Abstract
We describe in this study punchless, a nonpathogenic mutant from the rice blast fungus M. grisea, obtained by plasmid-mediated insertional mutagenesis. As do most fungal plant pathogens, M. grisea differentiates an infection structure specialized for host penetration called the appressorium. We show that punchless differentiates appressoria that fail to breach either the leaf epidermis or artificial membranes such as cellophane. Cytological analysis of punchless appressoria shows that they have a cellular structure, turgor, and glycogen content similar to those of wild type before penetration, but that they are unable to differentiate penetration pegs. The inactivated gene, PLS1, encodes a putative integral membrane protein of 225 aa (Pls1p). A functional Pls1p-green fluorescent protein fusion protein was detected only in appressoria and was localized in plasma membranes and vacuoles. Pls1p is structurally related to the tetraspanin family. In animals, these proteins are components of membrane signaling complexes controlling cell differentiation, motility, and adhesion. We conclude that PLS1 controls an appressorial function essential for the penetration of the fungus into host leaves.
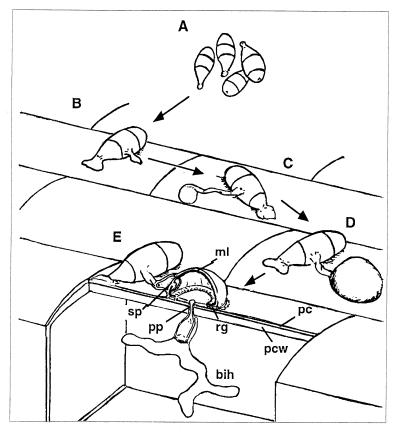
Plant pathogenic fungi are a major threat for crop production worldwide. Several of these fungal pathogens depend on the formation of a specialized cell, called an appressorium, for the successful penetration of host plant surfaces (1, 2). This penetration process is typical of fungal leaf spots such as rice blast caused by the filamentous ascomycete Magnaporthe grisea (3). This disease is disseminated by water-splashed conidia (ref. 4; Fig. 1A). After their adhesion to the host leaf by means of a strong glue (ref. 5; Fig. 1B), conidia germinate and differentiate appressoria at the tips of their germ tubes (Fig. 1C). This developmental process is induced by chemical and physical stimuli from the host surface such as hardness, hydrophobicity, or cutin monomers (2, 3). This dome-shaped, thick-walled, and melanized cell (Fig. 1D) promotes the differentiation of a specialized hypha called the penetration peg that is involved in penetration of plant surfaces (ref. 6; pp in Fig. 1E). The differentiation of appressoria and penetration pegs is the result of complex morphogenetic processes that involve major developmental and metabolic changes (2, 3, 6). Inside the epidermal plant cell, the penetration peg differentiates into a bulbous infection hypha that invades the whole cell and colonizes the leaf.
Figure 1.
Penetration of host leaves by the rice blast fungus. (A) Conidia are disseminated in water drops splashing from a sporulating lesion. (B) A strong glue sticks conidia to the host surface. (C) Appressorium differentiates rapidly after conidium germination. (D) Mature appressorium is a melanized, dome-shaped and thick-walled cell. (E) Penetration peg breaches the plant cuticle and cell wall by mechanical force. After penetration, a bulbous infection hypha invades the epidermal cell. Abbreviations: ml, melanin layer; sp, septum; pp, penetration peg; rg, ring of conidial glue; bih, bulbous infection hypha; pc, plant cuticle; pcw, plant cell wall.
To isolate genes involved in this infection process, we generated a collection of M. grisea pathogenicity mutants by using plasmid-mediated insertional mutagenesis. This strategy was successful for the cloning of pathogenicity genes in Colletotrichum lindemuthianum (7), Ustilago maydis,** and M. grisea (8, 9). In this study, we isolated punchless, a nonpathogenic mutant defective in penetration of plant surfaces. We show that the PLS1 gene is inactivated in punchless and encodes a putative membrane protein related to the tetraspanin family (10–12). The phenotypic and cytological analysis of punchless strongly suggests that PLS1 is essential for the differentiation of the penetration peg required for penetration.
Materials and Methods
Fungal Strains and Growth Conditions.
Media composition, M. grisea maintenance, transformation, and sexual crosses were as described (13, 14). P1.2 (MAT1.2) and M4 (MAT1.1) are M. grisea strains from the Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD). As are all M. grisea isolates pathogenic on rice, P1.2 and M4 are also pathogenic on barley. P1.2 was used as the recipient strain for plasmid-mediated insertional mutagenesis and M4 as the wild-type parent in crosses. The nonpathogenic buf1− mutant used as a control for the wounded leaf assay (14). A collection of 1,000 hygromycin-resistant transformants was obtained from transformation of 107 P1.2 protoplasts with 1 μg of the plasmid pAN7.1 (15).
Phenotypic Analysis and Cytology.
