Abstract
Background
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children. Bone pain is an important symptom that can be severe. Eosinophilia without any other abnormal laboratory findings is rare in ALL. Strongyloides stercoralis in ALL causes disseminated fatal disease.
Case Presentation
This 9-year-old girl presented with bone pain in lumbar region. Bone pain was the only symptom. The patient didn't have organomegaly. The BM samples were studied by flow cytometry, which showed pre-B cell ALL. Larva of Strongyloides stercoralis was found in fecal examination. Plain chest x ray showed bilateral para-cardiac infiltration. Strongyloidiasis was treated before starting chemotherapy. After two days treatment with Mebendazol the patient developed cough, dyspnea, respiratory distress and fever. The treatment changed to Ivermectin for 2 days. Chemotherapy started five days after diagnosis of leukemia.
Conclusion
The patient complained merely of bone pain in lumbar region without any other signs and symptoms. Peripheral blood smear showed eosinophilia without any other abnormality. Stool examination showed Strongyloides stercoralis larvae. We suggest that all patients diagnosed as ALL in tropical and subtropical regions should be evaluated for parasitic infection especially with Strongyloides stercoralis.
Keywords: Acute Lymphoblastic Leukemia, Eosinophilia, Strongyloidiasis
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children. Pallor, fatigue, bone pain, hepatomegaly, splenomegaly, lymphadenopathy, petechiae, purpura, bleeding, and fever are commonly present. Bone pain can be severe and is often associated with close to normal peripheral blood counts[1]. This finding may contribute to delayed diagnosis. Eosinophilia of >600/µL is abnormal in the vast majority of cases[2]. Eosinophilia without any other abnormal laboratory finding is rare in ALL[3, 4]. Acute eosinophilic leukemia (a variant of the M4 phenotype), precursor B-cell ALL with T[5, 14], and precursor T-cell lymphoblastic lymphoma with T[8, 13] may be associated with eosinophilia[5–7]. A broad variety of allergic, infectious, neoplastic, and idiopathic diseases are associated with increased blood and/or tissue eosinophils. The most common infectious causes for secondary eosinophilia are tissue invasive parasites such as Strongyloides stercoralis, hookworm, and Toxocara canis. Strongyloidiasis is endemic in tropical and subtropical regions and occurs sporadically in temperate areas[8]. Diagnosis requires identification of larvae in feces, body fluids, or biopsy of involved tissues. S. stercoralis infection 38 percent of patients with eosinophilia in some areas has been reported[9]. Manifestation of Strongyloides can range from asymptomatic eosinophilia in the immunocompetent host to disseminated disease with septic shock in the immunocompromised host[10]. It is thought that chemotherapy promotes the maturation of larvae from a quiescent rhabditiform stage. Successful management of hyperinfection syndrome requires early detection and initiation of therapy. Ivermectin can treat fulminant Strongyloidiasis[11].
