Abstract
Objective
Atrial septal defect (ASD) device closure is routinely done under the guide of transesophageal or intracardiac echocardiography which are expensive techniques and not easily affordable in developing countries.
Methods
Using metallic devices, we attempted 32 ASD device closures under transthoracic echocardiography.
Findings
Of those, 30 procedures were successful (94 %). In two patients with relatively large ASD we encountered difficulty in positioning the device. These patients were referred for surgical closure.
Conclusion
ASD device closure can be carried out successfully in most patients under transthoracic echocardiography in situations where transesophageal or intravenous echocardiographies are not available or affordable.
Keywords: Transthoracic echocardiography, Amplatzer septal occluder, ASD sizing, Interventional cardiology
Introduction
Transcatheter closure of atrial septal defect (ASD), first described by King et al in 1976 [1], is now an accepted method of therapy for this disease. Although this method of therapy may be more expensive, it is preferable over surgery due to less traumatic burden and better cosmetic results [2, 3]. In addition to fluoroscopy, an echocardiographic imaging modality is necessary to guide the interventionist during this procedure. Transesophageal echocardiography (TEE) or intracardiac echocardiography (ICE) are standard methods for this purpose. However, both methods are relatively inconvenient since they either require general anesthesia (TEE) or high cost catheters and special training. Many patients or pediatric cardiology centers cannot afford high expenses of purchasing and maintaining these equipments. On the other hand, transthoracic echocardiography (TTE) is convenient and accessible everywhere without additional cost. There is little evidence in the literature about ASD closure under TTE guidance [4–7]. In this retrospective study, we report the results of ASD device closures in 32 consecutive patients less than 40 kg under TTE guidance.
Subjects and Methods
Subjects: From October 2006 to December 2010, transcatheter ASD closure was attempted in 37 patients with secundum ASD at an academic hospital. Of those, 5 weighed more than 40 kg and the procedures were guided by TEE. Therefore, they are excluded from this study. Inclusion criteria were the existence of an ASD with significant shunt which was suitable for device closure (see the procedure section). The cohort consisted of 24 females and 8 males. Median age at the procedure was 5 years (range 2–14), and median weight 15 kg (range 9–40). Mean pulmonary artery pressure (MPAP) was 17.6±4.1 mmHg, and pulmonary to systemic shunt 2.2±0.6 to one. Median defect size was 14 mm (range 6–28), stretched defect size 16 mm (range 8.5–30), and device size 16 mm (range 9–30).
Procedure: Indication of ASD closure was set when pulmonary flow was at least 1.5 times higher than systemic flow. Defect diameters were measured in subcostal sagittal and coronal, apical four- and five-chamber, and short-axis views. The defect rims to aorta, mitral and tricuspid valves, superior and inferior venae cavae (SVC and IVC), and right pulmonary veins were determined in the same views. A minimum of 5 mm for septal occluders with exemption of aortic rim was required for device closure. Total septal dimensions were also measured and if they were not at least 2 mm larger than device discs, device closure was not attempted. Any concomitant cardiovascular abnormality was searched to repair if possible by transcatheter techniques or to schedule for surgery. Three patients had severe pulmonary stenosis which was treated simultaneously by balloon valvoplasty.
After obtaining informed consent, the patients were sent to the catheterism laboratory. The procedures were done under deep sedation using ketamine and/or midazolam. Femoral vein was catheterized. After complete right heart catheterism, a pulmonary angiography was done at anteroposterior view to exclude any anomalous pulmonary venous drainage. At this stage, an intravenous heparin bolus (75units/kg) was administrated. Then, ASD stretched diameter was determined using sizing balloon catheters (AGA Medical Corporation, USA) in all patients except two. In these two smallest most recent patients, we preferred to use a device at least 15% larger than the defect size without balloon sizing to decrease the risk of defect stretching in small children.
We have used metallic occluders from three companies. Of 30 implanted occluders, 16 were Atrial Septal Occluder (Lifetech Scientific, PR China), 6 Amplatzer Septal Occluder (AGA Medical Corporation, USA) and 8 Cardio-O-Fix ASD Occluder (Starways Medical Technologies, PR China). To order an appropriate device size, we added 15% to the largest measured defect diameter on pre-procedure echocardiogram. However, during the procedure we changed the device size to a larger one only if the stretched diameter was more than 1 mm greater than the pre-selected device size. This occurred in 6 patients out of 30. We selected a larger device ±2mm of stretched diameter which needed repeated catheterization in one patient due to device unavailability.