Seedlings from the susceptible rice cultivars Maratelli and Sariceltic were cultivated for 20 days (70% relative humidity; 25°C day, 20°C night), and seedlings from barley cultivar Express were cultivated for 10 days (20°C day, 15°C night). Leaf segments 3 cm long were placed on water/1% (wt/vol) agar plus kinetin (2 mg/liter) and inoculated with a suspension of 105 conidia per ml by using cotton tips. Symptoms were recorded after 4–7 days at 23°C. Leaves were abraded with sandpaper or punctured with a needle before inoculation or infiltrated with a conidial suspension by using a syringe without a needle. Leaf segments were also inoculated with 50-μl droplets of a 5⋅105 conidia per ml suspension. Epidermis from a droplet-inoculated leaf was peeled with forceps after a superficial incision with a razor blade. Spray inoculation of rice leaves was performed as described (14). Appressoria were differentiated on membranes composed of Teflon (Goodfellow, Cambridge, U.K.), Gelbond (Sigma), or polyethylene terephthalate (PET; Rhodia, Lyon, France). Appressorial turgor was assayed (16) by using vinyl slides (Rinzl; Polylabo, Strasbourg, France). Penetration of membrane was assayed by using PUDO-193 cellophane (ref. 17; gift from T. Bourett, duPont de Nemours, Wilmington, DE). Droplet-infected leaves were fixed in a mixture of 2% (vol/vol) glutaraldehyde and 0.5% paraformaldehyde in 0.1 M, pH 7, McIlvaine citrate/phosphate buffer (18) at room temperature and embedded in Epon resin. Serial semithin sections (0.4-μm thick) were stained either with azur II/methylene blue (19) or with malachite green (1%, 80°C, 3 min) followed by a Schiff coloration. Serial sections were observed by using a Nikon Optiphot-2 light microscope. Serial sections corresponding to 30 wild-type and 60 punchless appressoria were analyzed. Confocal laser scanning microscopy was performed on appressoria differentiated on PET membranes or barley leaves by using a Leica TCS-SP2 microscope. Fluorescence of Pls1p fused with green fluorescent protein (GFP) was detected in transformed appressoria by using an excitation wavelength of 488 nm and an emission between 500 and 530 nm. Fluorescent probes for vacuoles such as Celltracker blue-CMAC and carboxy-DCFDA (Yeast Vacuole Markers Kit, Molecular Probes) were used at concentrations recommended by the supplier and viewed by using a Nikon Optiphot-2 microscope with UV epifluorescence and appropriate filters.
Plasmids and Nucleic Acids Methods.
Plasmid pAN7.1 (hygromycin resistance; ref. 15) and pCB1265 (bialaphos resistance; ref. 20) were used for fungal transformation. pBL is a modified pBluescript II SK (+) carrying a second NotI site in place of its KpnI site (gift from C. d'Enfert, Institut Pasteur, Paris). pBLHYG plasmid is a pBL plasmid with the hph cassette from pCB1003 (20) cloned at its EcoRI site. M. grisea genomic DNA extractions were performed as described (13). Total RNA was extracted by using the hot acid/phenol protocol (21). mRNA was isolated with Dynabeads (Dynal, Compiegne, France). A cDNA library was constructed with a Lambda Zap Express cDNA Kit and Gigapack-III Gold Packaging Extract (Stratagene). Southern blotting and cDNA and cosmid library screenings were performed according to standard procedures (21). A Ready-To-Go RT-PCR One-Tube Reaction Kit (Amersham Pharmacia) was used for reverse transcription (RT)-PCR. Sequencing was performed by Genome Express (Grenoble, France). Sequence analysis software packages used were DNA STRIDER-1.2 (Commissariat à l'Energie Atomique, Gif-sur-Yvette, France), blast (22), and Clustal-W (European Molecular Biology Laboratory, Heidelberg, Germany). Prediction of protein structure was performed at ExPASy (http://www.expasy.ch) by using hmmtop, thmm, Predict-Protein, TmPred, ProfileScan, and Scan Prosite.
Cloning and Expression of the PLS1 Gene.