Case Presentation
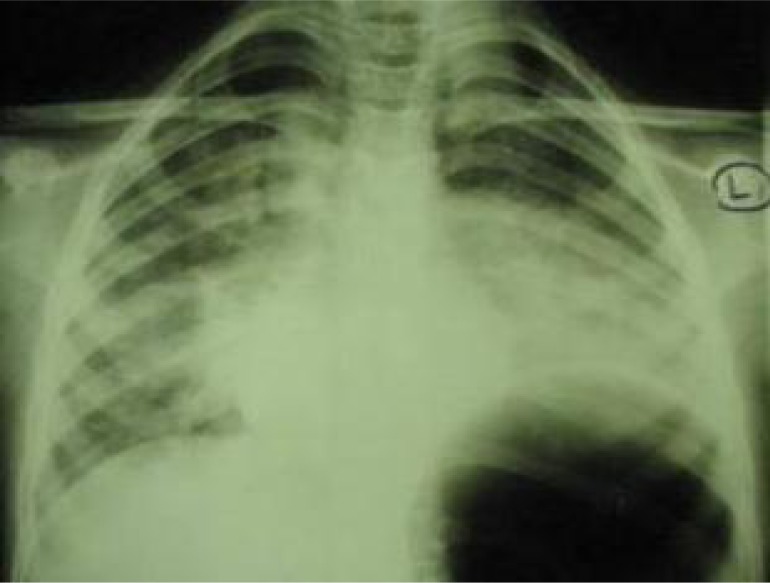
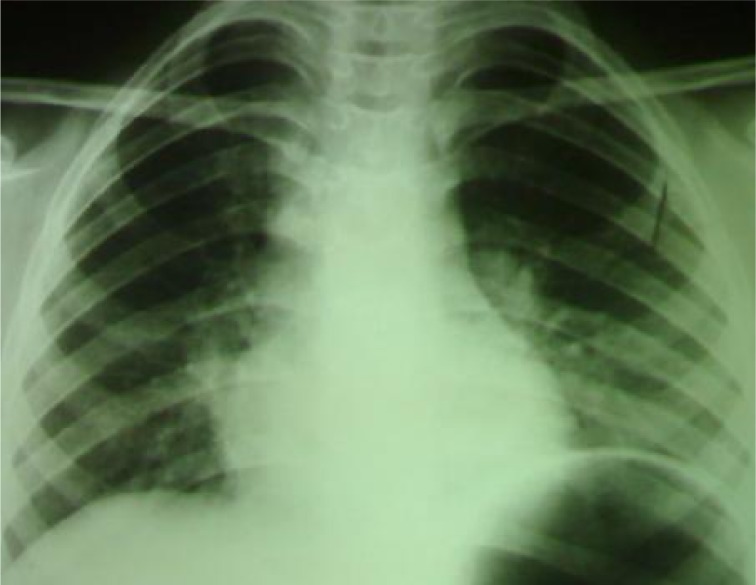
This 9-year-old girl presented with bone pain in lumbar region since 18 days before referring to our center. The patient had suffered a trauma in the lumbar region two months ago. Bone pain was the only symptom. Temperature was normal; no pallor. Normal heart sounds and respiratory rate. No lymphadeno pathy, hepato- or splenomegaly. Eosinophilia in peripheral blood smear was the first finding (Table 1). Abundant larvae of Strongyloides stercoralis were detected in fecal examination but no embryonated eggs in sputum. Radiology showed normal plain thoracolumbar x-ray and in plain chest X ray (Fig. 1). It was probable dissemination of Strongyloides stercoralis and initial phase of Loeffler's-like syndrome. Surprisingly the patient was asymptomatic in this stage. Light microscopic study of bone marrow (BM) specimen was compatible with ALL. Flow cytometry of BM specimen was consistent with pre-B cell ALL (Table 2). Cytogenetic studies of BM specimen showed 46XX. She received Mebendazol without cytotoxic agents. Two days later the patient developed cough, throat irritation, dyspnea, wheezing, respiratory distress and fever. Temperature was now 39°C. Infiltration in Chest X ray was increased. We replaced Mebendazol with Ivermectin 200 mcg/kg/day once daily for 2 days. Respiratory distress was improved 12 hours after initiation of this treatment. Chemotherapy started 5 days after established diagnosis of leukemia. In control examination after 7 and 28 days chemotherapy blast cells were less than 25% and 5% of BM cellularity respectively. Ivermectin therapy was repeated after 2 weeks. After 2 months of treatment, chest X-ray was improved (Fig. 2). The patient is now in complete remission.
Table 1.
Laboratory findings at admission
| WBC ×109/L | Hemoglobin gr/dl | Platelet ×109/L | Neutrophile | Lymphocyte | Eosinophil | ESR mm/hr | Stool exam | |
|---|---|---|---|---|---|---|---|---|
| At presentation | 5.600 | 12 | 307 | 19% | 36% | 45% | 6 | S.S.larvae |
WBC: Whit Blood Cell; ESR: Erythrocyte Sedimentation Rate; SS larve: Strongyloides Stercoralis
Fig. 1.
Chest X-ray shows Loeffler's-like syndrome due to concomitant ALL and Strongyloidiasis (left)
Table 2.
