Abstract
Objective
Angiopoietins are involved in the pathogenesis of a variety of human diseases. We tried to evaluate the application of pleural and serum Angiopoietin-1 and 2 in categorizing pleural effusions (PEs) into exudates and transudates in children.
Methods
Pleural fluid (PF) and serum Angiopoietin (Ang)-1 and Ang-2 were measured in 80 children with PEs (40 transudative and 40 exudative) by using enzyme-linked immunosorbent assay.
Findings
PF Ang-2 levels were significantly higher in pleural exudates than in transudates (P 0.012). PF Ang-2 levels were significantly higher than serum Ang-2 levels in patients with pleural exudates and transudates (P<0.001). PF Ang-2 levels were higher in tuberculous than in non-tuberculous pneumonic PEs and empyema (P=0.01). PF Ang-2 levels correlate with serum Ang-2 levels (P<0.003). PF Ang-1 levels were significantly lower than serum Ang-1 levels both in patients with exudates and those with transudates (P<0.001). Cutoff points of serum and PF Ang-2, differentiating between transudative and exudative effusions were 3ng/ml and 8ng/ml respectively. Predictive potentials of serum and PF Ang-2 cutoff points were: Sensitivity 90% and 95% respectively, specificity 92.50% and 97.50% respectively, positive predictive value 92.30% and 97.40% respectively and negative predictive value 90.20% and 95.10% respectively.
Conclusion
Ang-2 levels were elevated in exudative PEs and correlated with levels of markers of pleural inflammation and pleural vascular hyperpermeability. It could categorize PE to exudates and transudates with valuable discriminative properties. That was detected more obviously in pleural fluids than in serum.
Keywords: Pleural Effusions, Transudates, Exudates, Angiopoietin-1, Angiopoietin-2
Introduction
Pleural effusion (PE) is one of the most common diagnostic problems in clinical practice [1]. Distinguishing an exudate from a transudate is the first step in the diagnostic approach of a patient with PE [2, 3]. Currently, the criteria proposed by Light et al [4], have been generally accepted for this discrimination [4]. However, these criteria misclassified 20% to 30% of transudates as exudates that occur mostly in patients receiving diuretic therapy [5].
Several parameters such as pleural fluid (PF) cholesterol level, PF to serum cholesterol ratio [6], PF to serum bilirubin concentration ratio [7], alkaline phosphatase value[8], pleural cholinesterase, PF to serum cholinesterase ratio [9] and serum-pleural effusion albumin gradient [10] have been proposed in segregating the transudates from exudates more reliably than those of Light's criteria. The usefulness of biochemical criteria to separate pleural transudates from exudates is controversial.
PE can be caused by several mechanisms including increased permeability of the pleural membrane capillaries, increased pulmonary capillary pressure, decreased negative intrapleural pressure, decreased oncotic pressure and obstructed lymphatic flow [2]. The exact pathogenetic mechanisms of exudative PEs are unclear. Increased permeability of the pleural microvasculature is generally attributed to factors that are released in inflammatory pleural diseases[11].
Angiopoietins (Ang) play an important role in angiogenesis occurring under both physiologic and disease conditions [12]. Ang-1 and ang-2 function as ligands for Tie2, which is a receptor tyrosine kinase specifically expressed on endothelial cells [13–15]. Ang-1 stabilizes blood vessels by promoting the interaction between endothelial cells and the surrounding extracellular matrix [14]. Ang-2 antagonizes the stabilizing action of Ang-1 by binding to Tie2 competitively, which destabilizes vessels [15].
Ang-1 has anti-inflammatory and anti-permeability properties; it blocks the expression of adhesion molecules on the endothelial cell surface, leukocyte adherence on endothelial cells and transmigration into tissues, and interleukin-8 production by endothelial cells. In addition, Ang-1 inhibits vascular permeability caused by vascular endothelial growth factor (VEGF) and inflammatory agents [16–20]. By contrast, Ang-2 promotes vascular permeability[21]. Ang-2 destabilizes the endothelial cell monolayer integrity leading to the detachment of endothelial cells in vitro [22].
Furthermore, Ang-2 was found to induce edema formation and to exert a weak stimulatory effect on leukocyte migration when injected into a mouse paw [23].
The aim of this study was to evaluate the application of pleural and serum Angiopoietin-1 and 2 in categorizing PEs into exudates and transudates in children.
Subjects and Methods
Patients
Our study was performed on 80 children [categorized as exudates or transudates according to the criteria of Light [4] in Chest Unit of Pediatric Department and in Chest Department, Zagazig University Hospital and Outpatient Clinics in the same hospital between November 2006 and April 2008. They were 49 males and 31 females with a mean age of 4.76±1.50 years. Parental consent was taken to be eligible for enrollment into the study.
