Abstract
Objective
Neonatal sepsis (NS) is a common and life-threatening disorder in infants. Previous studies showed that interleukin-6 (IL-6) may be a valid non-invasive and rapid method for diagnosis of NS. We conducted this review to assess the validity of IL-6 for predicting NS.
Methods
This was a systematic review with meta-analysis. Embase, Medline and Web of Science databases were searched between January 1990 and December 2009. The search terms used were “cytokine”, “neonate”, “sepsis” and “interleukin-6". We used standard methods recommended for meta analyses of diagnostic test evaluations. The analysis was based on a summary ROC (SROC) curve. Meta-regression analysis was used to assess the effects of some confounding factors on the results of meta-analysis. Potential presence of publication bias was tested using funnel plots and the Egger test.
Findings
Meta-analysis was performed on 13 publications including 353 infants with sepsis and 691 control infants. The pooled sensitivity and specificity of IL-6 was 0.79 and 0.84, respectively. The maximum joint sensitivity and specificity (i.e., the Q value) in SROC curve was 0.82 and the area under curve (AUC) was 0.89 (95% CI: 0.84-0.94). Meta-regression analysis showed that the diagnostic accuracy of IL-6 was not affected by confounding variables. The evaluation of publication bias showed that the Egger test was not significant (P=0.07).
Conclusion
IL-6 seems to be a valid marker for predicting NS. It may be considered for early diagnosis of sepsis in neonatal care units.
Keywords: Interlukin-6, Cytokines, Sepsis, Neonate, Meta-analysis
Introduction
Neonatal sepsis (NS) is a common and life-threatening disorder, especially in preterm Infants [1]. Up to 10% of infants have an infection in the first month of life [2]. The rate of mortality and morbidity due to NS is very high [1, 2].
The prognosis and outcome of NS depend on early diagnosis and on-time and efficient antibiotic therapy [1]. Diagnosis of neonatal infection may be the greatest and most difficult challenge for a neonatologist [3]. In most of NS patients, the clinical manifestations are non-specific in earlier stages [4], thus rapid and non-invasive methods for diagnosis of the infected neonate is important in neonatal care [5].
Clinical judgment and laboratory tests such as complete blood cell count and the ratio between immature to total neutrophils (I/T ratio) showed to be useful in the early diagnosis of neonatal septic infection [4]. The microbiological cultures are the gold standard for diagnosis of neonatal sepsis but they are not available until at least up to 72h and do not identify most infected infants [1, 2].
In recent years, chemokines and pro-inflammatory cytokines such as tissue necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-8 and pro-calcitonin (PCT) have been introduced as early markers in infected infants [6, 7]. IL-6 is produced by monocytes, endothelial cells and fibroblasts [1]. The concentration of IL-6 rises rapidly after the onset of bacteremia, but its half life is short [8, 9]. Previous studies showed that IL-6 may be a valid non-invasive and rapid method for diagnosis of NS [8, 9]. The role of IL-6 in diagnosis of NS was reviewed in 2000 [6] but it did not include meta-analysis and could not determine the quantitative value of IL-6 for diagnosis of NS.
So, we conducted this systematic review and meta-analysis to assess the validity of IL-6 for predicting NS.
Subjects and Methods
Search strategy and study selection
The following electronic databases were searched between January 1990 and December 2009: Embase, Medline and Web of Science. The Cochrane Library also was reviewed to find relevant articles.
The search terms used were “cytokine”, “neonate”, “sepsis” and “intrleukin-6”. The reference lists from original and review articles were also searched. No language restrictions were considered. We excluded conference abstracts and letters to the journal editors because of the limited data presented in them. A study was included in the meta analysis when it was conducted on neonates (1-28 days old). It consisted of at least two groups (culture positive sepsis group and control group); assessed the diagnostic value of IL6 in neonatal sepsis and provided enough data allowing test results to be extracted for individual study subjects.
If an article did not include enough data for calculating sensitivity and specificity (two by two table) the corresponding author was asked to provide us necessary data. In case of no response from the corresponding author, a reminder was sent after 1 week. If necessary data was not available after this process, the study was excluded from meta-analysis.
Studies conducted on special groups of neonates (neonates with metabolic disorders, hemodialysis, necrotizing enterocolitis and intraventricular hemorrhage) were excluded. All studies were checked by two reviewers independently. Disagreements were resolved by consensus.
