Abstract
Objective
This study aimed to develop and test the validity of a risk score to be used as a simple tool to identify those children at high risk of sonographic non-alcoholic fatty liver disease (NAFLD).
Methods
This cross-sectional study was conducted among 962 participants aged 6–18 years in Isfahan, Iran. They consisted of three groups of nearly equal number of normal-weight, overweight and obese individuals. Coefficients of the logistic regression models were used to assign a score value for each variable and the composite sonographic NAFLD risk score was calculated as the sum of those scores. Performance of model was assessed by receiver operating characteristic (ROC) curve procedure.
Findings
Data of 931 participants was included in the analysis. The sonographic findings of 16.8% of participants were compatible with NAFLD. Age, sex, body mass index, waist circumference and serum triglycerides level were diagnosed as factors associated with NAFLD. The risk score was calculated as 50 for sonographic NAFLD.
Conclusion
This study, to the best of our knowledge is the first of its kind in the pediatric age group, focuses on predicting sonographic NAFLD from easily-measured factors. It may suggest an association of hypertriglyceridemic-waist phenotype with NAFLD in the pediatric age group.
Keywords: Fatty Liver, Risk Factors, Logistic Regression, Prediction, Prevention
Introduction
Non-alcoholic fatty liver disease (NAFLD) is considered as the most common cause of liver disease in the pediatric age group [1]. Overweight is known as the main predictor of NAFLD among children [2]. In view of the rapidly increasing trend of childhood obesity; it is becoming a health problem for children and adolescents [3].
Nonetheless, major variations exist in the prevalence of NAFLD. Both genetic and environmental factors are expected to be involved in predisposition to fatty liver in children and adolescents. In addition to its familial clustering,[4] some studies have documented that boys are at higher risk of NAFLD than girls[5, 6]. Moreover, the prevalence of NAFLD in children has marked ethnic variations, e.g. its incidence is greater in Hispanic children and adolescents than the white population, while black non-Hispanics are significantly less susceptible to NAFLD[6–8].
Limited information is available on predictive factors for NAFLD in children and adolescents, thus developing statistical models for predicting NAFLD would be of practical use in the pediatric age group. There are some challenges in defining NAFLD in population-based studies; although the gold standard requires liver biopsy, it is not practical in large studies. Imaging methods such as sonography are found to have acceptable level of specificity and sensitivity [9–11].
This study aimed to develop and validate a simple tool to identify those children and adolescents at high risk of sonographic NAFLD.
Subjects and Methods
The detailed methodology of this study is published before [12]; herein we report it in brief. The target population comprised school students, aged 6 to 18 years, in Isfahan the second large city of Iran. Necessary official arrangement was done with the Provincial Education and Training Organization, and after getting the approval from the Ethics Committee of the Isfahan Faculty of Medicine, and obtaining written consent from the students’ parents and oral assent from students, subjects were selected by multistage random cluster sampling. Then, based on their body mass index (BMI), they were categorized into three groups of normal weight, overweight (BMI of 85th to 94th percentile) and obese (BMI equal or greater than 95th percentile) [13], and a random sample of each BMI group was included in the study. A questionnaire was completed about socio-demographic factors and family history of chronic diseases, then students underwent physical examination, venous blood sampling and sonography.
Sonographic fatty liver was defined as increased echogenicity of the liver parenchyma to the extent that it was reported by ultrasound and disturbed the visibility of the portal vein and liver artery[14].
The whole dataset was randomly divided into two distinct parts: one part was used to construct the model (construction sample) and the other part to validate the model constructed from the first part (validation sample). Overall, 70 percent (670 cases) of data was used to construct model and 30 percent (260 cases) was used to validate the model.
To predict the NAFLD (absent/ present), a logistic regression model was used to compute the size effect (β-coefficients) for risk factors of NAFLD in this study. The risk factors used in the model as independent variables included age, sex, BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood sugar (FBS), triglycerides (TG), total cholesterol (TC), low density lipoprotein and high density lipoprotein cholesterol (LDL-C and HDL-C, respectively).