Bone marrow Immunophenotype that is consistent with pre B-ALL
| CD2 | CD3 | CD5 | CD10 | CD13 | CD14 | CD20 | CD45 |
|---|---|---|---|---|---|---|---|
| 4% | 4.8% | 4.9% | 93% | 8.2% | 0.2% | 76.9% | 85.9% |
Fig. 2.
Chest x ray with Loeffler's-like syndrome due to concomitant ALL and Strongyloidiasis after treatment (right)
Discussion
Our patient presented with bone pain in lumbar region without pallor, fatigue, fever, lymphadeno-pathy, hepatosplenomegaly, petechiae, purpura or bleeding that are common signs and symptoms in ALL [12]. Severe bone pain in children should be considered as an important sign of leukemia even though it may be associated with normal peripheral blood counts[13] and if noted, this may contribute to early diagnosis of the disease. In peripheral blood smear of our patient there was only eosinophilia without any blast cells or thrombocytopenia. Although stool examination showed Strongyloides stercoralis larvae and this could cause high eosinophil count, we should remember that eosinophilia is a herald for diagnosis of ALL especially when associated with severe bone pain. It is recommended to examine stool in tropical and subtropical regions in all patients diagnosed as ALL to evaluate parasitic infection especially Strongyloides stercoralis. Burgers et al reported of bone marrow transplantation (BMT) in an acute lymphoblastic leukemia with peripheral blood eosinophilia, who developed moderate to severe pulmonary symptoms probably by pulmonary infiltration of Strongyloides stercoralis after cytotoxic chemotherapy[14]. Although Strongyloides stercoralis hyperinfection syndrome (SHS) may develop in individuals with asymptomatic infection receiving immunosuppressive treatment, our patient probably had developed symptoms of SHS before starting chemotherapy, as she was an acquired immunodeficient patient (ALL). The patient became febrile and blood count showed severe neutropenia at that time. Blood culture was negative for bacteria. Some studies showed that SHS can predispose to Gram-negative sepsis and death[15, 16]. Therefore our treatment aimed to cover bacterial infection and Strongyloidiasis concomitantly. Bezares et al showed that disseminated Strongyloidia hyperinfection is fatal in acute leukemia[17]. Our patient became afebrile and recovered from respiratory distress several days after treatment. The patient is in complete remission after 15 months. We had started chemotherapy after treatment of Strongyloides stercoralis to prevent promotion of the maturation of larvae from a quiescent rhabditiform stage [18]. It‘s not clear that mebendazol induced severe leukopenia and dissemination of Strongyloides stercoralis or not? Ivermectin is preferred to other antihelmentic agents [19].
Conclusion
Before treating ALL we should rule out concomitant parasitic infections. It is preferred to treat Strongyloidiasis before starting chemo-therapy. We must consider Strongyloides stercoralis as a cause of fever and respiratory distress beside bacterial, fungal and viral infection in ALL in all phases of treatment. Strongyloides stercoralis may have been the unknown cause of death in many oncology centers in tropical regions. Finally, although high mortality rate is noted in disseminated Strongyloidiasis, it is still a curable disease when early diagnosis could be made and appropriate treatment applied.