Data extraction and quality assessment
Data collected from the studies included participant characteristics, type of study, sample size, test methods, sensitivity and specificity data, cutoff values, publication year, and methodological quality. The numbers of true-positive, false-positive, false-negative, and true-negative results were extracted for each study. If no data on the above criteria was reported in the primary studies, we requested the information from the authors.
We assessed the methodological quality of the studies using guidelines published by the quality assessment for studies of diagnostic accuracy (QUADAS) tool including 14 questions [10]. Questions with “yes”, “no” and “unknown” answer were scored as 1, -1 and 0, respectively. (maximum score, 14).
Statistical Analysis
We used standard methods recommended for metaanalyses of diagnostic test evaluations [11]. Analyses were performed using STATA version 10 and Meta-DiSc for Windows [12]. We computed the following measures of test accuracy for each study: sensitivity; specificity; positive likelihood ratio (PLR); negative likelihood ratio (NLR); and diagnostic odds ratio (DOR). The analysis was based on a summary ROC (SROC) curve [11, 13]. Sensitivity and specificity for the single test threshold identified for each study were used to plot an SROC curve [13, 14]. A random-effects model was used to calculate the average sensitivity, specificity and other measures across studies [15, 16].
The term heterogeneity when used in relation to meta-analyses refers to the degree of variability in results across studies. We used the chi-square and Fisher exact tests to detect statistically significant heterogeneity.
Meta-regression analysis was used to assess the effects of some factors on the results of meta-analysis. To assess the effects of these factors on the diagnostic ability of neonatal sepsis, we included them as covariates in univariate metaregression analysis (inverse variance weighted). The following 5 covariates entered the meta-regression analysis: time of sepsis onset (late onset versus others); study quality (QUADAS≥10 versus others); cut-off level for IL-6 assay (≥50 pg/ml versus others); control group status (healthy control versus others) and birth-weight of the neonates. Considering birth-weight, the neonates were divided into two groups. The first group consisted of neonates with very low birth-weight (VLBW) and the second one included neonates with low or normal birth-weight (others).
The relative DOR (RDOR) was calculated according to standard methods to analyze the change in diagnostic precision in the study per unit increase in the covariate [17, 18]. Since publication bias is of concern for metaanalyses of diagnostic studies, we tested for the potential presence of this bias using funnel plots and the Egger test [19].
Since the present study was a meta analysis that was based on published articles, to include the consents of patients and the approval of internal review boards was not applicable.
Findings
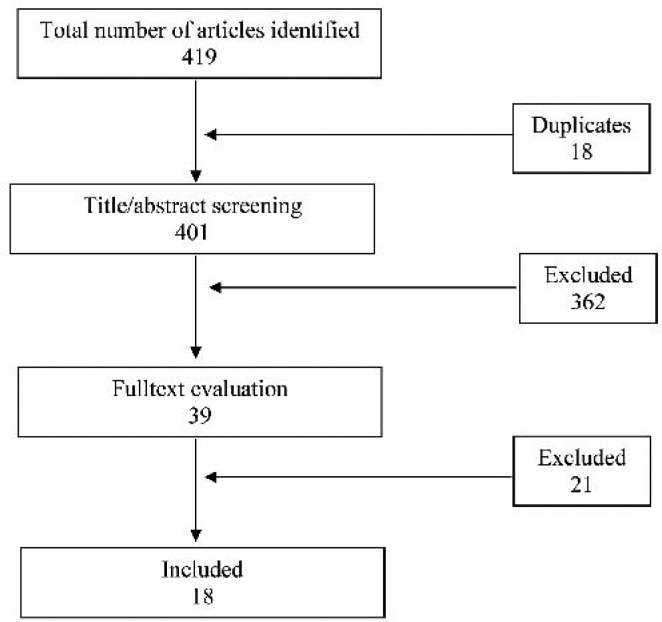
18 publications (No. 20-37) about the role of IL-6 for predicting NS were considered to have eligibility criteria to be included in the study (Fig. 1). We could not find enough data for analyzing 5 of 18 studies (Nos. 27, 28, 32, 33 and 35), so, they were excluded. Finally, 13 publications including 353 infants with sepsis and 691 control infants were analyzed. Table 1 shows summary data obtained from the studies.
Fig. 1.
Flow diagram of the process of identifying and including references for the systematic review
Table 1.