For calculating the risk score, logistic regression was refitted on the whole data using the categorical variables: sex (male/female), BMI (normal weight, overweight, and obese), age (two categories: 6-10-year-old considered as child and 11-18-year-old considered as adolescent) and waist circumference (two categories: normal: less than 90th percentile, abdominal obesity: equal or greater than 90th percentile). Then risk model was calculated using the coefficients obtained from logistic regression with categorical variables. Coefficients of the models were used to assign a score value for each variable and the composite NAFLD risk score was calculated as the sum of those scores.
The cut-off value for the risk score was determined using the receiver operating characteristic (ROC) curve procedure. The ROC curve was plotted for the NAFLD risk score, the sensitivity was plotted on the y-axis and the false positive rate (1- specificity) was plotted on x-axis. SPSS for Windows (version 16.0; SPSS Inc., Chicago, IL) was used for data analysis.
Findings
Data of 931 children and adolescents was complete and was included in the analysis; the study participants consisted of 518 (55.6%) girls and 413 (44.4%) boys with a mean (SD) age of 12.57 (3.3) years. The characteristics of study participants according to their BMI levels are presented in Table 1. The sonographic findings of 16.8% of participants were compatible with NAFLD. The β coefficients for the risk factors in the model constructed from construction sample are shown in Table 2.
Table 1.
Characteristics [mean (standard deviation)] of the study population according to the body mass index category
| Variables | Total | Normal weight | Overweight | Obese | P Value |
|---|---|---|---|---|---|
| Age (years) | 12.57 (3.3) | 12.08 (3.5) | 13.40 (3.0) | 12.23 (3.1) | 0.1 |
| Body mass index (Kg/m2) | 21.75 (5.5) | 16.65 (2.8) | 23.12 (2.9) | 27.59 (3.8) | <0.0001 |
| Waist circumference (cm) | 79.23 (14.4) | 66.71 (8.8) | 82.74 (8.8) | 93.33 (10.9) | <0.0001 |
| Alanine aminotransferase (U/L) | 19.50 (10.4) | 15.87 (7.8) | 19.62 (10.6) | 24.73 (11.3) | <0.0001 |
| Aspartate transaminase (U/L) | 24.04 (10.5) | 23.46 (9.2) | 23.33 (10.6) | 25.80 (11.8) | 0.003 |
| Alkaline phosphatase (U/L) | 525.43 (148.7) | 534.42 (157.6) | 494.54 (148.9) | 551.26 (131.5) | 0.08 |
| Triglycerides (mg/dL) | 112.93 (55.4) | 96.66 (40.1) | 110.51 (52.9) | 140.16 (66.8) | <0.0001 |
| Total cholesterol (mg/dL) | 169.60 (30.9) | 161.55 (26.7) | 168.12 (31.8) | 183.45 (30.9) | <0.0001 |
| HDL-cholesterol (mg/dL) | 45.75 (11.8) | 48.48 (13.0) | 44.44 (10.3) | 43.38 (10.8) | <0.0001 |
| LDL-cholesterol (mg/dL) | 101.74 (26.6) | 94.13 (23.5) | 102.12 (27.0) | 112.53 (26.6) | <0.0001 |
Table 2.
Coefficients for the risk factors in the logistic model in the construction sample
| Parameter | B | S.E. | Wald | df | P | Exp(B) |
|---|---|---|---|---|---|---|
| Sex | 0.423 | 0.205 | 4.25 | 1 | 0.04 | 1.527 |
| Age | 0.309 | 0.063 | 23.76 | 1 | 0.000001 | 1.362 |
| Body mass index (BMI) | 0.268 | 0.059 | 20.90 | 1 | 0.000005 | 1.307 |
| Waist circumference (WC) | 0.096 | 0.022 | 18.33 | 1 | 0.00002 | 1.101 |
| Triglycerides (TG) | 0.006 | 0.002 | 6.14 | 1 | 0.01 | 1.006 |
| Constant | −551.563 | 88.130 | 39.17 | 1 | 3.33E-22 | 0.000 |
In the sex variable, female group is defined as reference group
Log [p (fld=1)/1-p (fld=1)]=−551.563+0.423 *sex+0.268* BMI+0.006*TG+0.096*WC+0.309*age
Coefficients of this model, except intercept, were positive so each of them increased the probability of having NAFLD, also according to changes in log likelihood of model with removal of independent variable (Table 3); the variables of BMI, waist circumference and age caused the biggest change in log likelihood of the model.
Table 3.
