Abstract
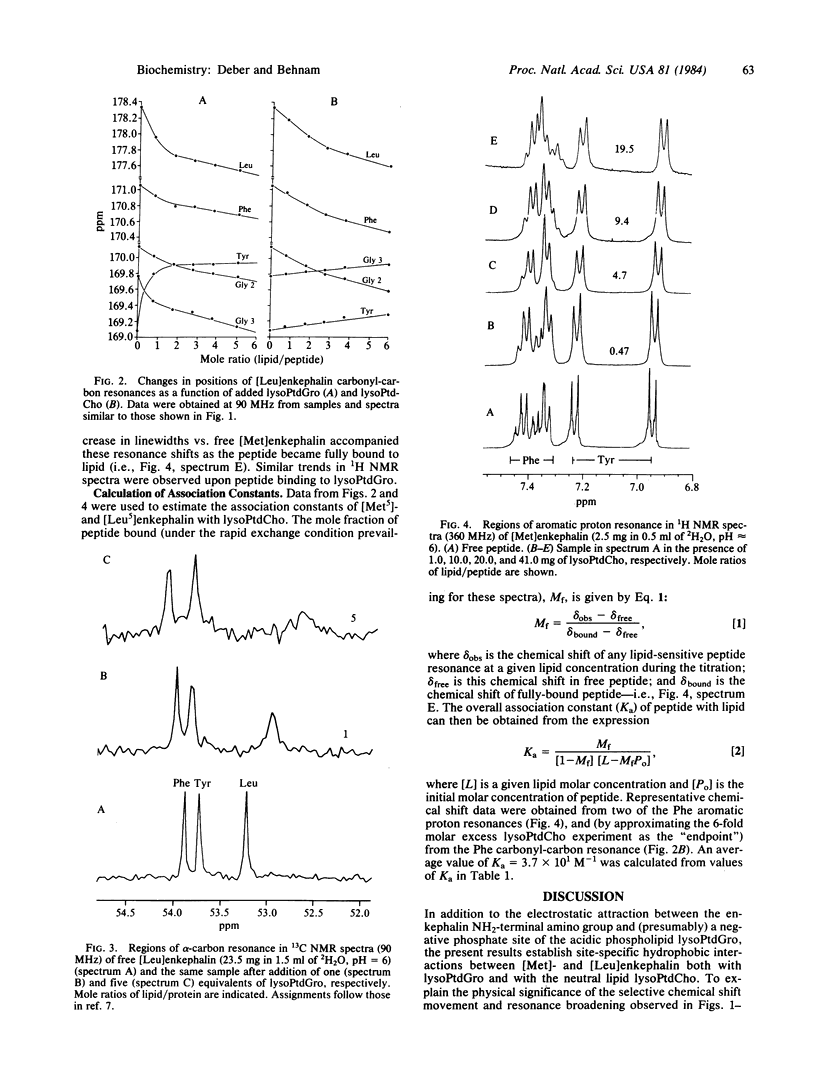
In the course of their biological function, peptide hormones must be transferred from an aqueous phase to the lipid-rich environment of their membrane-bound receptor proteins. We have investigated the possible influence of phospholipids in this process, using 360-MHz 1H and 90-MHz 13C NMR spectroscopy to examine the association of the opioid peptides [Met]- and [Leu]enkephalins (Tyr-Gly-Gly-Phe-Met/Leu) with phospholipid micelles. Binding of peptides to lipid was monitored in NMR spectra by selective chemical shift movements (e.g., the Phe aromatic ring protons) and residue-specific line broadening (e.g., of Met/Leu carbonyl- and alpha-carbon resonances). Results established that the zwitterionic hormones associate hydrophobically both with a neutral lipid (lysophosphatidylcholine) and (also electrostatically) with a negative lipid (lysophosphatidylglycerol). An association constant of Ka = 3.7 X 10(1) M-1 was calculated for the hydrophobic binding of enkephalin to lysophosphatidylcholine. NMR data suggested that enkephalin binds to the lipid with Met/Leu, Phe, and likely Tyr side-chain substituents associated with nonpolar interior regions of the micelle, whereas the COOH-terminal carboxylate moiety of the peptide is located in the surface of the lipid particle. An "attraction-interaction" model is proposed for hormone-lipid association wherein negative lipids attract the hormone electrostatically, while site-specific hydrophobic contacts facilitate its entry, concentration, and orientation into the lipid phase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood L. G., Salem N., MacNeil M., Butler M. Phospholipid changes in synaptic membranes by lipolytic enzymes and subsequent restoration of opiate binding with phosphatidylserine. Biochim Biophys Acta. 1978 Jul 25;530(1):35–46. doi: 10.1016/0005-2760(78)90124-8. [DOI] [PubMed] [Google Scholar]
- Abood L. G., Takeda F. Enhancement of stereospecific opiate binding to neural membranes by phosphatidyl serine. Eur J Pharmacol. 1976 Sep;39(1):71–77. doi: 10.1016/0014-2999(76)90114-x. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. O., Fishman P. H. Biotransducers of membrane-mediated information. Adv Enzymol Relat Areas Mol Biol. 1979;50:303–323. doi: 10.1002/9780470122952.ch6. [DOI] [PubMed] [Google Scholar]
- Brown L. R. Use of fully deuterated micelles for conformational studies of membrane proteins by high resolution 1H nuclear magnetic resonance. Biochim Biophys Acta. 1979 Oct 19;557(1):135–148. doi: 10.1016/0005-2736(79)90096-8. [DOI] [PubMed] [Google Scholar]
- Burgen A. S., Roberts G. C., Feeney J. Binding of flexible ligands to macromolecules. Nature. 1975 Feb 27;253(5494):753–755. doi: 10.1038/253753a0. [DOI] [PubMed] [Google Scholar]