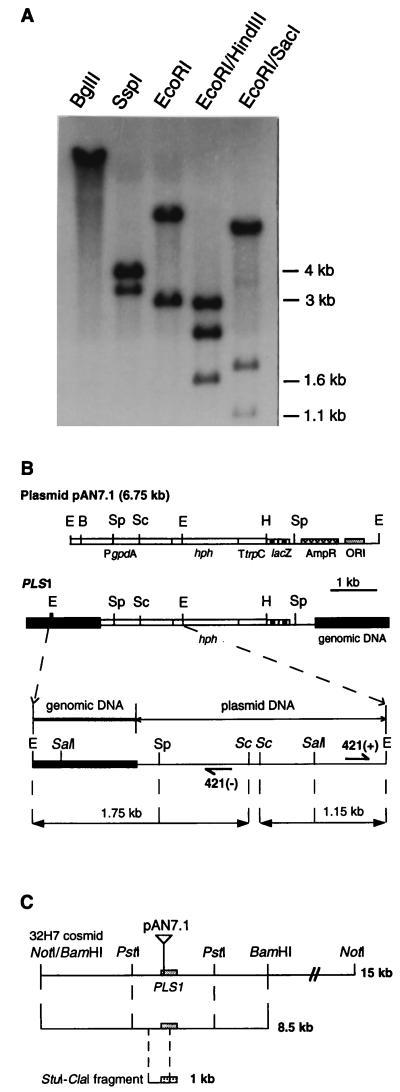
pAN7.1 junction fragments were cloned by inverse PCR (ref. 23; Fig. 3B) with primers 421(+) (TTGTGTGACTTTTGGTTACG) and 421(−) (TTATGTTTATCGGCACTTTG). A 0.35-kb EcoRI–SalI genomic fragment from this inverse PCR product was used to clone cosmid 32H7 from a M. grisea wild-type genomic library (Fig. 3B). An 8.5-kb BamHI fragment from cosmid 32H7 was cloned into plasmid pCB1265 (pCM421-8.5; Fig. 3C) and introduced by transformation into punchless. A 675-bp ORF (PLS1) was localized at the pAN7.1 insertion site in pCM421-8.5. A genomic 1-kb StuI–ClaI internal fragment from PLS1 (Fig. 3C) was used to screen a mycelial cDNA library (RNA extracted from mycelium grown in agitated liquid complete medium for 24 h). One full-length (1910-bp) PLS1 cDNA clone was rescued and sequenced. Primers were designed at the putative initiation and termination signals of PLS1 (pair 1: CGATACCGACCGACGAACCT and TCAAGACGCTCACAGAGTGC) and inside its coding sequence (pair 2: CCTGATGCTGGGCTTCTC and ACACATGCCGAAAACACCAG). These primer pairs were used for RT-PCR (pair 1, 50 cycles) followed by a nested PCR (pair 2, 35 cycles) using 2–4 μg of total RNA extracted from mycelium or infected barley epidermis (16–20 leaf fragments of 0.5 mm2 peeled at 30 min, and 4, 12, and 20 h after inoculation). PLS1 deletion mutants were obtained by transformation with vector pRM421. This vector was prepared by the replacement of the 1-kb StuI–ClaI fragment from pCM421-8.5 by the 1.4-kb SmaI–ClaI hph resistance gene from plasmid pBLHYG. GFP was fused to the N terminus of Pls1p under the control of PLS1 promoter and terminator (PLS1∷GFP fusion). NcoI and XbaI sites were introduced by PCR at the ATG site of PLS1 by using a 937-bp NruI subclone from pCM421-8.5 as a template. pEGFP (CLONTECH, Ozyme) was used to obtain a PCR product containing a NcoI site at the start of the ORF and a XbaI site at the end of the ORF. This PCR product was fused to the ATG of the modified PLS1–937-bp NruI subclone and reintroduced as a NruI fragment into pCM421-8.5.
Figure 3.
Cloning of pAN7.1 insertion locus from punchless. (A) punchless genomic DNA gel blot analysis. A single pAN7.1-hybridizing fragment was detected for BglII, which normally cuts once in pAN7.1. SspI hybridization pattern revealed a 4-kb fragment corresponding to a pAN7.1 internal fragment. Analysis of double-digest hybridization patterns showed that the origin of replication and a segment of the ampicillin-resistance gene of pAN7.1 were deleted during its integration. (B) Restriction map of the pAN7.1 insertion locus cloned by inverse PCR. Shaded box, M. grisea genomic DNA. Line and empty box, plasmid DNA. Abbreviations for restriction sites are E, EcoRI; B, BglII; H, HindIII; Sp, SspI; and SacI, Sc. 421(+) and 421(−) are the positions of primers used for inverse PCR. (C) Restriction map of wild-type genomic fragments (15 kb and 8.5 kb) from cosmid 32H7 used for complementation. The 8.5-kb BamHI fragment was cloned in pCB1265 to give plasmid pCM421-8.5. The StuI–ClaI fragment of 1 kb was used as a probe to identify PLS1 cDNA clones.
Results
punchless Is a Nonpathogenic Mutant Unable to Penetrate Host Leaf Surfaces.