Changes in log likelihood of logistic model with removal of independent variable
| Variables | Model Log Likelihood | Change in -2 Log Likelihood | df | Significance of the Change |
|---|---|---|---|---|
| Sex | −168.66 | 5.53 | 1 | 0.02 |
| Age | −189.93 | 48.06 | 1 | 0.00 |
| Body mass index | −174.10 | 16.42 | 1 | 0.00 |
| Waist circumference | −174.49 | 17.19 | 1 | 0.00 |
| Triglycerides | −169.34 | 6.89 | 1 | 0.01 |
Log: logarithm; df: degree of freedom;
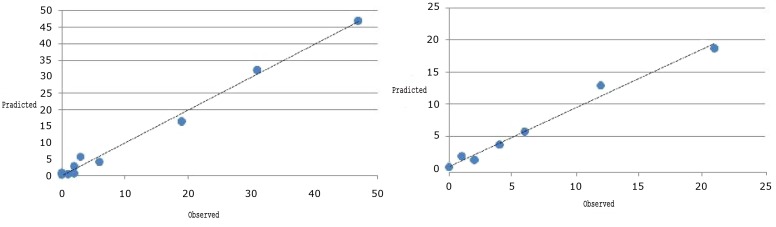
The Hosmer and Lemeshow goodness of fit criteria for this model was 5.17 with Chi-square distribution with 8 degrees of freedom and P-value of 0.747, and confirmed the good fit of the model. Fig 1a shows the great agreement between observed and predicted values in deciles of predicted probabilities with logistic model.
Fig. 1.
Goodness of fit of Hosmer and Lemeshow in Construction samples (1a) and validation samples (1b)
Performing the obtained model on validation sampling could classify 88.85% of cases correctly. The Hosmer and Lemeshow statistic for goodness of fit in the validation part was 5.34 with Chi-square and 8 degrees of freedom confirming the good fit of the model in validation part (Fig 1b). Moreover, the area under the ROC curve for predicted probability of the model was 0.921 with 0.891–0.961 confidence interval and confirmed a good performance of the model in prediction of NAFLD.
The risk model was calculated using the coefficients obtained from logistic regression with categorical variables. These coefficients are presented in Table 4. For easier use of this model without computer and calculator, every category of the variables was given a number according to the coefficients of the logistic model, so that coefficients of the model is multiplied by 10 and rounded to represent risk score for that especial variable. The coefficients are presented in Table 5. The risk score for categories which are not in the table is zero.
Table 4.
The logistic regression coefficients for categorical variables
| B | SE | Wald | df | Sig | Exp(B) | |
|---|---|---|---|---|---|---|
| Sex | 0.29 | 0.23 | 1.58 | 1.00 | 0.21 | 1.34 |
| Body mass index | 119.01 | 3.00 | 0.00 | |||
| 5 th–84 thpercentile | 0.19 | 1.23 | 0.02 | 1.00 | 0.88 | 0.82 |
| 85 th–94 thpercentile | 2.21 | 1.03 | 4.60 | 1.00 | 0.03 | 9.10 |
| >85 thpercentile | 4.53 | 1.02 | 19.75 | 1.00 | 0.00 | 34.40 |
| Waist circumference >90 thvs.<90 thpercentile | 1.38 | 0.31 | 19.61 | 1.00 | 0.00 | 3.96 |
| Age >11 vs.<10.9years | 0.27 | 0.29 | 0.92 | 1.00 | 0.04 | 1.32 |
| Constant | −5.05 | 1.03 | 24.26 | 1.00 | 0.00 | 0.01 |
Table 5.
Coefficients of risk score model
| Independent variables | Category | Risk score | |
|---|---|---|---|
| Sex | Male | 3 | |
| Body mass index | 5th–84th percentile | 18.54–22.99 | 2 |
| 85th–94th percentile | 23–27.54 | 22 | |
| >85th percentile | >27.55 | 45 | |
| Waist circumference | >90th percentile | 13 | |
| Age | >11 years | 2 | |
Risk Score=2* male+ 2* Normal+ 22* overweight +45* obese+13* Waist Circ+2* age
If all the variables be in reference category total risk score is zero (minimum risk) and if they all be in the last category maximum risk is calculated. To assess the performance of the risk score model and determine the cut point of risk score, ROC curve is used, the area under the curve for predicted risk by this model is 0.88 with confidence interval of 0.873–0.923 and P-value less than 0.05 which indicates that using of the model for prediction is beneficial. Also considering the specificity and sensitivity of the ROC analysis in respect of different risk values, risk score of 50 with sensitivity of 86.3% and specificity of 84.6% selected as cut point, so if the risk score for a child or adolescent is greater than 50 there is a high risk of having sonographic fatty liver.