Metallic occluder implantation was carried out as described by Hijazi [8] with some modifications. Transthoracic real-time echocardiography was done during several steps of the procedure using either an Esaote MyLab 50CV or Toshiba UI TH-370A echocardiographs. First, stretched defect size was measured when shunting across the defect was eliminated on subcostal view during slow balloon inflation (static sizing, stop-flow diameter) [9, 10]. Second, TTE was used to confirm correct left atrial disc position on the defect (subcostal view). Then aortic, mitral, pulmonary, IVC, SVC, right pulmonary venous, and coronary sinus flows; position of the device; and the presence of any residual shunt or missed additional defects were investigated by TTE (Fig. 1). Fluoroscopy was done between these steps to deploy the discs and to doubly check the position of the device. Simultaneous echocardiography and fluoroscopy was used in some difficult situations. But we tried to limit these occasions because of x-ray hazards to the echocardiographer (Fig. 2). Care was taken to prevent contamination of the catheter introduction area while performing echocardiography and changing the position of the camera. The device was released under fluoroscopy.
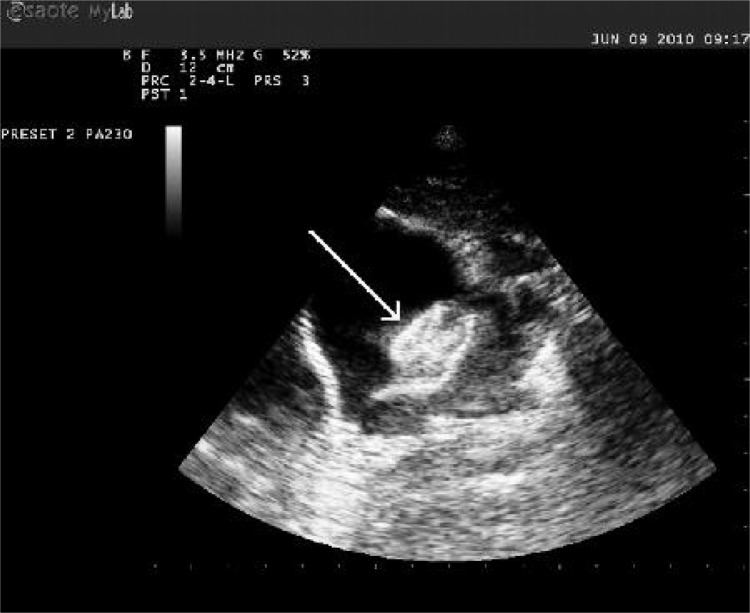
Fig. 1.
Echocardiographic image of the atria, interatrial septum and septal occluder (white arrow) during catheterization, showing optimal position of the occluder.
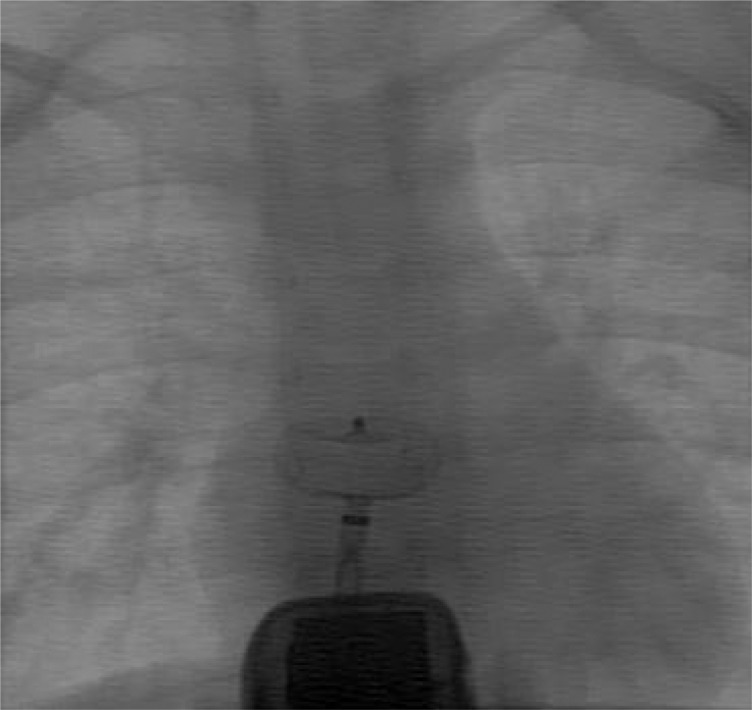
Fig. 2.