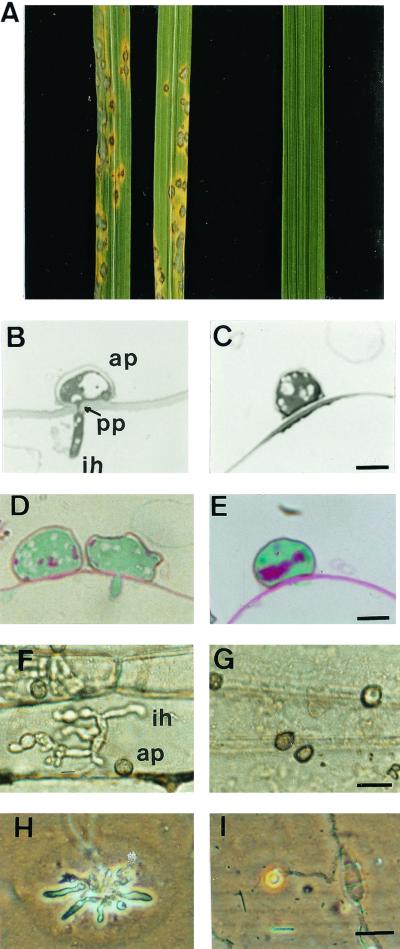
M. grisea nonpathogenic mutant punchless was identified among 1,000 hygromycin-resistant transformants by its inability to cause disease symptoms on rice (Fig. 2A) and barley. Analysis of progeny from a cross between punchless and wild-type strain M4 showed that the loss of pathogenicity cosegregated with hygromycin resistance (data not shown), demonstrating the tagging of a pathogenicity gene by the plasmid used for transformation. punchless is fertile in crosses, has a normal filamentous growth and sporulation rate, and has no specific nutritional requirements. It differentiates melanized appressoria similar to wild type on hydrophobic artificial surfaces (vinyl, PET, Teflon, hydrophobic side of Gelbond) and on barley or rice leaves. Colonization of barley epidermal cells by wild type and punchless was investigated after peeling of leaf epidermis. Twenty-four hours after inoculation with wild-type strain, bulbous infection hyphae were detected inside epidermal cells. These cells were completely filled with hyphae 48 h after inoculation (Fig. 2F). Twenty-four hours after inoculation with punchless, appressoria were observed on the leaf surface, but infectious hyphae were not detected inside epidermal cells (Fig. 2G), even 6 days after inoculation (data not shown). Serial sections of barley leaves inoculated with wild type or punchless were examined for the presence of penetration pegs and infectious hyphae. Twenty hours after inoculation, 80% of wild-type appressoria have differentiated a penetration peg (Fig. 2B) and 50% showed extensive vacuolization. We did not detect penetration pegs or infectious hyphae below punchless appressoria (Fig. 2C). A significant number of punchless appressoria (35%) were collapsed compared with wild type (5%), but this collapse was not observed with punchless appressoria formed on artificial membranes. punchless appressoria that remained turgid on the leaf surface (65%) had a normal subcellular structure (mitochondria and nucleus) and a visible melanin layer (as observed by transmission electron microscopy; data not shown), although they were less vacuolized than wild type. Malachite green/Schiff staining of appressoria was performed to assess their viability and glycogen content. In wild-type appressoria, glycogen was almost completely degraded 20 h after inoculation (slight red staining within the appressorium, Fig. 2D), as previously reported for appressoria during penetration of a cellophane membrane (17). Cytoplasm of punchless appressoria was stained green as in wild type, and glycogen was detected as a strong red staining located in the lower part of the appressoria (Fig. 2E). Such an accumulation of glycogen was also observed in wild-type appressoria before penetration (8–16 h after inoculation; data not shown). Penetration of artificial membranes by wild type and punchless was assayed by using a cellophane membrane. The wild-type strain differentiated appressoria on cellophane that penetrated 4 days after inoculation, as shown by pseudoinfectious hyphae growing within the membrane under the appressoria (5 days; Fig. 2H), as previously described (17). We did not detect any penetration of punchless into the cellophane (Fig. 2I), even 8 days after inoculation. Appressorial turgor was estimated by using various osmolytes (glycerol, polyethylene glycols of molecular weight 4,000 and 8,000). The turgor of 24-h-old punchless appressoria differentiated on artificial membranes (vinyl slides) was similar to wild-type appressorial turgor (7 MPa) and published values (16).
Figure 2.
punchless phenotypic analysis. (A) Infection assay on rice. Left, punchless mutant; right, P1.2 wild type. Typical lesions were observed for wild type 7 days after inoculation. (B and C) Sections of barley epidermal cells 20 h after inoculation with P1.2 (B) or punchless (C). A penetration peg (pp) and an infectious hypha are visible below the wild-type appressorium (ap). (Bar = 5 μm.) (D and E) Stained sections of barley leaves 20 h after inoculation with P1.2 (D) or punchless (E). Glycogen is detected only in punchless appressorium as shown by a red staining. (Bar = 5 μm.) (F and G) Barley epidermal strips 2 days after inoculation with P1.2 (F) or punchless (G). Bulbous infection hyphae (ih) are visible under the wild-type appressorium (ap). (Bar = 20 μm.) (H and I) Penetration of cellophane by P1.2. (H) or punchless (I). Five days after inoculation, pseudoinfection hyphae are visible inside the cellophane under the wild-type appressorium. (Bar = 20 μm.)
To compare punchless with other known M. grisea pathogenicity mutants, we evaluated its pathogenicity on wounded rice or barley leaves. As a control we used a buf1− mutant that differentiates nonmelanized appressoria with a low turgor. This mutant is unable to penetrate intact leaf surfaces but can infect wounded leaves (14, 24–26). punchless failed to infect wounded rice or barley leaves whatever the inoculation protocol (superficial abrasion, needle puncture, injection through leaf sheaths, infiltration into the leaf), whereas the buf1 mutant was pathogenic on all type of wounded leaves
punchless Is Defective in a Gene Encoding a Tetraspanin-Like Protein (PLS1).