References
- 1.Cabot RC, Scully RE, Mark EJ, et al. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 32–2000-A boy with vertebral compression fractures. N Engl J med. 2000;343(16):1168–76. doi: 10.1056/NEJM200010193431607. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment. Mayo Clin Proc. 2005;80:75–83. doi: 10.1016/S0025-6196(11)62962-5. [DOI] [PubMed] [Google Scholar]
- 3.Tancrède-Bohin E, Ionescu MA, Salmonière PdL, et al. Prognostic value of blood eosinophilia in primary cutaneous T-cell lymphomas. Arch Dermatol. 2004;140(9):1057–61. doi: 10.1001/archderm.140.9.1057. [DOI] [PubMed] [Google Scholar]
- 4.Wilson F, Tefferi A. Acute lymphocytic leukemia with eosinophilia: two case reports and a literature review. Leukemia Lymphoma. 2005;46(7):1045–50. doi: 10.1080/10428190500085537. [DOI] [PubMed] [Google Scholar]
- 5.Loffler H, Gassmann W, Haferlach T. AML M1 and M2 with eosinophilia and AML M4Eo: Diagnostic and clinical aspects. Leuk Lymphoma. 1995;18(Suppl 1):61–3. doi: 10.3109/10428199509075305. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133(5):468–92. doi: 10.1111/j.1365-2141.2006.06038.x. [DOI] [PubMed] [Google Scholar]
- 7.Inhorn RC, Aster JC, Roach SA. A syndrome of lymphoblastic lymphoma, eosinophilia, and myeloid hyperplasia/malignancy associated with t(8;13)(p11;q11): description of a distinctive clinicopathologic entity. Blood. 1995;85(7):881–7. [PubMed] [Google Scholar]
- 8.Brito-Babapulle F. The eosinophilias, including the idiopathic hypereosinophilic syndrome. Br J Haematol. 2003;121(2):203–23. doi: 10.1046/j.1365-2141.2003.04195.x. [DOI] [PubMed] [Google Scholar]
- 9.Nutman TB, Ottesen EA, Ieng S, et al. Eosinophilia in Southeast Asian refugees: Evaluation at a referral center. J Infect Dis. 1987;155(2):309–13. doi: 10.1093/infdis/155.2.309. [DOI] [PubMed] [Google Scholar]
- 10.Nucci M, Portugal R, Pulcheri W, et al. Strongyloidiasis in patients with hematologic malignancies. Clin Infect Dis. 1995;21(3):675–7. doi: 10.1093/clinids/21.3.675. [DOI] [PubMed] [Google Scholar]
- 11.Takashima N, Yazawa S, Ishihara A, et al. Fulminant strongyloidiasis successfully treated by subcutaneous Ivermectin: an autopsy case. Rinsho Shinkeigaku. 2008;48(1):30–5. doi: 10.5692/clinicalneurol.48.30. [DOI] [PubMed] [Google Scholar]
- 12.Margulin JF, Philip-Steuber C, Podlack DG. Acute Lymphoblastic Leukemia. In: Pizzo PA, Podlack DG, editors. Principles and Practice of Pediatric Oncology. 5 ed. Lippincott Williams & Wilkins; 2005. pp. 558–9. [Google Scholar]
- 13.Jonsson OG, Sartain P, Ducore JM, et al. Bone pain as an initial symptom of childhood acute lymphoblastic leukemia: association with nearly normal hematologic indexes. Pediatrics. 1990;117(2 Pt 1):233–7. doi: 10.1016/s0022-3476(05)80535-9. [DOI] [PubMed] [Google Scholar]
- 14.Burgers JA, Sluiters JF, de Jong DW, et al. Pseudoparasitic pneumonia after bone marrow transplantation. Neth J Med. 2001;59(4):170–6. doi: 10.1016/s0300-2977(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 15.Mora CS, Segami MI, Hidalgo JA. Strongyloides stercoralis hyperinfection in systemic lupus erythematosus and the antiphospholipid syndrome. Semin Arthritis Rheum. 2006;36(3):135–43. doi: 10.1016/j.semarthrit.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Yu JJ, Lu SC, Wu IH, et al. Disseminated Strongyloides stercoralis infection mimicking pneumonia. J Formos Med Assoc. 1995;94(Suppl 2):S162–5. [PubMed] [Google Scholar]
- 17.Bezares RF, Carreras LO, Marin CA, et al. Fatal strongyloides stercoralis hyperinfection in acute leukaemia. Lancet. 1983;1(8322):481. doi: 10.1016/s0140-6736(83)91484-8. [DOI] [PubMed] [Google Scholar]
- 18.Walsh TJ, Roilides E, Groll AH, Gonzales C, Pizzo PA. Infectious Complications in Pediatric Cancer Patients. In: Pizzo PA, Podlack DG, editors. Principles and Practice of Pediatric Oncology. 5 ed. Lippincott Williams & Wilkins; 2005. pp. 1304–5. [Google Scholar]
- 19.Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, et al. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. The American journal of tropical medicine and hygiene. 1996;55(5):477. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]