- Bösch C., Brown L. R., Wüthrich K. Physicochemical characterization of glucagon-containing lipid micelles. Biochim Biophys Acta. 1980 Dec 12;603(2):298–312. doi: 10.1016/0005-2736(80)90376-4. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Garbay-Jaureguiberry C., Roques B. P., Oberlin R. Preferential conformation of the endogenous opiate-like pentapeptide Met-enkephalin in DMSO-D6 solution determined by high field H NMR. Biochem Biophys Res Commun. 1976 Jul 26;71(2):558–565. doi: 10.1016/0006-291x(76)90823-8. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M., Lacy J. E., Thompson K. F., Rockwell A. L., Watnick P. I. Conformations of model peptides in membrane-mimetic environments. Biophys J. 1982 Jan;37(1):275–284. doi: 10.1016/S0006-3495(82)84676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godici P. E., Landsberger F. R. The dynamic structure of lipid membranes. A 13C nuclear magnetic resonance study using spin labels. Biochemistry. 1974 Jan 15;13(2):362–368. doi: 10.1021/bi00699a022. [DOI] [PubMed] [Google Scholar]
- Hagen D. S., Weiner J. H., Sykes B. D. Investigation of solvent accessibility of the fluorotyrosyl residues of M13 coat protein in deoxycholate micelles and phospholipid vesicles. Biochemistry. 1979 May 15;18(10):2007–2012. doi: 10.1021/bi00577a026. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hebdon G. M., LeVine H., 3rd, Sahyoun N. E., Schmitges C. J., Cuatrecasas P. Specific phospholipids are required to reconstitute adenylate cyclase solubilized from rat brain. Proc Natl Acad Sci U S A. 1981 Jan;78(1):120–123. doi: 10.1073/pnas.78.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby V. J. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sci. 1982 Jul 19;31(3):189–199. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- Hughes D. W., Stollery J. G., Moscarello M. A., Deber C. M. Binding of myelin basic protein to phospholipid micelles. J Biol Chem. 1982 May 10;257(9):4698–4700. [PubMed] [Google Scholar]
- Jarrell H. C., Deslauriers R., McGregor W. H., Smith I. C. Interaction of opioid peptides with model membranes. A carbon-13 nuclear magnetic study of enkephalin binding to phosphatidylserine. Biochemistry. 1980 Jan 22;19(2):385–390. doi: 10.1021/bi00543a021. [DOI] [PubMed] [Google Scholar]
- Jones C. R., Gibbons W. A., Garsky V. Proton magnetic resonance studies of conformation and flexibility of enkephalin peptides. Nature. 1976 Aug 26;262(5571):779–782. doi: 10.1038/262779a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Bridges K., Tsunoo H., Blumenthal R., Weinstein J. N., Ashwell G. Reconstitution of the hepatic asialoglycoprotein receptor with phospholipid vesicles. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5087–5091. doi: 10.1073/pnas.77.9.