Characteristics of studies dealing with the role of IL-6 for predicting neonatal sepsis
| Study/ year | Assay method | Cut-off (pg/ml) | Sensitivityr (CI 95%) | Specificity (CI 95%) | DOR (CI 95%) | Quality score (QUADAS) |
|---|---|---|---|---|---|---|
| Buck et al/1994[20] | ELISA | 10 | 0.727 (0.390- 0.940) | 0.778 (0.644- 0.880) | 9.333 (2.138- 40.750) | 8 |
| Ng et al/1997[21] | ELISA | 31 | 0.886 (0.733- 0.968) | 0.955 (0.845- 0.994) | 162.75 (28.009- 945.68) | 13 |
| Berner et al/1998[22] | Double sandwich EIA | 100 | 0.875 (0.710- 0.965) | 0.93 (0.872- 0.968) | 93.333 (26.806- 324.97) | 11 |
| Kuster et al/1998[23] | ELISA | 25 | 0.857 (0.673- 0.960) | 0.85 (0.621- 0.968) | 34 (6.723- 171.94) | 13 |
| Dollner et al/2001[24] | ELISA | 33 | 0.818 (0.482- 0.977) | 0.692 (0.386- 0.909) | 10.125 (1.466- 69.935) | 11 |
| Krueger et al/2001[25] | ChIIA | 80 | 0.872 (0.726- 0.957) | 0.902 (0.837- 0.947) | 62.246 (20.729- 186.92) | 14 |
| Martin et al/2001[26] | ChIIA | 160 | 0.962 (0.693- 1.000) | 0.69 (0.454- 0.871) | 55.769 (2.849- 1091.7) | 11 |
| Gonzalez et al/2003[29] | ELISA | 18 | 0.667 (0.223- 0.957) | 0.81 (0.581- 0.946) | 8.5 (1.131- 63.871) | 10 |
| Laborada et al/ 2003[30] | ChII | 18 | 0.756 (0.597- 0.876) | 0.737 (0.603- 0.845) | 8.68 (3.442- 21.890) | 12 |
| Santana Reyes et al/ 2003[31] | ELISA | 30 | 0.612 (0.462- 0.748) | 0.803 (0.720- 0.871) | 6.453 (3.099- 13.439) | 13 |
| Verboon-Maciolek et al/2006[34] | ChIIA | 60 | 0.676 (0.502- 0.820) | 0.759 (0.565- 0.897) | 6.548 (2.192- 19.556) | 13 |
| Caldas et al/2008[36] | ChIIA | 25.8 | 0.775 (0.615- 0.892) | 0.87 (0.664- 0.972) | 22.963 (5.537- 95.232) | 11 |
| Beceiro Mosquera et al/2009[37] | Lateral Flow Immunoassay | 53 | 0.909 (0.587- 0.998) | 0.806 (0.625- 0.925) | 41.667 (4.434- 391.56) | 9 |
IL: interleukin; DOR: Diagnostic odds ratio; QUADAS: Quality assessment for studies of diagnostic accuracy; ELISA: Enzyme-linked immunosorbent assay; ChIIA: Chemiluminescence immunoassay; EIA= Enzyme immunoassay
Charachteristics of the studies
In 4 of 13 studies, control groups included healthy neonates. The designs of 6 studies were case-control and the remaining 7 were cross-sectional. In all of 13 studies, both male and female neonates were included. Mean gestational ages were 32.1 (range: 27.1-38.8) and 33.2 (range: 27.7-40) weeks in sepsis and control groups, respectively. Mean birth weights were 1780 (range: 904-3502) and 1927 (range: 1060-3628) grams in sepsis and non-sepsis neonates, respectively.