Diagnostic Criteria
Patients with PEs were included in this study. Forty of all cases were defined as transudates [4] and sub classified as regards etiology to heart failure (15 patients), renal hydrothorax (13 patients) and liver cirrhosis (12 patients). In exudative effusion group (40 patients) there were 11 tuberculous effusions, 17 non-tuberculous para-pneumonic effusions and 12 empyema patients.
The diagnosis of heart failure was made on clinical grounds based on history, physical examination, chest radiograph, electrocardiogram with response to diuretic therapy and confirmed by echocardiographical evidence of left ventricular systolic dysfunction (left ventricular ejection fraction ≤40%), evidence of severe valvular disease or evidence of severe left ventricular diastolic dysfunction according to the American Heart Association guidelines [24].
Hepatic hydrothorax was defined as transudative effusion due to cirrhosis in presence of ascites with absence of any other causes of PE.
Renal hydrothorax (nephrotic patients) was defined as transudative effusion due to renal dysfunction (presence of heavy proteinuria, hyperlipidemia, hypoalbuminemia and edema) in absence of any other causes of PE.
Tuberculous pleuritis was diagnosed if Ziehl-Nelseen stains (three cases) or Lowenstein Jensen cultures of PF (three cases), or sputum (two cases) were positive or an exudative lymphocytic effusion with high adenosine deaminase level (>40 U/L) (three cases) cleared in response to antituberculous therapy.
Parapneumonic effusions refer to patients presented with pleural exudates [4] and associated with bacterial pneumonia which was diagnosed by clinical, radiological and laboratory findings (including positive bacterial culture in pleural fluids) [25].
Empyema was considered to be present if there was a finding of a PE on the chest radiograph coupled with aspiration of frank pus [25].
Biochemical measurements
The following biochemical parameters were determined simultaneously in all samples [serum and pleural fluid] including total protein level, LDH concentration and total cholesterol concentration. Pleural fluid/serum protein ratio, pleural fluid/serum LDH ratio and pleural fluid/serum cholesterol ratio were calculated.
Light's criteria [4] were applied (the fluid was defined as exudates if it fulfilled at least one of the following criteria: pleural/serum ratio of total proteins greater than 0.5; pleural/serum ratio of total lactate dehydrogenase greater than 0.6 or pleural lactate dehydrogenase greater than two thirds of upper limit of normal for serum lactate dehydrogenase [i.e. >400 IU/L according to the kits used). PF cholesterol as well as the serum-PF gradients for albumin and total proteins (serum albumin or protein concentration minus PF albumin or protein concentration) were determined in all samples. A PF cholesterol ≤45 mg/dL and a serum-effusion albumin and protein gradient ≥12g/L and 31g/L respectively were consistent with a transudate [26].
All these biochemical measurements in serum and PF specimens were carried out on Hitachi autoanalizer [mode l902, Japan] using standarized photometric method specifically, total protein was measured by Biuret method and LDH by an optimized UV kinetic method.
PF pH was measured using arterial blood gas machine just after aspiration of pleural fluid. Blood for measurement of protein, LDH, cholesterol was drawn at the same time as pleural fluid.
All PF samples underwent biochemical, cytological and microbiological analysis shortly after thoracocentesis. Both PF and serum samples were centrifuged at 4°C; aliquots of supernatant were frozen at −80°C until Ang-1 and Ang-2 levels were estimated.
Angiopoietin Ang-1 and Ang-2 levels estimation
The levels of Ang-2 in PE and serum were measured by enzyme-linked immunosorbent assay using a duoset methodology [R&D Systems; Minneapolis, MN]. Ang-1 protein levels were measured by a sandwich noncompetitive enzyme-linked immunosorbent assay consisting of a primary mouse antihuman Ang-1 antibody and a secondary biotinylated goat antihuman Ang-1 antibody [both from R&D Systems]. The minimum detectable dose of the assays for Ang-1 and Ang-2 were 200 and 65 pg/mL respectively.
Exclusion Criteria
In this study, all patients with effusions of undetermined origin, effusions with more than one cause, chylothorax and hemothorax were excluded.
Statistical Analysis
SPSS for windows, version 11 was used for data analysis. Values were expressed as means±SD. Chi-square test, Student's t-test and analysis of variance (ANOVA) test were used. Multiple comparison analysis by the least significant difference (LSD) was used to detect statistical difference between two means when ANOVA test referred to significances. Correlation between variables was assessed.
Receiver operating characteristic (ROC) curve analysis was used to determine the discriminative properties of various cutoff levels of serum and pleural Ang-2 levels. A P-value of <0.05 was considered significant.
Findings
Causes of PEs in 80 patients, who were included in this study, are demonstrated in Table 1. There were no significant differences as regards age, weight and gender of the studied cases (P=0.1, P=0.1 and P=0.09, respectively) (Table 2). Biochemical profile of pleural fluids showed significant differences between transudates and exudates (P< 0.01) (Table 2).
