Abstract
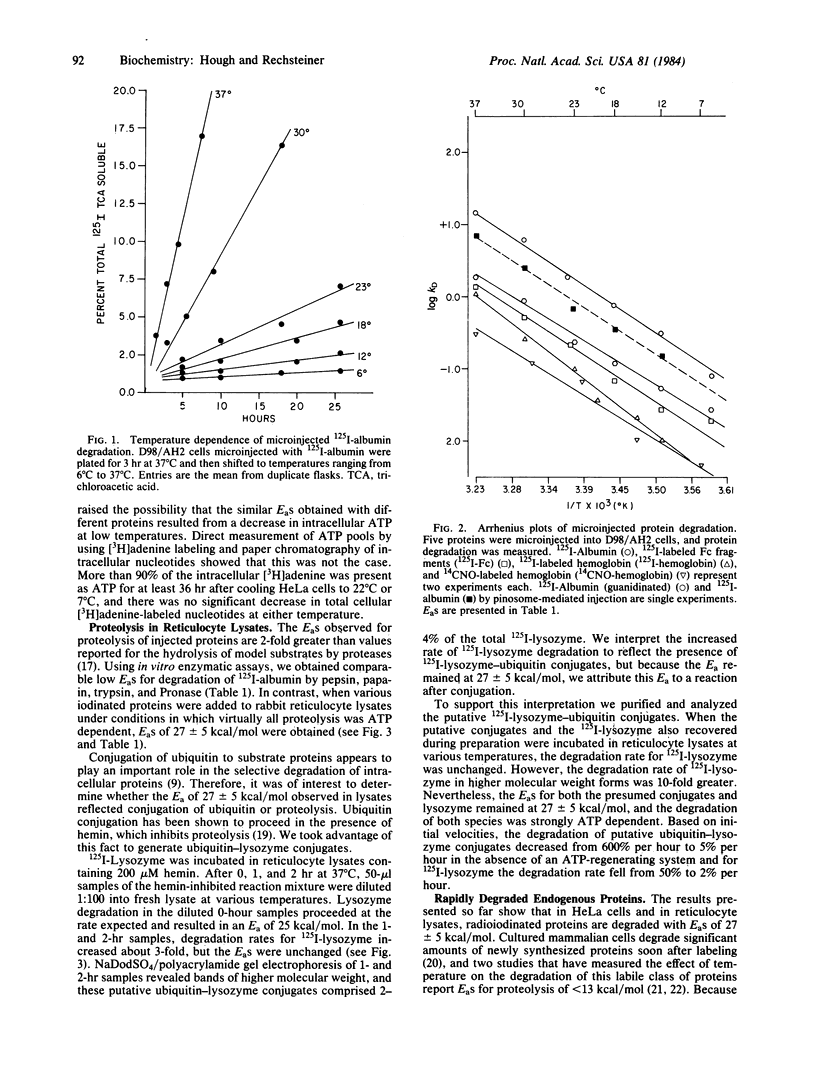
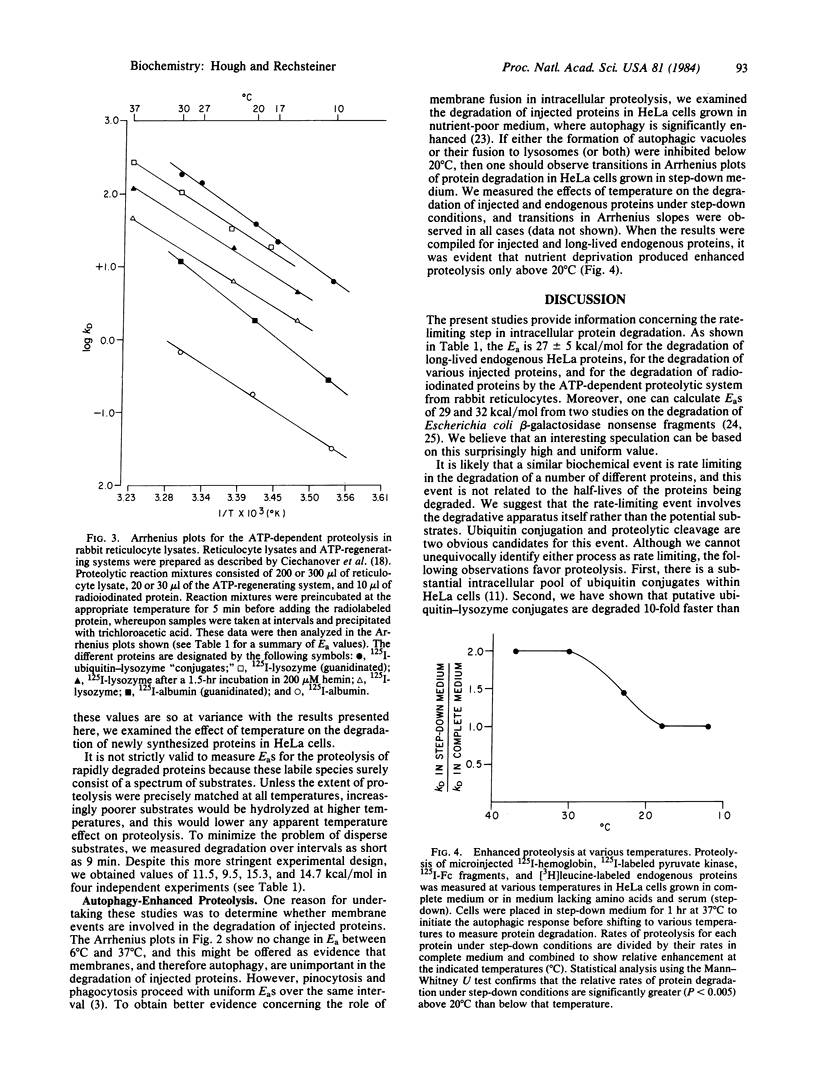
Bovine serum albumin, pyruvate kinase, hemoglobin, and the Fc fragment of IgG were labeled and introduced into HeLa cells by erythrocyte-mediated microinjection. Degradation of the injected proteins was then measured in cells cultured at temperatures between 6 degrees C and 37 degrees C. Arrhenius plots revealed a constant Ea of 27 +/- 5 kcal/mol over this temperature interval. Similarly, the apparent Ea for the degradation of long-term endogenously labeled HeLa proteins was 22-26 kcal/mol. Both local protein unfolding and proteolysis by defined enzymes, such as trypsin or papain, proceed with EaS between 5 and 15 kcal/mol. The 2-fold higher values obtained in this study indicate that protein unfolding or simple proteolysis is not rate limiting in the degradation of injected or long-lived endogenous HeLa proteins. Moreover, the relatively uniform EaS suggest that a similar biochemical event is rate limiting in the degradation of a specific protein independent of its half-life. This event may involve a reaction in the ATP-dependent proteolytic pathway from rabbit reticulocyte lysates because we observed that EaS for ATP-dependent proteolysis in this system were also 27 +/- 5 kcal/mol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Sargus M. J., Venkatesan S., Shinozuka H. Role of the vacuolar apparatus in augmented protein degradation in cultured fibroblasts. J Cell Physiol. 1978 Jan;94(1):77–86. doi: 10.1002/jcp.1040940110. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A, pH and Temperature dependence of tripeptide hydrolysis. Biochemistry. 1971 Jul 20;10(15):2892–2897. doi: 10.1021/bi00791a015. [DOI] [PubMed] [Google Scholar]
- Bates P. J., Coetzee G. A., Van der Westhuyzen D. R. The degradation of endogenous and exogenous proteins in cultured smooth muscle cells. Biochim Biophys Acta. 1982 Nov 24;719(2):377–387. doi: 10.1016/0304-4165(82)90113-1. [DOI] [PubMed] [Google Scholar]