Diagnostic accuracy
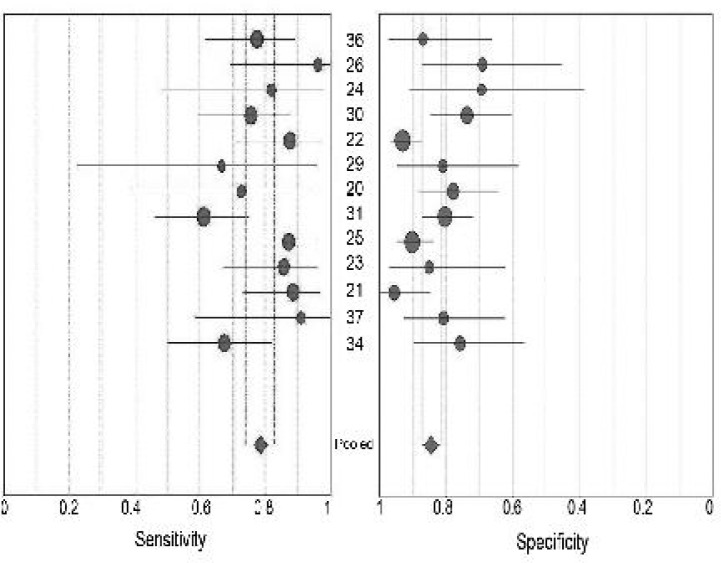
Table 1 and Fig. 2 show the results from included studies on sensitivity and specificity of IL-6 for predicting neonatal sepsis. PLR ranged from 2.66 to 19.47 (pooled, 4.55; 95% CI: 3.27-6.32) while NLR ranged from 0.06 to 0.48 (pooled, 0.26; 95% CI: 0.18-0.36). The pooled value of DOR was 20.74 with 95% CI of 10.83 to 39.7 (ranged from 6.45 to 162.75). χ2 values of sensitivity, specificity, PLR, NLR and DOR were 22.45 (P=0.03), 32.27 (P=0.001), 34.12 (P=0.001), 27.02 (P=0.008) and 33.96 (P=0.001).
Fig. 2.
Forest plot of estimates of sensitivity and specificity of IL-6 for predicting neonatal sepsis.
•=point estimates of sensitivity and specificity from each study; error bars=95% CIs; numbers= reference numbers of studies cited in the reference list. Pooled estimates were as follows: sensitivity, 0.79 (95% CI, 0.74 to 0.83); specificity, 0.84 (95% CI, 0.81 to 0.87)
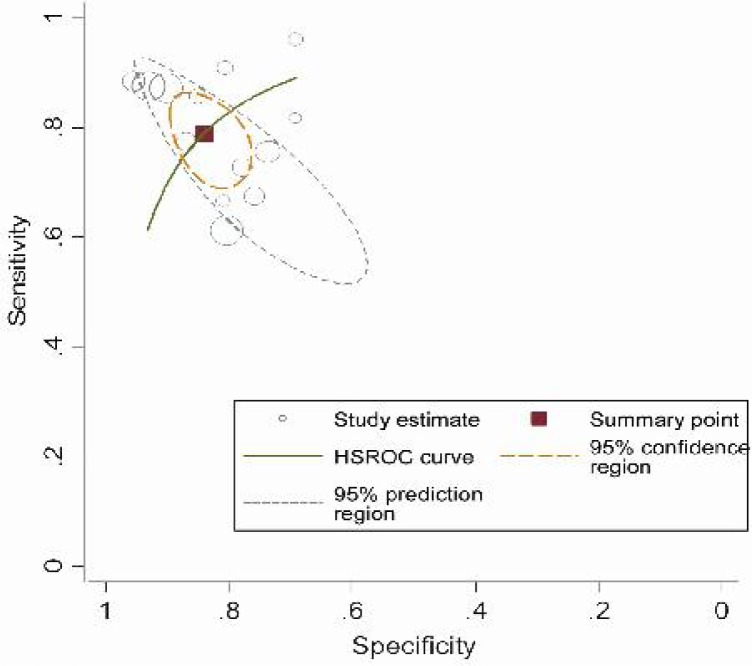
We found that the SROC curve is positioned near the upper left corner of the curve, and that the maximum joint sensitivity and specificity (i.e., the Q value) was 0.82; and the area under curve (AUC) was 0.89 (95% CI: 0.84-0.94) (Fig. 3).
Fig. 3.
Summary ROC curve for assessment of the diagnostic accuracy of IL-6 to predict neonatal sepsis.
Meta-regression analysis
The 5 covariates entered into the meta-regression did not have significant effect of the diagnostic accuracy of IL-6 (Table 2).
Table 2.