A genomic clone (cosmid 32H7) was recovered from a wild-type cosmid library with a probe corresponding to the locus tagged by plasmid pAN7.1 in punchless (Fig. 3 A and B). A 8.5-kb BamHI restriction fragment (Fig. 3C) from cosmid 32H7 was able to restore pathogenicity of punchless when introduced by transformation. Sequencing of the genomic and cDNA clones corresponding to the plasmid insertion site revealed an ORF of 675 bp interrupted by two introns (78 bp, 96 bp) that encodes a putative protein of 225 aa. The gene corresponding to this ORF was named PLS1 for PunchLeSs. A putative polyadenylation signal (AUUUUAU) was localized in its 3′ untranslated region. A gene replacement experiment was carried out to demonstrate that the deletion of PLS1 leads to a mutant phenotype similar to punchless. Genomic Southern blot analysis of the transformants obtained with linearized pRM421 showed that 40% (24 of 60) resulted from the deletion of a 1-kb StuI–ClaI fragment from PLS1 and its replacement by one copy of the hph resistance gene (data not shown). All these ΔPLS1 mutants displayed the same nonpathogenic phenotype as punchless.
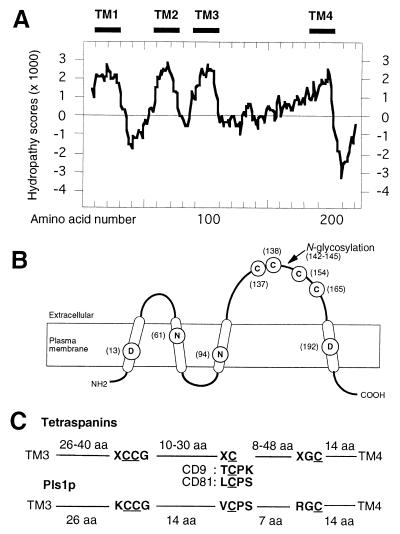
blast or fasta searches did not reveal any homology between PLS1 and sequences from databases. Profile Scan analysis (ExPASy) identified Pls1p as a member of the tetraspanin family despite a low homology at the amino acid level (identities less than 15%). The main structural hallmarks of the tetraspanin family are the presence of four transmembrane domains (TM), the orientation across plasma membrane of the intra- and extracellular domains, the presence of a large extracellular domain (ECL2, >50 aa) between TM3 and TM4 with three conserved cysteine-based patterns, and a small C-terminal domain rich in charged/polar amino acids (10–12). TmPred, tmhmm, and hmmtop software predicted four transmembrane domains in Pls1p (TM1 to TM4; Fig. 4A). Three of these domains were clustered at the N terminus of the protein and separated from a fourth one by a large hydrophilic loop of 74 aa (putative ECL2 of Pls1p). A plasma membrane localization was predicted for Pls1p, with intracytoplasmic N and C termini and two extracytoplasmic loops (Fig. 4B). The three cysteine-based patterns typical of tetraspanins were identified in the ECL2 domain of Pls1p (Fig. 4 B and C) with a spacing consistent with that of tetraspanins. The C-terminal domain of Pls1p (22 aa) is mainly (72%) composed of charged/polar amino acids, as in tetraspanins. This analysis shows that Pls1p has all structural hallmarks of animal tetraspanins and suggests that it could be classified as a fungal tetraspanin-like protein despite its low sequence homology to these proteins.
Figure 4.
Predicted secondary structure of Pls1p. (A) Prediction of Pls1p transmembrane domains. Hydropathy plot using the Kyte and Doolittle algorithm. Bars, putative transmembrane domains (TM1, -2, -3, and -4). (B) Predicted secondary structure of Pls1p. Cysteine-based patterns conserved among tetraspanins are CCG at position 137, CP at position 154, and GC at position 165. Charged amino acids located in transmembrane domains are at positions 13 (D), 61 (N), 94 (N), and 192 (D). Transmembrane domains are indicated as open cylinders. (C) Cysteine-based patterns in ECL2 of Pls1p and animal tetraspanins. The distance between each conserved cysteine-based pattern is given in aa. Variations of the XCPK/S pattern in tetraspanins CD9 and CD81 are given under Pls1p pattern.
Pls1p Is Expressed During Infection and Is Localized to Appressorial Membranes.