- Bigelow S., Hough R., Rechsteiner M. The selective degradation of injected proteins occurs principally in the cytosol rather than in lysosomes. Cell. 1981 Jul;25(1):83–93. doi: 10.1016/0092-8674(81)90233-6. [DOI] [PubMed] [Google Scholar]
- Brahm J. Temperature-dependent changes of chloride transport kinetics in human red cells. J Gen Physiol. 1977 Sep;70(3):283–306. doi: 10.1085/jgp.70.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. T., Kuehl L., Rechsteiner M. Conjugation of ubiquitin to denatured hemoglobin is proportional to the rate of hemoglobin degradation in HeLa cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5857–5861. doi: 10.1073/pnas.79.19.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- Craig N., Fahrman C. Regulation of protein synthesis by temperature in mammalian cells. Non-involvement of the plasma membrane. Biochim Biophys Acta. 1977 Feb 3;474(3):478–490. doi: 10.1016/0005-2787(77)90276-3. [DOI] [PubMed] [Google Scholar]
- Cupo P., El-Deiry W., Whitney P. L., Awad W. M., Jr Stabilization of proteins by guanidination. J Biol Chem. 1980 Nov 25;255(22):10828–10833. [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Funk J., Wunderlich F., Kreutz W. Thermotropic 'two-stage' liquid crystalline equilibrium crystalline lipid phase separation in microsomal membranes. Biochim Biophys Acta. 1982 Sep 9;690(2):306–309. doi: 10.1016/0005-2736(82)90336-4. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. Hemin inhibits ATP-dependent ubiquitin-dependent proteolysis: role of hemin in regulating ubiquitin conjugate degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6845–6848. doi: 10.1073/pnas.78.11.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendil K. B. Intracellular degradation of hemoglobin transferred into fibroblasts by fusion with red blood cells. J Cell Physiol. 1980 Dec;105(3):449–460. doi: 10.1002/jcp.1041050309. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980 Jun;85(3):754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. M., Sharon N., Koshland D. E. PURIFIED MUSCLE PROTEINS AND THE WALKING RATE OF ANTS. Proc Natl Acad Sci U S A. 1959 Jun;45(6):785–791. doi: 10.1073/pnas.45.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- McGarry T., Hough R., Rogers S., Rechsteiner M. Intracellular distribution and degradation of immunoglobulin G and immunoglobulin G fragments injected into HeLa cells. J Cell Biol. 1983 Feb;96(2):338–346. doi: 10.1083/jcb.96.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., DeMartino G. N., Goldberg A. L. The effect of protease inhibitors and decreased temperature on the degradation of different classes of proteins in cultured hepatocytes. J Cell Physiol. 1979 Dec;101(3):439–457. doi: 10.1002/jcp.1041010311. [DOI] [PubMed] [Google Scholar]
- Okada C. Y., Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Poole B., Wibo M. Protein degradation in cultured cells. The effect of fresh medium, fluoride, and iodoacetate on the digestion of cellular protein of rat fibroblasts. J Biol Chem. 1973 Sep 10;248(17):6221–6226. [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983 Jul;116(1):103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- SIMPSON M. V. The release of labeled amino acids from the proteins of rat liver slices. J Biol Chem. 1953 Mar;201(1):143–154. [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman L., Goldberg A. L. Protease La from Escherichia coli hydrolyzes ATP and proteins in a linked fashion. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4883–4887. doi: 10.1073/pnas.79.16.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. S., Strickhart F. S., Kicha L. P. The effect of temperature on monoxygenase reactions in the microsomal membrane. Biochim Biophys Acta. 1977 Mar 1;465(2):362–370. doi: 10.1016/0005-2736(77)90085-2. [DOI] [PubMed] [Google Scholar]