Discussion
The risk score calculated and validated in this study can be used as a screening tool for identifying children and adolescents at high risk for sonographic NAFLD. This study, which to the best of our knowledge is the first of its kind in the pediatric age group, focuses on predicting fatty liver from factors that are easy to measure with available and non-invasive methods without using imaging instruments.
Currently, there is an increasing interest in predicting the probability of adverse events for various medical purposes. Accurately predicting the probability of adverse events allows for effective patient risk stratification, thus permitting more appropriate medical care to be delivered[15]. Furthermore, accurately predicting the probability of an adverse event allows for risk-adjusted outcomes to be compared across health care providers. We found that the indexes for generalized and abdominal obesity, i.e. BMI and waist circumference, and triglycerides can be used to predict sonographic NAFLD among children and adolescents. This might be new confirmatory evidence about the epidemiologic and clinical significance of the hypertriglyceridemic-waist (HW) phenotype among children and adolescents. It is well-documented that the atherogenic triad of high apolipoprotein B, hyperinsulinemia and high serum small-dense LDL-C is predictable by the HW phenotype [16].
Moreover, studies among adults of different ethnicities have shown that HW combination can be considered as a simple tool for prediction of the cardiovascular risk clustering [17, 18].
HW phenotype is also associated with low-grade inflammation among apparently healthy non-diabetic individuals; this could be an underlying mechanism for atherogenesis [19]. The existence of HW phenotype in patients with type 2 diabetes was able to categorize a subgroup with higher degree of visceral adiposity and a greater degree of subclinical atherosclerosis that may be related to the pro-atherogenic lipoprotein changes[20]. As proposed by Després et al, visceral fat accumulation is a marker of the relative inability of subcutaneous fat to act as a protective metabolic sink, leading to ectopic fat deposition, thus “HW phenotype is thought to represent an altered, dysfunctional, and highly lipolytic adipose tissue that is a major culprit abnormality behind the metabolic syndrome and associated cardiometabolic risk, independently from classical cardiovascular disease risk factors such as age, sex, and plasma LDL cholesterol levels”[21].
The clustering of metabolic abnormalities is also documented in the pediatric age group[22, 23] even in children with 6 years of age. It is noteworthy to mention that HW and NAFLD have common lifestyle determinants among children and adolescents [23, 24].
The considerably high prevalence of overweight among young Iranian children[25], prevention and control of weight disorders and related complications, as NAFLD, should be considered as a priority for health policy makers and pediatricians.
The considerably high prevalence of overweight among young Iranian children [25], prevention and control of weight disorders and related complications, as NAFLD, should be considered as a priority for health policy makers and pediatricians.
Limitations and strengths: We should acknowledge that our study population was healthy looking children, and it was unethical to consider an invasive method for screening fatty liver; however this is usual for population-based studies to assess sonographic fatty liver, especially for the pediatric age group. The main limitation of this study is its cross-sectional nature. However its strength is its novelty in the pediatric age group in providing a risk score to be used as a screening tool for prediction of NAFLD from cross-sectional data.
Conclusion
This study, which to the best of our knowledge is the first of its kind in the pediatric age group, focuses on predicting sonographic NAFLD from factors that are easy to measure. The risk score calculated and validated in this study can be used as a screening tool. It might be confirmatory evidence on the association of HW phenotype with NAFLD in children and adolescents. This combination might be used in population-based studies as an accurate and easy tool for screening children who are at risk of NAFLD.
Acknowledgment
This study was funded as a thesis by the Vice Chancellery for Research, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest
None to declare.