Table 1.
Different causes of pleural effusion in our study
| Etiology | Number of patients (%) N=80 |
|---|---|
| Transudates | 40 (50) |
| Congestive heart failure | 15 (37.5) |
| Renal hydrothorax (nephritic) | 13 (32.5) |
| Hepatic hydrothorax | 12 (30.0) |
| Exudates | 40 (50) |
| Tuberculous | 11 (27.5) |
| Parapneumonic | 17 (42.5) |
| Empyema | 12 (30.0) |
Table 2.
Demographic, clinical and pleural fluid data of the studied cases
| Characters | Transudates (n=40) | Exudates (n=40) | P-value |
|---|---|---|---|
| Age (years) | 4.6 (±1.1) | 4.9 (±1.9) | 0.1 |
| Weight (kg) | 17.0 (±3.7) | 16.1 (±3.0) | 0.2 |
| Gender (Male/Female) | 25/15 | 24/16 | 0.09 |
| Bilateral effusions (No of cases) | 15 | 5 | 0.002 |
| Pleural fluid protein (g/L) | 18.0 (±4.9) | 48.0 (±11.6) | 0.006 |
| Pleural fluid /Serum protein | 0.32 (±0.12) | 0.64 (±0.17) | 0.007 |
| Pleural fluid LDH (IU/L) | 260 (±45) | 713 (±96) | 0.009 |
| Pleural fluid /Serum LDH | 0.41 (±0.09) | 0.65 (±0.18) | 0.003 |
| Pleural fluid cholesterol (mg/dL) | 23.0 (±4.1) | 85.0 (±23.6) | 0.001 |
| Protein gradient (g/L) | 42.0 (±11.3) | 25.0 (±4.5) | 0.006 |
| Albumin gradient (g/L) | 20.0 (±4.8) | 8.0 (±2.0) | 0.005 |
| RBCs (cells/µL) | 210 (±65) | 1200 (±134) | 0.004 |
| Nucleated cell count (cells/µL) | 600 (±54) | 1875 (±231) | 0.002 |
| pH | 7.37 (±0.08) | 7.24 (±0.14) | 0.009 |
| Glucose (mg/dl) | 124.0 (±22.7) | 68.0 (±12.5) | 0.01 |
| Pleural fluid /Serum glucose | 1.14 (±0.12) | 0.71 (±0.07) | 0.01 |
LDH: Lactate dehydrogenase; RBC: Red Blood Cell
PF Ang-2 levels were significantly higher in pleural exudates than in transudates (P=0.01) (Table 3). PF Ang-2 levels were higher than serum Ang-2 levels in patients with pleural exudates and patients with transudates and that was highly significant (P<0.001)(Table 3). PF Ang-1 levels were significantly lower than serum Ang-1 levels both in patients with exudates and those with transudates (P<0.001), otherwise, we did not detect significant differences between our groups as regards PF and serum Ang-1 levels (P=0.2 and P=0.09, respectively)(Table 3).
Table 3.
Angiopeptin 1 and 2 levels in patients with pleural exudates and pleural transudates
| Angiopoietin | Fluid | Pleural exudates group | Pleural transudates group | P-value |
|---|---|---|---|---|
| Angiopoietin-1 | Serum (ng/ml) | 13.90 (± 2.34) | 13.97 (±2.26) | 0.09 |
| Pleural (ng/ml) | 0.62 (±0.19) | 0.65 (±0.12) | 0.2 | |
| P-value | 0.001 | 0.001 | ||
| Angiopoietin-2 | Serum (ng/ml) | 5.08 (±1.27) | 2.23 (±0.77) | 0.01 |
| Pleural (ng/ml) | 20.71 (±3.05) | 4.15 (±1.05) | 0.01 | |
| P-value | 0.001 | 0.001 |
We also compared various etiologies of PE for differences in PF Ang-2 levels which were significant (P=0.01). Ang-2 levels were higher in tuberculous than in non-tuberculous pneumonic PEs and empyema exudates (P=0.02). By contrast, there were no significant differences in pleural Ang-2 levels between patients with pleural transudates due to different etiologies (P>0.05) (Table 4).
Table 4.
Mean level (Standard deviation) of Angiopeptin 2 in patients with pleural effusions of different etiologies
| Etiology | Serum Ang-2 (ng/ml) | Pleural Ang-2 (ng/ml) |
|---|---|---|
| Heart failure | 2.22 (0.69)a | 4.10 (0.85)a |
| Hepatic hydrothorax | 2.32 (0.84)a | 4.25 (1.10)a |
| Renal hydrothorax | 2.14 (0.79)a | 4.09 (1.20)a |
| Parapneumonic exudates | 4.96 (1.31)b | 15.87 (3.41)b |
| Empyema exudates | 5.10 (1.21)b | 19.42 (2.24)c |
| Tuberculosis exudates | 5.17 (1.29)b | 26.85 (3.51)d |
| P-value (for ANOVA test) | 0.01 | 0.01 |
ANOVA and multicomparison analysis with LSD (the least significant difference)
Ang: Angiopeptin; ANOVA: Analysis of variance
aa, bb= non-significant (P>0.05); ab, ac, ad=Significant (P<0.05). bc, bd, cd =Significant (P<0.05).
