Abstract
Objective
Atopic dermatitis (AD) is the most common chronic relapsing skin disease seen in infancy and childhood. The intestinal microbiota play an important role in immune development and may play a role in the development of allergic disorders. Manipulation of the intestinal microbiota by synbiotics may therefore offer an approach to the prevention or treatment of AD and allergic diseases. We studied the clinical and immunologic effects of a new symbiotic (a mixture of seven probiotic strains of bacteria and Fructooligosaccharide) in infants and children with AD.
Methods
In a randomized, double-blind, placebo-controlled study, 40 infants and children aged 3 months to 6 years with AD received either a synbiotic or placebo for 8 weeks. The Severity Scoring of Atopic Dermatitis (SCORAD) index was recorded at baseline and also at 4 and 8 weeks of treatment.
Findings
There was no significant difference between the probiotic and placebo group in baseline characteristics including sex, age, family history, corticosteroid usage and prick testing. Mean age was 23 months. The synbiotic group showed a significantly greater reduction in SCORAD than did the placebo group (P=0.001). No specific effect was demonstrated of the probiotics employed on cytokine profile (P=0.4, P=0.6). Egg white was the most common (45%) allergen followed by peanut and cow's milk.
Conclusion
This study provides evidence that a mixture of seven strains of probiotics and Fructooligosaccharide can clinically improve the severity of AD in young children. Further studies are needed to investigate the effects on underlying immune responses and the potential long term benefits for patients with AD.
Keywords: Atopic dermatitis, Synbiotic, Cytokine, Children, Randomized Controlled Trial
Introduction
Atopic dermatitis (AD) is the most common chronic relapsing skin disease seen in infancy and childhood which affects 10-20% of children worldwide [1,2]. Current treatment strategies include allergen avoidance, topical steroids, moisturizing agents etc. Some patients are resistant to treatment or undergo drug side effects and some may have other allergies such as asthma at presentation or in the future. A novel treatment that alters basic immunologic mechanisms of allergies should be sought.
Although several mechanisms are thought to be involved, lack of microbial challenges during infancy is known to skew the immune status toward the development of allergic diseases [3].
Probiotics are defined as viable micro organisms, sufficient amounts of which reach the intestine in an active state and thus exert positive health effects. Prebiotics are substances that promote the growth of beneficial bacteria, and Synbiotic is a mixture of probiotics and prebiotis[4]. The onset of AD could be prevented by probiotics and this treatment strategy should be also effective even when AD is already established. There has been speculation that exposure to these microbial agents in early life, when immune maturation is critical, could play an important role in maturation of type 1 T helper cell (TH1) immune responses and could inhibit the development of allergic type 2 T helper cell (TH2) responses and IgE antibody production [5]. Probiotics have a strain-dependent capability to endow T cells with regulatory properties (Tregs). Such induction of Tregs by probiotics may involve APCs (monocytes, dendritic cells), or a direct action on T cells, and may take place in the intestine, where these cells encounter commensal bacteria [6–8].
The discovery of TH17, a new lineage of TH cells that produce IL-17 and effects of microbiota on the balance between these cells and Tregs development is a new era for research [9]. A number of studies have found conflicting results concerning the effect of synbiotics on atopic dermatitis. Optimal dosage, frequency and duration of treatment, single strain or a mixture of probiotics and effects of prebiotics should be determined because different probiotic strains vary in their ability to modulate the immune system [10].
In a randomized, double-blind, placebo-controlled trial, we aimed on studying the clinical and immunologic effects of a mixture of seven strains of probiotic bacteria (Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus) and Fructooligosaccharide in the treatment of AD in infancy and early childhood.
Subjects and Methods
This randomized, double blind, placebo controlled trial was carried out between November 2007 and March 2009 in the Allergy department of Qaem University Hospital, Mashhad, Iran. Sixty two children aged 3 months to 6 years with mild to severe AD were recruited from outpatient clinics. Children were ineligible for the study if they had prior exposure to probiotics, were currently taking a course of antibiotics, or had other major medical problems. Fifty two cases met the inclusion criteria and entered the study whereas ultimately 40 patients completed the study protocol.