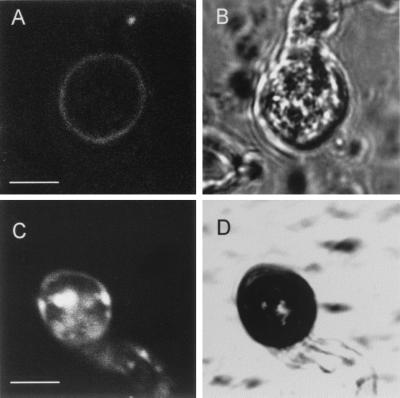
Using RT-PCR, we showed that PLS1 was expressed during infection of barley leaves by M. grisea (data not shown). We used a GFP fusion protein to survey Pls1p expression during infection and to determine its localization in the fungal cell. The Pls1p-GFP fusion was expressed under the control of PLS1 promoter and terminator. This construct was functional in M. grisea, as it successfully complemented the pathogenicity defect of the ΔPLS1 mutant. These transformants were analyzed for their GFP fluorescence by classical epifluorescence and confocal laser scanning microscopy. GFP fluorescence was detected only in appressoria differentiated either on artificial membranes (Teflon, PET, cellophane) or on leaves (Fig. 5 A and C) before penetration (8–20 h). In a few appressoria, we observed a faint fluorescence at the periphery of the appressorial cell that should correspond to the labeling of the plasma membrane (Fig. 5A, class I). Most appressoria (80%) had highly fluorescent round intracellular compartments (Fig. 5C, class II) corresponding to vacuoles (as shown by accumulation of the specific fluorescent probes CMAC and carboxy-DCFDA, data not shown). These appressoria also displayed a faint fluorescence at their cell periphery (membrane localization). Such a strong vacuolar fluorescence associated with a faint fluorescence at the cell periphery was already observed in M. grisea appressoria expressing a functional GFP fusion with the plasma membrane Pth11p (27). As suggested for Pth11p, we propose that Pls1p is present in small amounts in the appressorial plasma membrane and that it accumulates in the vacuole as a result of recycling through endocytosis or missorting.
Figure 5.
Expression and localization of Pls1p in the appressorium during infection of barley leaves. A transformant expressing a Pls1p-GFP fusion under the control of the PLS1 promoter and terminator was inoculated to detached barley leaves and observed by confocal laser scanning microscopy. Two classes of appressoria were observed on the leaf surface 12–14 h after inoculation. (A) GFP fluorescence of class I appressorium. (Bar = 4 μm.) A faint fluorescence was detected at the appressorium periphery. (B) Same view as in A under bright light. (C) GFP fluorescence of class II appressorium. A strong fluorescence was detected in vacuoles and a faint fluorescence was detected at the appressorium periphery. (Bar = 4 μm.) (D) Same view as in C under bright light. Most appressoria (80%) observed belonged to class II.
Discussion
Using plasmid-mediated insertional mutagenesis, we isolated punchless, a nonpathogenic mutant from the rice blast fungus M. grisea. punchless is nonpathogenic on leaves of rice and barley, as its appressoria were unable to penetrate host leaf surfaces. punchless had a normal mycelial growth, sporulation rate, and mating behavior. punchless differentiated conidia similar to wild type and melanized appressoria that built up as much turgor as wild type. Overall, punchless is indistinguishable from wild type except for its lack of pathogenicity. These observations suggest that punchless is defective in a function that is specific of the infection process. Interestingly, punchless differs from most other M. grisea nonpathogenic mutants, which are also altered in morphological or physiological traits observable on mycelial cultures, spores, or appressoria (2, 3, 8, 14, 24, 25, 27–29).
Cytological investigations showed that punchless appressoria differentiated on leaves were similar to mature wild-type appressoria before penetration (high turgor, melanization, accumulation of glycogen) but they did not differentiate penetration pegs or degrade their glycogen. These phenotypic observations suggest that punchless is blocked at an early stage of appressorium-mediated penetration, as penetration peg emergence is correlated with glycogen degradation in wild type (17). Three other M. grisea nonpathogenic mutants have been reported to differentiate appressoria that cannot penetrate intact host surfaces (nonfunctional appressoria): buf1− is nonmelanized and unable to generate turgor (6, 24, 26), cpkA− is defective in the catalytic subunit of a cAMP-dependent protein kinase (25), and mps1− is defective in a mitogen-activated protein kinase involved in the cell wall integrity signaling pathway (29). These mutants cannot penetrate intact leaf surfaces but retain the ability to infect wounded leaves. punchless differed from these mutants, as it failed to infect wounded leaves. We speculated that buf1−, cpkA−, and mps1− appressoria retain the ability to differentiate an appressorium and a penetration peg that has the ability to invade wounded but not intact plant surfaces. According to this hypothesis, we explain the inability of punchless to invade wounded leaves only as a consequence of its lack of penetration peg differentiation.