One of the infrequent situations where fluoroscopy and echocardiography were used simultaneously.
Follow up: Median follow up duration was 5 (range 1–28) months. Eight patients did not return later than the first follow up visit (one month after the procedure). Aspirin was administered to all patients for 6 months (3–5 mg/kg daily). Physical examination, electrocardiography, chest radiography and TTE were performed at each scheduled follow up visit.
Statistical Analysis: Descriptive statistics such as mean, median, standard deviation, and frequency were calculated for each demographic and clinical characteristic whenever appropriate.
Findings
Immediate: Of 32 attempted ASD transcatheter closures, the procedure was successful in 30 (94%). Mean device to defect ratio was 1.2±0.12 in the successful closures. Eight of 30 ratios (27%) were less than 1.125 and 3 (10%) were more than 1.33.
In the first failed patient (2.5 years of age, 12 kg), the measured defect size by echo was 10 mm. During the procedure, the defect could be stretched up to 16 mm. We first tried to deploy a 12mm metallic device and then a 14 mm one, but both trials were unsuccessful. As patient's total septal diameter (around 30 mm) was not large enough to accept a bigger device, we ended device closure. In the second failed patient (8.5 years of age, 18 kg), the unstretched and stretched defect diameters were 26 and 29mm, respectively. We tried to implant a 30mm device but we could not position it on the septum. We referred both patients for surgical ASD closure which was done successfully.
One patient had a small residual shunt the day after the procedure. That disappeared one month later at the first follow up. No major complication had occurred.
The echocardiography windows in all of our patients were excellent. Differentiating between SVC flow disturbance over sizing balloon and small residual shunts was difficult in some patients.
Follow up: Mild mitral regurgitation developed in 3 patients after device occlusion. No pericardial effusion, device embolization, or neurologic problems were seen.
Discussion
TEE is the standard adjunct echocardiography modality during ASD closure. Even some advocate ASD closure only under TEE guidance without using fluoroscopy [11]. However, this modality has some disadvantages when used for children. Not only the purchase but also the maintenance of small TEE probes is generally more costly. In addition, TEE in children requires general anesthesia due to patient intolerance which adds to the cost and complexity of the equipments.
ICE is the other accepted echocardiographic modality for ASD device closure [12–14]. The main disadvantage of ICE is its cost. In the past, the need for at least an 11 Fr sheath for the 10.5 Fr AcuNav catheter (Acuson Corporation, Simens Medical, USA) was another drawback. The introduction of the new 8 Fr catheter largely eliminated this problem.
Although both TEE and ICE provide constant and high quality images during ASD device closure, their cost disadvantages make them unaffordable for the hospitals and patients in developing world. Instead, we used TTE for guiding ASD device closure. This modality has several disadvantages as well. First, the quality of images is less than those of TEE and ICE. Second, there are a risk of infection due to the proximity of catheterism site and subcostal region. Third, simultaneous use of fluoroscopy and echocardio-graphy can expose the echocardiographer to a large radiation dose.
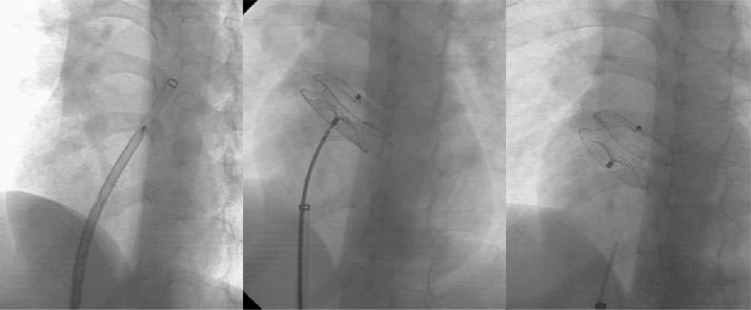
Although the image quality of TTE is suboptimal, we showed that it could be enough for successful device implantation. The procedures were successful in 94% of attempts. We had two failed procedures while attempting metallic device implantation. Underestimation of the defect size, balloon overstretching, small size of the patient, and use of TTE may lead to the procedural failure in the first patient. Our limited experience in closure of large defects, relatively small size of the patient and TTE guidance were possible causes for the failure in the second case. However, we later occluded another large defect in another patient successfully (Fig. 3). Left atrial disc of large metallic occluders often prolapse to the RA due to their perpendicular positioning. Several techniques or special equipments were suggested to prevent this problem during large ASD (defect size more than 25 mm) closure [15–17]. We used a straight long sheath without any curve at its end (William Cook Europe, Denmark) instead of the traditional curved ones to deliver a 30-mm Amplatzer septal occluder in this patient. Device was positioned easily in this way.