Participants were randomly divided into two groups. Patients in the probiotic group received synbiotic containing 1 billion Colony Forming Unit (CFU) of Protexin Restore: a mixture of Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus and Fructooligosaccharide (Protexin healthcare, Somerset, United Kingdom), in the form of freeze dried powder twice daily for 8 weeks. The control group received placebo powder without probiotics which was matched for size, shape, and volume of contents and manufactured by the same company, twice daily for the same duration. Both supplements were dispensed as a stable powder in identical individual 1 g sachets, reconstituted by parents with 5–10 ml of water or breast milk and administered orally as a suspension. During the study all patients received the optimal treatment for AD by a single physician.
Participants were visited at baseline (1st visit), at week 4 (2nd visit) and at the end of intervention at week 8 (3rd visit). A detailed history was obtained at baseline with follow up questionnaires at each of the other visits.
Severity Scoring of Atopic Dermatitis (SCORAD) index assessment was also performed at each visit by a single clinician who was blind to the intervention. This SCORAD scores the extension, intensity, and subjective symptoms including pruritus and sleep-loss[11]. A 5 ml blood sample was collected from each participant at baseline and after 8 weeks. At each time point mononuclear cells were isolated from peripheral blood samples and cultured under polyclonal stimulation; the supernatant was assessed by ELISA for quantifying γ-interferon (INF-γ) and interlokin-4 (IL-4) concentrations based on the kit's instructions (Sanquin Blood Supply Foundation, Amsterdam, the Netherlands). Skin prick tests (SPTs) with common food allergens (egg white, cow's milk, peanut, tomato, pepper, fish, beef and wheat) and house dust mites were carried out on the patient's first visit.
One parent of each infant gave a written informed consent. The study protocol was approved by the local ethics committee of Mashhad University of Medical Sciences. This study had no conflict of interest on either side.
Twelve participants withdrew from the study; five within the first 4 weeks and other seven during the second month; two due to significant improvement, another 2 stopped taking the drug as a result of deterioration in their clinical condition from their parents' point of view, these two patients had severe AD with multiple food allergies, mainly to cow's milk. Five withdrew because of little treatment response and two children withdrew due to refusal of the suspension and one because of frequent antibiotic consumption. Forty patients were available for analysis. All statistical analysis was performed using SPSS (Version 11.5). A P-value <0.05 was considered statistically significant for all analyses.
Findings
There was no significant difference between the probiotic and placebo group in baseline characteristics including sex, age, family history, corticosteroid usage and prick testing (Table 1). Their mean age was 23 months, twenty five were males and 15 were females. The great majority (95%) of children had been breastfed.
Table 1.
Comparing the baseline demographic characteristics of the study and control group
| Variable | Study group | Control group | P-value |
|---|---|---|---|
| Males | 11 | 14 | 0.56 |
| Females | 8 | 7 | 0.49 |
| Mean age | 28.68±40.86 | 22.76±34.03 | 0.43 |
| Positive family history | 78% | 76% | 1 |
| Positive corticosteroid usage | 44% | 52% | 0.53 |
| Skin Prick testing | 56% | 54% | 1 |
Thirty three participants (81.7%) had at least one parent with a history of allergy (food allergy, asthma, allergic rhinitis or AD). Among the 12 patients who did not complete the study, most unsatisfied cases were from the placebo group who had low educated parents and did not take the optimal AD treatment seriously enough and had only focused on the placebo powder.
Only two from the study group withdrew due to significant improvement, another two children withdrew due to refusal of the suspension and one because of frequent antibiotic consumption. No significant side effects were observed in either group.
