 Mar 29;159(3822):1481–1483. doi: 10.1126/science.159.3822.1481. [DOI] [PubMed] [Google Scholar]
- Best A. N. Composition and Characterization of tRNA from Methanococcus vannielii. J Bacteriol. 1978 Jan;133(1):240–250. doi: 10.1128/jb.133.1.240-250.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Stadtman T. C. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1403–1407. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Stadtman T. C. Selenium-containing tRNAGlu from Clostridium sticklandii: correlation of aminoacylation with selenium content. Proc Natl Acad Sci U S A. 1982 Jan;79(2):374–377. doi: 10.1073/pnas.79.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C. W., Kuo K. C., McCune R. A., Gerhardt K. O., Agris P. F. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J Chromatogr. 1982 Jul 9;230(2):297–308. [PubMed] [Google Scholar]
- Hartmanis M. G., Stadtman T. C. Isolation of a selenium-containing thiolase from Clostridium kluyveri: identification of the selenium moiety as selenomethionine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4912–4916. doi: 10.1073/pnas.79.16.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S. Selective modification of 4-thiouridylate residue in Escherichia coli transfer RNA with cyanogen bromide. Biochim Biophys Acta. 1970 Apr 15;204(2):389–399. doi: 10.1016/0005-2787(70)90158-9. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J. Specific incorporation of selenium into lysine- and glutamate- accepting tRNAs from Escherichia coli. J Biol Chem. 1983 Jul 25;258(14):8637–8641. [PubMed] [Google Scholar]