We demonstrated by complementation of punchless with wild-type PLS1 gene and by targeted deletion of PLS1 in wild type that the punchless phenotype correlates with the inactivation of this gene. PLS1 encodes a putative integral membrane protein of 225 aa (Pls1p) with all of the structural hallmarks of the tetraspanin family (10–12). The Pls1p-GFP fusion was detected only in appressoria differentiated on leaves before and during penetration (8–20 h). This expression pattern supports the hypothesis deduced from the phenotypic analysis of punchless that gives to PLS1 a key role in the control of a function required before and during the early stage of penetration. Using this Pls1p-GFP fusion, we have shown that Pls1p is located in the appressorial plasma membranes. We observed that the Pls1-GFP fusion was also targeted to appressorial vacuoles. Such vacuolar targeting of the fusion protein could result either from the recycling of membrane proteins by endocytosis or from the partial missorting of the fusion protein. This vacuolar targeting is apparently not detrimental to the function of Pls1p-GFP, as this construct was shown to complement the ΔPLS1 mutant. The structural similarity between Pls1p and tetraspanins and the membrane localization of Pls1p strongly suggest that it belongs to this family of proteins. Pls1p appears to be the first tetraspanin-like protein described outside of the animal kingdom, since we could not detect any tetraspanin-like encoding genes in the yeast Saccharomyces cerevisiae genome by using either homology searches or structural analysis of yeast proteins with four transmembrane domains. In animals, tetraspanins are involved in cell differentiation, migration, and adhesion (10–12). They associate with other membrane proteins, including integrins, into signaling complexes (30). As a result of tetraspanin overexpression or their triggering by specific monoclonal antibodies, major changes in animal cell shape (aggregation or migration) and signaling pathways (calcium increase, tyrosine phosphorylation, and actin network modification) were observed (12). Three mutants defective for a tetraspanin were reported, the late bloomer (lbl) mutant from Drosophila melanogaster (31) and CD81 (32, 33) and CD9 (34, 35) mutants from mouse. The lbl mutant displayed a delay in synapse formation (31). In the cd81− mutant, B lymphocyte proliferation induced by IgM receptor cross-linking was reduced (32). cd81− mutants showed also a reduction in IgM receptor expression (33). The cd9− mutant displayed a severe reduction in female fertility as a result of a defect in sperm–egg fusion, a process involving a CD9–integrin association (34, 35). The differences observed between the phenotypes of these tetraspanin mutants suggest that these related proteins are involved in the control of different cellular processes. The inactivation of the fungal tetraspanin-like encoding gene PLS1 abolished a function required for host surface penetration that results in the absence of penetration peg differentiation. This differentiation process is characterized by a polarized growth initiated at the appressorium base. In M. grisea, actin was shown to accumulate at the base of the appressorium and in the penetration peg, suggesting a reorganization of the actin cytoskeleton during such a polarized growth (36). Because some animal tetraspanins are involved in signaling pathways mediating actin reorganization (37, 38), the present results led us to speculate that PLS1 could be involved in a similar signaling pathway in fungi that could control actin cytoskeleton reorganization at the base of the appressorium before penetration peg emergence. As described for animal tetraspanins, Pls1p could be associated with other membrane proteins in complexes that might include an integrin-like protein. If such membrane complexes are present in fungi, they could open new paths to investigate the morphogenesis of fungal infectious structures.
Acknowledgments
We are grateful to M. Dufresne and T. Langin (Institut de Biotechnologie des Plantes, Université Paris-Sud, Orsay, France), N. Tongue and N. J. Talbot (Biological Sciences, University of Exeter, Exeter, U.K.) for their assistance in PLS1 gene expression analysis and cDNA cloning. We also thank D. Grunwald (Département de Biologie Moléculaire et Structurale–Canaux Ioniques Signalisation, Commissariat à l'Energie Atomique-Grenoble, France) for his precious help in confocal scanning microscopy and B. Favery (Institut National de la Recherche Agronomique, Antibes, France) for setting up transformation. We acknowledge C. Derpierre (Fongicides, Aventis CropScience, Lyon, France), S. Sibuet (Biotechnologies, Aventis CropScience, Lyon, France), C. Duchartre (Institut de Génétique et Microbiologie, Université Paris-Sud, Orsay, France), and J. Milazzo and H. Adreit (Phytopathologie, Centre de Coopération Internationale en Recherche Agronomique pour le Développement-Cultures Annuelles, Montpellier, France) for their excellent technical assistance. We also thank D. Pauron (Institut National de la Recherche Agronomique, Antibes, France) for his independent analysis of Pls1p, H. Böhnert, D. Job, and R. Douce (Unité Mixte de Recherche 1932 Centre National de la Recherche Scientifique-Aventis, Lyon, France), and J. Pillmoor and R. Bohlmann (Biotechnologies, Aventis CropScience, Lyon, France) for comments on the manuscript. P.-H.C. was supported by a fellowship from the Bioavenir Program (Ministère de l'Education Nationale, de la Recherche et de la Technologie-Aventis CropScience, Lyon, France).
Abbreviations
- PET
polyethylene terephthalate
- GFP
green fluorescent protein
- RT-PCR
reverse transcription–PCR
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AX058235 and AX058239).
Böhnert, H., Basse, C., Aichinger, C. & Kahmann, R., Fourth European Congress on Fungal Genetics, April 5–10, 1998, Leon, Spain, p. 257 (abstr.).