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Hughes J. Some thoughts on the significance of enkephalin, the endogenous ligand. Life Sci. 1975 Jul 1;17(1):91–96. doi: 10.1016/0024-3205(75)90243-x. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Bösch C., Brown L. R., Wüthrich K. Physicochemical studies of the protein-lipid interactions in melittin-containing micelles. Biochim Biophys Acta. 1979 Sep 21;556(2):244–264. doi: 10.1016/0005-2736(79)90046-4. [DOI] [PubMed] [Google Scholar]
- Lee N. M., Smith A. P. A protein-lipid model of the opiate receptor. Life Sci. 1980 May 5;26(18):1459–1464. doi: 10.1016/0024-3205(80)90266-0. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972 Apr 11;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Massey J. B., Gotto A. M., Jr, Pownall H. J. Dynamics of lipid-protein interactions. Interaction of apolipoprotein A-II from human plasma high density lipoproteins with dimyristoylphosphatidylcholine. J Biol Chem. 1980 Nov 10;255(21):10167–10173. [PubMed] [Google Scholar]
- Simonds W. F., Koski G., Streaty R. A., Hjelmeland L. M., Klee W. A. Solubilization of active opiate receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4623–4627. doi: 10.1073/pnas.77.8.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., McDonald B. J. Association of myelin basic protein with detergent micelles. Biochim Biophys Acta. 1979 Jun 13;554(1):133–147. doi: 10.1016/0005-2736(79)90013-0. [DOI] [PubMed] [Google Scholar]
- Stimson E. R., Meinwald Y. C., Scheraga H. A. Solution conformation of enkephalin. A nuclear magnetic resonance study of 13C-enriched carbonyl carbons in [Leu5]-enkephalin. Biochemistry. 1979 May 1;18(9):1661–1671. doi: 10.1021/bi00576a005. [DOI] [PubMed] [Google Scholar]
- Tancrède P., Deslauriers R., McGregor W. H., Ralston E., Sarantakis D., Somorjai R. L., Smith I. C. A carbon-13 nuclear magnetic resonance study of the molecular dynamics of methionine-enkephalin and alpha-endorphin in aqueous solution. Biochemistry. 1978 Jul 11;17(14):2905–2914. doi: 10.1021/bi00607a032. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Ikeda K., Yang J. T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981 Feb 3;20(3):566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Yang J. T. Conformation of insulin and its fragments in surfactant solutions. Biochim Biophys Acta. 1981 Feb 27;667(2):285–293. doi: 10.1016/0005-2795(81)90194-x. [DOI] [PubMed] [Google Scholar]
- Zetta L., Cabassi F. 270-MHz 1H nuclear-magnetic-resonance study of met-enkephalin in solvent mixtures. Conformational transition from dimethylsulphoxide to water. Eur J Biochem. 1982 Feb;122(1):215–222. doi: 10.1111/j.1432-1033.1982.tb05869.x. [DOI] [PubMed] [Google Scholar]