The primary outcome measure was change in the severity of AD as assessed by the SCORAD index. The synbiotic group showed a significantly greater reduction in SCORAD than did the placebo group especially between the first and second visits (P=0.001) and between the first and third visits (P=0.005) (Table 2).
Table 2.
The results of the General Linear Model for the changes of SCORAD in the 1st and 2nd visits, 1st and 3rd visits, and also 2nd and 3rd visits between both groups
| SCORAD | Control group Mean (SD) | 95% Confidence Interval | Study group Mean (SD) | 95% Confidence Interval | F | P value |
|---|---|---|---|---|---|---|
| visit 1 and 2 | −11.06(10.96) | −14.05– (−9.15) | −29.51 (19.09) | −33.78– (−25.24) | 12.6 | 0.001 |
| visit 1 and 3 | −20.10(8.63) | −22.3– (−18.07) | −39.2(24.22) | −44.61– (−33.79) | 8.94 | 0.005 |
| visit 2 and 3 | −7.65(8.33) | −9.58–(−5.72) | −9.4(11.14) | −11.89– (−6.91) | 0.22 | 0.6 |
SCORAD: Severity Scoring of Atopic Dermatitis
No significant effect was demonstrated of the synbiotic employed in this study on cytokine production of either INF-γ or IL-4 (Table 3, 4).
Table 3.
Comparing Mean and Standard Deviation (SD) of cytokines changes between baseline and the final visit in the two groups
| Cytokines | Control group Mean (SD) | Study group Mean (SD) | D | 95% Confidence Interval | t | P value |
|---|---|---|---|---|---|---|
| Interleukin 4 | 0.33 (2.60) | 0.38 (1.26) | 0.05 | 0.05–1.31 | 0.85 | 0.4 |
| γ-Interferon | 153.33 (509.33) | 77.70 (225.94) | −75.63 | −75.63– 256.21 | 0.47 | 0.6 |
Table 4.
Comparing the Mean (SD) cytokines level before and after treatment in both groups
| Cytokines | Groups | Before treatment Mean (SD) | After treatment Mean (SD) | Test results | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | T-student | P value | ||
| Interleukin 4 | Study group | 3.267± 1.64 | 2.96± 0.86 | 1.04 | 0.3 |
| Control group | 3.528± 1.75 | 3.77± 2.40 | 0.46 | 0.7 | |
| D | −0.26 | −0.81 | |||
| 95% CI | −0.261 (-1.08) | −0.81 (-1.17) | |||
| Test result | t=0.46; P=0.6 | t=1.09; P=0.3 | |||
| γ-Interferon | Study group | 196.10 ±176.60 | 102.59 ± 100.38 | 0.78 | 0.4 |
| Control group | 339.80 ±284.80 | 189.035 ± 344.93 | 1.50 | 0.1 | |
| D | −143.70 | −86.44 | |||
| 95% CI | −143.70 -153.39 | −86.44-166.11 | |||
| Test result | Z=1.36; P=0.2 | Z=0.38; P=0.7 | |||
SD: Standard Deviation / CI: Confidence Interval
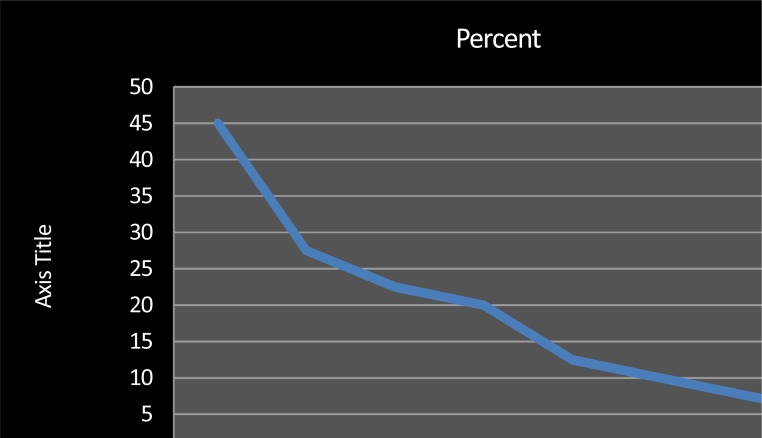
Food allergen sensitization was common with 23 (58%) cases having a positive skin prick testing for common food allergen extracts with egg white being the most common allergen (45%) followed by peanut and cow's milk (Fig. 1).