References
- 1.Emmett R W, Parbery D G. Annu Rev Phytopathol. 1975;13:147–167. [Google Scholar]
- 2.Dean R A. Annu Rev Phytopathol. 1997;35:211–234. doi: 10.1146/annurev.phyto.35.1.211. [DOI] [PubMed] [Google Scholar]
- 3.Hamer J E, Talbot N J. Curr Opin Microbiol. 1998;1:693–697. doi: 10.1016/s1369-5274(98)80117-3. [DOI] [PubMed] [Google Scholar]
- 4.Ou S H. Rice Diseases. 2nd Ed. Commonwealth Agricultural Bureaux, Kew, U.K.: Commonwealth Mycological Institute; 1985. pp. 109–201. [Google Scholar]
- 5.Hamer J E, Howard R J, Chumley F G, Valent B. Science. 1988;239:288–290. doi: 10.1126/science.239.4837.288. [DOI] [PubMed] [Google Scholar]
- 6.Howard R J, Valent B. Annu Rev Microbiol. 1996;50:491–512. doi: 10.1146/annurev.micro.50.1.491. [DOI] [PubMed] [Google Scholar]
- 7.Dufresne M, Bailey J, A, Dron M, Langin T. Mol Plant–Microbe Interact. 1998;11:99–108. doi: 10.1094/MPMI.1998.11.2.99. [DOI] [PubMed] [Google Scholar]
- 8.Sweigard J, Carroll A M, Farall L, Chumley F G, Valent B. Mol Plant–Microbe Interact. 1998;11:404–412. doi: 10.1094/MPMI.1998.11.5.404. [DOI] [PubMed] [Google Scholar]
- 9.Urban M, Bhargarva T, Hamer J E. EMBO J. 1999;18:512–521. doi: 10.1093/emboj/18.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horejsi V, Vlcek C. FEBS Lett. 1991;288:1–4. doi: 10.1016/0014-5793(91)80988-f. [DOI] [PubMed] [Google Scholar]
- 11.Wright M D, Tomlinson M G. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 12.Maecker H T, Todd S C, Levy S. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 13.Daboussi M J, Djeballi A, Gerlinger C, Blaiseau P L, Bouvier I, Cassan M, Lebrun M-H, Parisot D, Brygoo Y. Curr Genet. 1989;15:453–456. doi: 10.1007/BF00376803. [DOI] [PubMed] [Google Scholar]
- 14.Silué D, Tharreau D, Talbot N J, Clergeot P-H, Notteghem J-L, Lebrun M-H. Physiol Mol Plant Pathol. 1998;53:239–251. [Google Scholar]
- 15.Punt P J, Oliver R P, Dingemanse M A, Pouwels P H, van den Hondel C A. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 16.Howard R J, Ferrari M A, Roach D H, Money N P. Proc Natl Acad Sci USA. 1991;88:11281–11284. doi: 10.1073/pnas.88.24.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard R J, Bourett T M. Can J Bot. 1990;68:329–342. [Google Scholar]
- 18.Pépin R, Boumendil J. Cytologia. 1982;47:359–372. [Google Scholar]
- 19.Richardson K C, Jarrett L, Finke E H. Stain Technol. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- 20.Sweigard J, Chumley F G, Carroll A M, Farrall L, Valent B. Fungal Genet Newsletter. 1997;44:52–53. [Google Scholar]
- 21.Ausubel F M, Brent R, Kingston R E, Moore D, D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 22.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Ochmann H, Gerber A L, Hartl D L. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chumley F G, Valent B. Mol Plant–Microbe Interact. 1990;3:135–143. doi: 10.1094/MPMI.1998.11.5.404. [DOI] [PubMed] [Google Scholar]
- 25.Xu J-R, Urban M, Sweigard J A, Hamer J E. Mol Plant–Microbe Interact. 1997;10:187–194. [Google Scholar]
- 26.Howard R J, Ferrari M A. Exp Mycol. 1989;13:403–418. [Google Scholar]
- 27.DeZwaan T M, Carrol A, Valent B, Sweigard J. Plant Cell. 1999;11:2013–2030. doi: 10.1105/tpc.11.10.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balhadère P V, Foster A J, Talbot N J. Mol Plant–Microbe Interact. 1999;12:129–142. [Google Scholar]
- 29.Xu J-R, Staiger C J, Hamer J E. Proc Natl Acad Sci USA. 1998;95:12713–12718. doi: 10.1073/pnas.95.21.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemler M E. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- 31.Kopczynski C C, Davis G W, Goodman C S. Science. 1996;271:1867–1870. doi: 10.1126/science.271.5257.1867. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, Müller U, Campbell K S. EMBO J. 1997;16:4217–4225. doi: 10.1093/emboj/16.14.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsitikov E N, Gutierrez-Ramos J C, Geha R S. Proc Natl Acad Sci USA. 1997;94:10844–10849. doi: 10.1073/pnas.94.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 35.Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, et al. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 36.Bourett T M, Howard R J. Protoplasma. 1992;168:20–26. [Google Scholar]
- 37.Lagaudrière-Gesbert C, Lebel-Binay S, Hubeau C, Fradelizi D, Conjeaud H. Eur J Immunol. 1998;28:4332–4344. doi: 10.1002/(SICI)1521-4141(199812)28:12<4332::AID-IMMU4332>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Berditchevski F, Odintsova E. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]