Fig. 3.
Successful implantation of a 30-mm Amplatzer septal occluder using a long sheath with straight tip. Left, forwarding the device in the straight long sheath; middle, implanted device before release; right, final position of the device
Great care was paid to antiseptic management, especially while changing camera position. The patient's body was uncovered until his or her umbilicus for echocardiography. The echocardiographer tried not to touch the sterilized catheterization area either by hand or echocardiography probe. We did not encounter any infective complication. The interventionist should accept the problem of frequent camera position changes and possible resultant extra time. The echocardiographist also should accept the need for simultaneous echocardiography and fluoroscopy in rare occasions (Fig. 2).
Several reports on successful ASD closure under TTE guidance exist. Kardon et al reported 56 successful procedures. However, they occluded all patients with multiple defects using TEE [5]. Sun et al performed 14 successful closures of atrial septal aneurysms associated with ASD [6]. An unknown number of ASD closures in the report of Fischer et al were done under TTE guidance [4]. Zaquot et al used TTE during ASD closure in 2 patients due to surgically repaired transesophageal fistula in one and technical failure of TEE in the other [7]. Behjati et al used TTE for ASD closure in 63 patients [18]. They were successful in 57 procedures but failed in 6 including one device dislodgement and one embolization.
Three-dimensional echocardiography is increasingly recognized as a useful tool for delineating the shape of the ASD and number of the defects before ASD closure. In addition, it can be additive or even superior to other modalities in guiding the procedure [19, 20].
We used metallic occluders from several companies with different prices. All sizes of these devices cannot be available in our catheterization laboratory at the same time. Therefore, we developed a protocol to select device size and purchase it before the procedure. We changed our selected size after balloon sizing only in 6 out of 30 patients. Our sizing protocol complies with the general trend to implant smaller devices [9, 10]. We encountered no major complication from device over- (erosion, pericardial effusion) or undersizing (device embolization), although more than one third of device/defect ratios were out of the safe zone (1.125–1.333) proposed by Rastogi et al [21].
Limitations of our study were use of devices from different companies, unavailability of all device sizes, and lack of a control group.
Conclusion
We conclude that ASD device closure can be safely done under TTE where more sophisticated echocardiographic modalities (TEE, ICE) are not available or affordable by the hospital or patient. However, for patients with relatively large defects we recommend either using TEE or ICE.
Acknowledgment
The authors acknowledge Drs Arjmandnia and Khosroshahi for their help, the patient who participated in this study.
Conflict of Interest
None
References
- 1.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976;235(23):2506–9. [PubMed] [Google Scholar]
- 2.Zeinaloo AA, Meraji SM, Zanjani KS, Mirzaaghayan MR. Device occlusion versus surgery for closure of congenital heart defects: cost issues in Iran. J Tehran Heart Center. 2008;3(3):141–4. [Google Scholar]
- 3.Molaei A MS, Nakhostin Davari P, Aarabi Moghaddam MY, Shah Mohammadi A. Assessment of the results of secundum atrial septal defect closure by two methods: surgery (right thoracotomy) and intervention (transcatheter Amplatzer Septal Occluder) Iranian Heart J. 2010;11(2):55–8. [Google Scholar]
- 4.Fischer G, Smevik B, Kramer HH, Bjornstad PG. Catheter-based closure of atrial septal defects in the oval fossa with the Amplatzer device in patients in their first or second year of life. Catheter Cardiovasc Interv. 2009;73(7):949–55. doi: 10.1002/ccd.21866. [DOI] [PubMed] [Google Scholar]