Fig. 1.
Frequency distribution of positive skin prick test to common food allergens and mites in all the studied patients
Discussion
The increasing incidence of allergic diseases is a worldwide problem. Solving this problem needs new strategies that should be safe, effective and available for all. Influencing the gut microbiota by administration of synbiotics to prevent and treat allergy is an alternative[12].
This study provided an opportunity to examine the clinical and immunological effects of a new synbiotic on AD in infancy and early childhood.
The primary outcome measure was change in the severity of AD as assessed by the SCORAD index. Considering that all of the patients entering the study received the optimal treatment for AD, we expected improvement in both groups; but the probiotic group showed a significantly greater reduction in SCORAD than the placebo group (Table 1). The significant difference in SCORAD between the two groups occurred between the 1st and 2nd visits (P=0.001) and the 1st and 3rd visits (P=0.005).
These results are in agreement with the meta-analyses by Michail SK et al in which the data from 10 studies (n=678) were available to analyze and an overall statistically significant difference favoring probiotics compared with placebo in reducing the SCORAD score was approved[13]. Other studies failed to show efficacy of probiotics or synbiotics in treating atopic dermatitis[14–16]. There are several reasons for difference in effects of probiotics and synbiotics in these studies including difference in dose and strains of probiotics used, duration of intervention, characteristics of the studied cases, sample size, and technical differences in the measurements. Therefore, further studies are required to explore strain-specific effects and mechanisms of different probiotic bacteria on allergic patients.
Kim et al studied whether supplementation of probiotics at 4-8 weeks before delivery and continuing until 6 months after delivery can prevent the development of eczema in infants at high risk and showed the prevalence of eczema at 1 year in the probiotic group was significantly lower than in the placebo group[17,18].
From the collected Skin Prick test data it was proved that egg antigen sensitization with a 45% frequency is the most common food allergen in atopic children, followed by peanut, cow's milk and tomato with 30%, 25% and 22% prevalence, respectively. This, similar to other reports, emphasizes high rate of food allergen sensitization in atopic children[2,6].
In this study, no specific effect was demonstrated of the probiotics employed on cytokine production of either the predominant T-helper type 1 (TH1) cytokine INF-γ, or the TH2 cytokine IL-4 after polyclonal stimulation. This is in agreement with the study by Rosenfeldt et al where no significant changes in cytokines (IL-2, IL-4, IL-10 and INF-γ) were found during 6 weeks of treatment[19]. These results differ from those of Pohjavuori et al who were able to demonstrate an increase in INF-γ production in peripheral blood mononuclear cells in infants treated with Lactobacillus GG [20]. The limitation of our study was the short duration of patients' follow up.
In this Double blind Placebo-Controle trial, application of a highly effective topical treatment and an elimination diet may have prevented us from fully appreciating the effects of probiotic preparations. It could also be argued that treatment or follow-up was too short. On the other hand, SCORAD was greatly reduced and low after the 8-week follow-up period.
Conclusion
This study provides evidence that a mixture of seven probiotic strains and Fructooligo-saccharide may clinically improve the severity of AD in young children. Considering the numerous beneficial effects of probiotics, their simple administration route and their low side effects, assessing the effect of these treatments on other allergies such as food allergy, asthma and allergic rhinitis and outcome of AD is a new era for future researches.