Metaregression analysis of the effects of some covariates on diagnostic precision of IL-6 for predicting neonatal sepsis
| Covariates | Number of studies | Coefficient | RDOR (CI95%) | P value |
|---|---|---|---|---|
| Time of sepsis onset (late onset) | 4 | −0.26 | 0.77 (0.15–3.83) | 0.72 |
| Study quality (QUADAS ≥10) | 11 | 0.98 | 2.65 (0.28–25.1) | 0.5 |
| Cut-off level (≥50 pg/ml) | 5 | −0.88 | 0.41 (0.09–1.98) | 0.24 |
| Birth-weight (VLBW) | 3 | 0.84 | 2.31 (0.34–5.88) | 0.35 |
| Control group status (healthy controls) | 4 | 0.06 | 1.06 (0.18–6.14) | 0.94 |
IL: interleukin; RDOR: Relative diagnostic odds ratio; QUADAS: Quality assessment for studies of diagnostic accuracy VLBW: Very low birth-weight
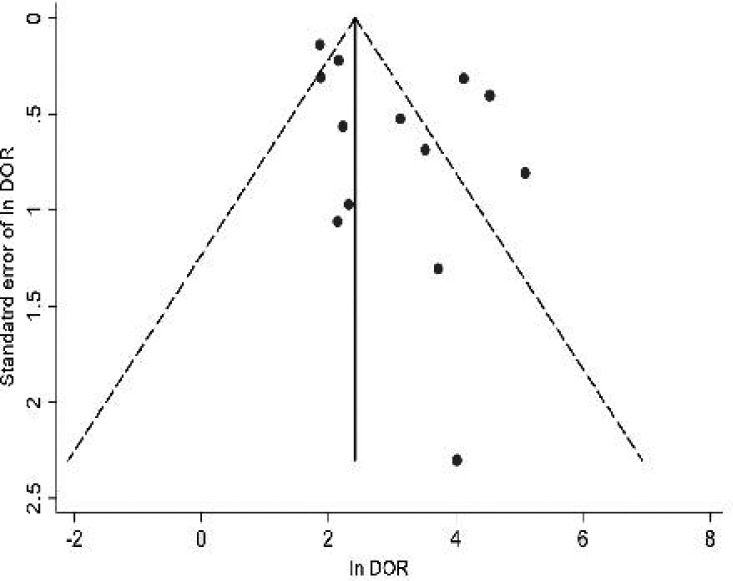
The evaluation of publication bias showed that the Egger test was not significant (P=0.07). The funnel plots for publication bias also show no asymmetry (Fig. 4). These results indicate no potential for publication bias.
Fig. 4.
Funnel plot for the assessment of potential publication bias in IL-6 assay.
•=Each study in the metaanalysis; center line = SDOR. The result of the Egger test for publication bias was not significant (P= 0.07).
Discussion
This meta-analysis was conducted to assess the validity of IL-6 for predicting NS. The sensitivity of IL-6 assay ranged from 0.61 [31] to 0.96 [26]. Martin et al reported the specificity of IL-6 as 0.69, while it was as high as 0.95 in another study by Ng et al [21]. We found a wide range for DOR of IL-6 (8.5 to 162.75). These differences in sensitivity, specificity and DOR of IL-6 for predicting NS may be due to various factors. Method of study, method of IL-6 assay, cut-off levels for IL-6 assay, neonates characteristics (e.g. birthweight) may be some possible explanations for the discrepancy. The discrepancy in some studies seemed to be due to low sample size [22, 38].
The pooled values of sensitivity (0.79), specificity (0.84) and DOR (20.74) showed favorable accuracy of IL-6 for predicting NS. Laboratory examination in previous studies similarly suggested that IL-6 in the umbilical cord blood increases in newborn infants who developed sepsis [39]. The Q value in SROC curve was 0.82 indicating high overall accuracy of IL-6 for predicting NS.
The results of meta-regression showed that the diagnostic accuracy of IL-6 for predicting NS was not affected by study quality, cut-off levels of IL-6 assay, birth-weight of neonates and control group status. Our results also showed no significant publication bias. So, it can be concluded that the observed accuracy of IL-6 for predicting NS was not confounded by other variables and IL-6 may be considered as a promising marker for diagnosis of NS. The risks of blood sampling for IL-6 assessment are minimal and restricted to the risks of blood sampling. The only relative contraindication of multiple blood sampling is anemia of prematurity [6]. Previous studies suggested that the small amount of blood sample required for cytokines assessment may not contribute significantly to the development of anemia [38]. The major limitation of IL-6 assay, especially manual methods, is the relatively long time as well as the interobserver error [40].
However, newer automatic methods made the measurement of interleukins simpler and more accurate [41].