References
- 1.Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26(4):409–15. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Sartorio A, Del Col A, Agosti F, et al. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61(7):877–83. doi: 10.1038/sj.ejcn.1602588. [DOI] [PubMed] [Google Scholar]
- 3.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28(1):13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]
- 4.Carter-Kent C, Feldstein AE. Non-alcoholic steatohepatitis over multiple generations. Dig Dis Sci. 2010;55(5):1494–7. doi: 10.1007/s10620-009-0896-z. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, McGreal N, Deutsch R, et al. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5):561–5. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Deutsch R, Rauch JB, et al. Obesity, insulin resistance, and other clinicopathological correlates of pediatric non-alcoholic fatty liver disease. J Pediatr. 2003;143(4):500–5. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 8.Quiros-Tejeira RE, Rivera CA, Ziba TT, et al. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI>or=95th percentile. J Pediatr Gastroenterol Nutr. 2007;44(2):228–36. doi: 10.1097/MPG.0b013e31802d4acc. [DOI] [PubMed] [Google Scholar]
- 9.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2010 Nov 2; doi: 10.3109/07853890.2010.518623. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Roberts E. Nonalcoholic steatohepatitis in children. Curr Gastroenterol Rep. 2003;5(3):253–9. doi: 10.1007/s11894-003-0028-4. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadinia AR, Bakhtavar K, Ebrahimi-Daryani N, et al. Hepatic vessel doppler indices: a study on non- Alcoholic liver disease. Tehran University Medical Journal. 2009;67(2):112–7. In Persian. [Google Scholar]
- 12.Kelishadi R, Cook SR, Adibi A, et al. Association of the components of the metabolic syndrome with non-alcoholic fatty liver disease among normal-weight, overweight and obese children and adolescents. Diabetol Metab Syndr. 2009;1:29. doi: 10.1186/1758-5996-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 14.Wilson SR, Withers CE. The Liver. In: Rumack CM, Wilson SR, Charboneau JW, editors. Diagnostic Ultrasound. 3rd ed. St. Louis: Elsevier Mosby; 2005. pp. 95–6. [Google Scholar]
- 15.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136(6):727–33. [PubMed] [Google Scholar]
- 16.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small dense LDL) in men? Circulation. 2000;102(2):179–84. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 17.LaMonte MJ, Ainsworth BE, DuBose KD, et al. The hypertriglyceridemic waist phenotype among women. Atherosclerosis. 2003;171(1):123–30. doi: 10.1016/j.atherosclerosis.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Hiura Y, Acklin F, Newman J, et al. Hypertriglyceridemic waist as a screening tool for CVD risk in indigenous Australian women. Ethn Dis. 2003;13(1):80–4. [PubMed] [Google Scholar]
- 19.Rogowski O, Shapira I, Steinvil A, et al. Low-grade inflammation in individuals with the hypertriglyceridemic waist phenotype: another feature of the atherogenic dysmetabolism. Metabolism. 2009;58(5):661–7. doi: 10.1016/j.metabol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Sam S, Haffner S, Davidson MH, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care. 2009;32(10):1916–20. doi: 10.2337/dc09-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Després JP, Cartier A, Côté M, Arsenault BJ. The concept of cardiometabolic risk: Bridging the fields of diabetology and cardiology. Ann Med. 2008;40(7):514–23. doi: 10.1080/07853890802004959. [DOI] [PubMed] [Google Scholar]
- 22.Esmaillzadeh A, Mirmiran P, Azizi F. Clustering of metabolic abnormalities in adolescents with the hypertriglyceridemic waist phenotype. Am J Clin Nutr. 2006;83(1):36–46. doi: 10.1093/ajcn/83.1.36. [DOI] [PubMed] [Google Scholar]
- 23.Alavian SM, Motlagh ME, Ardalan G, et al. Hypertriglyceridemic waist phenotype and associated lifestyle factors in a national population of youths: CASPIAN Study. J Trop Pediatr. 2008;54(3):169–77. doi: 10.1093/tropej/fmm105. [DOI] [PubMed] [Google Scholar]
- 24.Mager DR, Patterson C, So S, et al. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr. 2010;64(6):628–35. doi: 10.1038/ejcn.2010.35. [DOI] [PubMed] [Google Scholar]
- 25.Motlagh ME, Kelishadi R, Amirkhani MA, et al. Double burden of nutritional disorders in young Iranian children: findings of a nationwide screening survey. Public Health Nutr. 2010;14(4):605–10. doi: 10.1017/S1368980010002399. [DOI] [PubMed] [Google Scholar]