Acknowledgment
This study was performed with the grant of the vice chancellor for research, Mashhad University of Medical Sciences. We also wish to thank Dr Amirian, Dr Layegh and Ms Boruk for their invaluable help in performing the study. This study is registered in Iranian Registry of Clinical Trials (IRCT) ID: IRCT201010204976N1
Conflict of Interest
None
References
- 1.Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomized controlled trial. Arch Dis Child. 2005;90(9):892–7. doi: 10.1136/adc.2004.060673. Epub 2005 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson Textbook of Pediatrics. 18th ed. Philadelphia: Saunders; 2007. [Google Scholar]
- 3.Jung T, Stingl G. Atopic dermatitis: Therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol. 2008;122(6):1074–81. doi: 10.1016/j.jaci.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 4.De Vrese M, Schrezenmeir J. Probiotics, Prebiotics, and Synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 5.Ghadimi D, Fölster-Holst R, de Vrese M, et al. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology. 2008;213(8):677–92. doi: 10.1016/j.imbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Adkinson NF, Jr, Bochner BS, Busse WW, et al. Middleton's Allergy: Principles and Practice. 7th edn. Philadelphia: Mosby; 2009. [Google Scholar]
- 7.Fink LN. Induction of regulatory T cells by probiotics: potential for treatment of allergy? Clinl Exper Allergy. 2010;40(1):5–8. doi: 10.1111/j.1365-2222.2009.03408.x. [DOI] [PubMed] [Google Scholar]
- 8.Feleszko W, Jaworska J, Rha RD, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mecha-nisms in a murine model of asthma. Clin Exp Allergy. 2007;37(4):498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 9.Mucid D, Salek-Ardekani SH. Regulation of TH17 cells in the mucosal surfaces. J Allergy Clin Immunol. 2009;123(5):997–1003. doi: 10.1016/j.jaci.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol. 2008;606:423–54. doi: 10.1007/978-0-387-74087-4_17. [DOI] [PubMed] [Google Scholar]
- 11.Gelmetti C, Colonna C. The value of SCORAD and beyond. Towards a standardized evaluation of severity? Allergy. 2004;59(Suppl 78):61–5. doi: 10.1111/j.1398-9995.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 12.Rijkers GT, Bengmark S, Enck P, et al. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr. 2010;140(3):671S–6. doi: 10.3945/jn.109.113779. [DOI] [PubMed] [Google Scholar]
- 13.Michail SK, Stolfi A, Johnson T, Onady GM. Efficacy of probiotics in the treatment of pediatric atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101(5):508–16. doi: 10.1016/S1081-1206(10)60290-6. [DOI] [PubMed] [Google Scholar]
- 14.Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009;39(8):1117–27. doi: 10.1111/j.1365-2222.2009.03305.x. [DOI] [PubMed] [Google Scholar]
- 15.Kopp MV, Salfeld P. Probiotics and prevention of allergic disease. Curr Opin Clin Nutr Metab Care. 2009;12(3):298–303. doi: 10.1097/MCO.0b013e32832989a3. [DOI] [PubMed] [Google Scholar]
- 16.van der Aa LB, Heymans HS, van Aalderen WM, et al. The Synbad Study Group. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy. 2010;40(5):795–804. doi: 10.1111/j.1365-2222.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JY, Kwon JH, Ahn SH, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol. 2009;21(2 Pt 2):e386–93. doi: 10.1111/j.1399-3038.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 18.Niers L, Martín R, Rijkers G, et al. The effects of selected probiotic strains on the development of eczema (the PandA study) Allergy. 2009;64(9):1349–58. doi: 10.1111/j.1398-9995.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeldt V, Benfeldt E, Nielsen SD, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111(2):389–95. doi: 10.1067/mai.2003.389. [DOI] [PubMed] [Google Scholar]
- 20.Pohjavuori E, Viljanen M, Korpela R, et al. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy. J Allergy Clin Immunol. 2004;114(1):131–6. doi: 10.1016/j.jaci.2004.03.036. [DOI] [PubMed] [Google Scholar]