- 5.Kardon RE, Sokoloski MC, Levi DS, Perry JS, Schneider DJ, 2nd, Allada V, et al. Transthoracic echocardiographic guidance of transcatheter atrial septal defect closure. Am J Cardiol. 2004;94(2):256–60. doi: 10.1016/j.amjcard.2004.03.080. [DOI] [PubMed] [Google Scholar]
- 6.Sun ZL, Xie QY, Yang TL, et al. Transthoracic echocardiography in transcatheter closure of atrial septal aneurysm combined with secoundum-type atrial septal defect. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33(8):755–60. [PubMed] [Google Scholar]
- 7.Zaqout M, Suys B, De Wilde H, et al. Transthoracic echocardiography guidance of transcatheter atrial septal defect closure in children. Pediatr Cardiol. 2009;30(7):992–4. doi: 10.1007/s00246-009-9456-8. [DOI] [PubMed] [Google Scholar]
- 8.Hijazi ZM. Catheter closure of atrial septal and ventricular septal defects using the Amplatzer devices. Heart Lung Circ. 2003;12(Suppl 2):S63–72. doi: 10.1046/j.1443-9506.2003.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- 9.Amin Z, Hijazi ZM, Bass JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63(4):496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 10.Carlson KM, Justino H, O'Brien RE, Dimas VV, Leonard GT, Jr, Pignatelli RH, et al. Transcatheter atrial septal defect closure: modified balloon sizing technique to avoid overstretching the defect and oversizing the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2005;66(3):390–6. doi: 10.1002/ccd.20443. [DOI] [PubMed] [Google Scholar]
- 11.Ewert P, Berger F, Daehnert I, et al. Transcatheter closure of atrial septal defects without fluoroscopy: feasibility of a new method. Circulation. 2000;101(8):847–9. doi: 10.1161/01.cir.101.8.847. [DOI] [PubMed] [Google Scholar]
- 12.Koenig P, Cao QL, Heitschmidt M, et al. Role of intracardiac echocardiographic guidance in transcatheter closure of atrial septal defects and patent foramen ovale using the Amplatzer device. J Interv Cardiol. 2003;16(1):51–62. doi: 10.1046/j.1540-8183.2003.08003.x. [DOI] [PubMed] [Google Scholar]
- 13.Koenig PR, Abdulla RI, Cao QL, Hijazi ZM. Use of intracardiac echocardiography to guide catheter closure of atrial communications. Echocardiography. 2003;20(8):781–7. doi: 10.1111/j.0742-2822.2003.03039.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Cao QL, Koenig PR, et al. Intracardiac echocardiography to guide closure of atrial septal defects in children less than 15 kilograms. Catheter Cardiovasc Interv. 2006;68(2):287–91. doi: 10.1002/ccd.20824. [DOI] [PubMed] [Google Scholar]
- 15.Dalvi BV, Pinto RJ, Gupta A. New technique for device closure of large atrial septal defects. Catheter Cardiovasc Interv. 2005;64(1):102–7. doi: 10.1002/ccd.20248. [DOI] [PubMed] [Google Scholar]
- 16.Varma C, Benson LN, Silversides C, Yip J, Warr MR, Webb G, et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter Cardiovasc Interv. 2004;61(1):131–9. doi: 10.1002/ccd.10700. [DOI] [PubMed] [Google Scholar]
- 17.Wahab HA, Bairam AR, Cao QL, Hijazi ZM. Novel technique to prevent prolapse of the Amplatzer septal occluder through large atrial septal defect. Catheter Cardiovasc Interv. 2003;60(4):543–5. doi: 10.1002/ccd.10686. [DOI] [PubMed] [Google Scholar]
- 18.Behjati M, Mirhosseini S, Hosseini S, Rajaei S. Transcatheter closure of atrial septal defect with Amplatzer device in children and adolescents: Short and midterm results; an Iranian experience. Iran J Pediatr. 2011;21(2):166–172. [PMC free article] [PubMed] [Google Scholar]
- 19.Dardas PS, Ninios VN, Mezilis NE, et al. Percutaneous closure of atrial septal defects: immediate and mid-term results. Hellenic J Cardiol. 2010;51(2):104–12. [PubMed] [Google Scholar]
- 20.Johri AM, Witzke C, Solis J, et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr. 2011;24(4):431–7. doi: 10.1016/j.echo.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi N, Smeeton NC, Qureshi SA. Factors related to successful transcatheter closure of atrial septal defects using the Amplatzer septal occluder. Pediatr Cardiol. 2009;30(7):888–92. doi: 10.1007/s00246-009-9452-z. [DOI] [PubMed] [Google Scholar]