Conclusion
In summary, IL-6 is a valid marker for predicting NS. It may be considered for early diagnosis and control of sepsis in neonatal care units.
Acknowledgment
The results presented in this article were part of a thesis conducted in Golestan University of Medical Sciences (GOUMS) for obtaining a speciality degree in pediatrics. This study was reviewed and approved by the Review Board of GOUMS.
Conflict of Interest
None
References
- 1.Stoll BJ. Infection in neonatal infants. In: Kliegman R, Behrman R, Jenson H, et al., editors. Nelson Textbook of Pediaterics. Philadelphia: Saunders; 2007. pp. 794–811. [Google Scholar]
- 2.Boskabadi H, Maamouri G, Tavakol Afshari J, et al. Serum interleukin-8 level as a diagnostic marker in late neonatal sepsis. Iran J Pediatr. 2010;20(1):41–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Volante E, Moretti S, Pisani F, Bevilacqua G. Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med. 2004;16(Suppl2):13–6. doi: 10.1080/14767050410001727116. [DOI] [PubMed] [Google Scholar]
- 4.Ucar B, Yildiz B, Aksit MA, et al. Mediators Inflamm. 2008. Serum amyloid A, procalcitonin, tumor necrosis factor-alpha, and interleukin-1beta levels in neonatal late-onset sepsis. Article ID:737141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra UK, Jacobs SE, Doyle LW, Garland SM. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F208–12. doi: 10.1136/adc.2004.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehr S, Doyle LW. Cytokines as markers of bacterial sepsis in newborn infants: a review. Pediatr Infect Dis J. 2000;19(9):879–87. doi: 10.1097/00006454-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Ng PC, Li K, Leung TF, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–9. doi: 10.1373/clinchem.2005.062075. [DOI] [PubMed] [Google Scholar]
- 8.Kingsmore SF, Kennedy N, Halliday HL, et al. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008;7(10):1863–75. doi: 10.1074/mcp.M800175-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam HS, Ng PC. Biochemical markers of neonatal sepsis. Pathology. 2008;40(2):141–8. doi: 10.1080/00313020701813735. [DOI] [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deville WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 14.Lau J, Ioannidis JP, Balk EM, et al. Diagnosing acute cardiac ischemia in the emergency department: a systematic review of the accuracy and clinical effect of current technologies. Ann Emerg Med. 2001;37:453–60. doi: 10.1067/mem.2001.114903. [DOI] [PubMed] [Google Scholar]
- 15.Irwig L, Tosteson AN, Gatsonis C, et al. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994;120:667–76. doi: 10.7326/0003-4819-120-8-199404150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Vamvakas EC. Meta-analyses of studies of the diagnostic accuracy of laboratory tests: a review of the concepts and methods. Arch Pathol Lab Med. 1998;122(8):675–86. [PubMed] [Google Scholar]
- 17.Suzuki S, Moro-oka T, Choudhry NK. The conditional relative odds ratio provided less biased results for comparing diagnostic test accuracy in meta-analyses. J Clin Epidemiol. 2004;57(5):461–9. doi: 10.1016/j.jclinepi.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Westwood ME, Whiting PF, Kleijnen J. How does study quality affect the results of a diagnostic meta-analysis? BMC Med Res Methodol. 2005;8:20. doi: 10.1186/1471-2288-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck C, Bundschu J, Gallati H, et al. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics. 1994;93(1):54–8. [PubMed] [Google Scholar]
- 21.Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F221–7. doi: 10.1136/fn.77.3.f221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berner R, Niemeyer CM, Leititis JU, et al. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44(4):469–77. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Küster H, Weiss M, Willeitner AE, et al. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352(9136):1271–7. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 24.Døllner H, Vatten L, Linnebo I, et al. Inflammatory mediators in umbilical plasma from neonates who develop early-onset sepsis. Biol Neonate. 2001;80(1):41–7. doi: 10.1159/000047118. [DOI] [PubMed] [Google Scholar]
- 25.Krueger M, Nauck MS, Sang S, et al. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Biol Neonate. 2001;80(2):118–23. doi: 10.1159/000047130. [DOI] [PubMed] [Google Scholar]
- 26.Martin H, Olander B, Norman M. Reactive hyperemia and interleukin 6, interleukin 8, and tumor necrosis factor-alpha in the diagnosis of early-onset neonatal sepsis. Pediatrics. 2001;108(4):E61. doi: 10.1542/peds.108.4.e61. [DOI] [PubMed] [Google Scholar]
- 27.Santana C, Guindeo MC, González G, et al. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr. 2001;90(10):1176–81. doi: 10.1080/080352501317061602. [DOI] [PubMed] [Google Scholar]
- 28.Berner R, Fürll B, Stelter F, et al. Elevated levels of lipopolysaccharide-binding protein and soluble CD14 in plasma in neonatal early-onset sepsis. Clin Diagn Lab Immunol. 2002;9(2):440–5. doi: 10.1128/CDLI.9.2.440-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez BE, Mercado CK, Johnson L, et al. Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med. 2003;31(1):60–8. doi: 10.1515/JPM.2003.009. [DOI] [PubMed] [Google Scholar]
- 30.Laborada G, Rego M, Jain A, et al. Diagnostic value of cytokines and C-reactive protein in the first 24 hours of neonatal sepsis. Am J Perinatol. 2003;20(8):491–501. doi: 10.1055/s-2003-45382. [DOI] [PubMed] [Google Scholar]
- 31.Santana Reyes C, García-Muñoz F, Reyes D, et al. Role of cytokines (interleukin-1beta, 6, 8, tumour necrosis factor-alpha, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr. 2003;92(2):221–7. doi: 10.1111/j.1651-2227.2003.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 32.Hodge G, Hodge S, Haslam R, et al. Rapid simultaneous measurement of multiple cytokines using 100 microl sample volumes--association with neonatal sepsis. Clin Exp Immunol. 2004;137(2):402–7. doi: 10.1111/j.1365-2249.2004.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng PC, Li K, Leung TF, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–9. doi: 10.1373/clinchem.2005.062075. [DOI] [PubMed] [Google Scholar]
- 34.Verboon-Maciolek MA, Thijsen SF, Hemels MA, et al. Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatr Res. 2006;59(3):457–61. doi: 10.1203/01.pdr.0000200808.35368.57. [DOI] [PubMed] [Google Scholar]
- 35.Kurt AN, Aygun AD, Godekmerdan A. Mediators Inflamm. 2007. Serum IL-1beta, IL-6, IL-8, and TNF-alpha levels in early diagnosis and management of neonatal sepsis. Article ID:31397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldas JP, Marba ST, Blotta MH, et al. Accuracy of white blood cell count, C-reactive protein, interleukin-6 and tumor necrosis factor alpha for diagnosing late neonatal sepsis. J Pediatr (Rio J) 2008;84(6):536–42. doi: 10.2223/JPED.1838. [DOI] [PubMed] [Google Scholar]
- 37.Beceiro Mosquera J, Sivera Monzo CL, Oria de Rueda Salguero O, et al. Usefulness of a rapid serum interleukin-6 test combined with C-reactive protein to predict sepsis in newborns with suspicion of infection. An Pediatr (Barc) 2009;71(6):483–8. doi: 10.1016/j.anpedi.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Messer J, Eyer D, Donato L, et al. Evaluation of interleukin-6 and soluble receptors of tumor necrosis factor for early diagnosis of neonatal infection. J Pediatr. 1996;129(4):574–80. doi: 10.1016/s0022-3476(96)70123-3. [DOI] [PubMed] [Google Scholar]
- 39.Miller LC, Isa S, LoPreste G, et al. Neonatal interleukin-1-beta, interleukin-6, and tumor necrosis factor: cord blood levels and cellular production. J Pediatr. 1990;117(6):961–5. doi: 10.1016/s0022-3476(05)80145-3. [DOI] [PubMed] [Google Scholar]
- 40.de Bont ES, Martens A, van Raan J, et al. Diagnostic value of plasma levels of tumor necrosis factor alpha (TNF alpha) and interleukin-6 (IL-6) in newborns with sepsis. Acta Paediatr. 1994;83(7):696–9. doi: 10.1111/j.1651-2227.1994.tb13121.x. [DOI] [PubMed] [Google Scholar]
- 41.Franz AR, Steinbach G, Kron M, et al. Reduction of unnecessary antibiotic therapy in newborn infants using interleukin-8 and C-reactive protein as markers of bacterial infections. Pediatrics. 1999;104(3 pt 1):47–53. doi: 10.1542/peds.104.3.447. [DOI] [PubMed] [Google Scholar]