Although serum Ang-2 levels were significantly higher in patients with exudates than those with transudates (P=0.01), serum Ang-2 levels showed no differences between different etiologies of transudates or exudates (P>0.05) (Table 4].
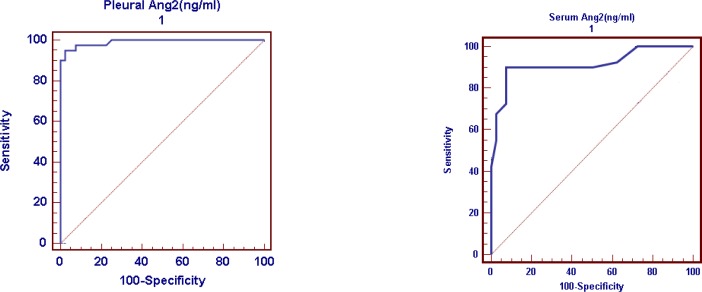
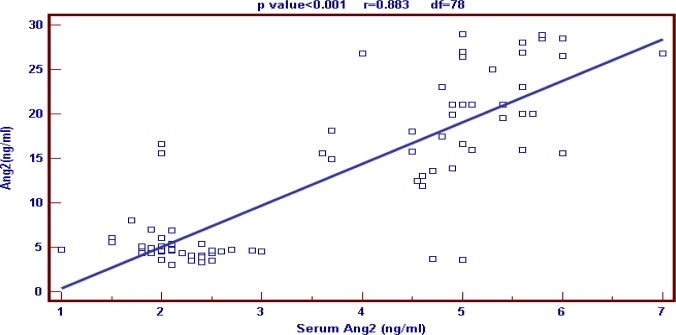
ROC curve was used to detect cutoff points for both serum and PF Ang-2, differentiating between transudative and exudative PEs, which were 3ng/ml and 8ng/ml respectively (Fig. 1). Predictive values of serum Ang-2 cutoff point were as the following: Sensitivity 90%, specificity 92.5%, positive predictive value 92.3% and negative predictive value 90.2% (Table 5). Predictive values of pleural Ang-2 cutoff point were as the following: Sensitivity 95%, specificity 97.5%, positive predictive value 97.4% and negative predictive value 95.1% (Table 5). Our results showed that there was highly significant positive correlation between serum and pleural Ang-2 levels in patients presented with PE (P<0.001)(Fig. 2).
Fig. 1.
Receiver operating characteristic curves for Angiopeptin-2 (Ang-2) levels in serum and pleural fluid to differentiate between transudative and exudative pleural effusions
Table 5.
Predictive potential of serum and pleural Ang-2 cutoff points
| Parameters | Pleural Ang-2 | Serum Ang -2 |
|---|---|---|
| Area under ROC curve | 0.991 | 0.915 |
| Standard error | 0.011 | 0.033 |
| 95% confidence interval | 0.94–1.00 | 0.83–1.00 |
| Significance level P(area=0.05) | 0.0001 | 0.0001 |
| Cut-off point (ng/ml) | 8 | 3 |
| Sensitivity | 95 | 90 |
| Specificity | 97.5 | 92.5 |
| Positive predictive value (%) | 97.4 | 92.3 |
| Negative predictive value (%) | 95.1 | 90.2 |
Ang: Angiopeptin; ROC: Receiver operating characteristic
Fig. 2.
Correlation between serum and pleural levels of angiopeptin-2 (Ang-2)
Although PF Ang-2 levels showed significant positive correlation with PF RBC count, PF nucleated cell count, PF total protein levels and PF LDH levels, it showed significant negative correlation with PF pH, PF glucose levels and PF/serum glucose ratio (Table 6). PF Ang-1 levels showed highly significant negative correlations with PF protein and PF/serum protein ratio (Table 6).
Table 6.
Correlation of Ang-1 and Ang-2 levels with clinical and laboratory data
| Pleural fluid | Serum fluid | |||||||
|---|---|---|---|---|---|---|---|---|
| Ang-1 | Ang-2 | Ang-1 | Ang-2 | |||||
| R | P | r | P | r | P | r | P | |
| Age | 0.12 | 0.1 | 0.21 | 0.06 | 0.19 | 0.08 | 0.16 | 0.08 |
| Weight | 0.14 | 0.09 | 0.20 | 0.06 | 0.17 | 0.07 | 0.14 | 0.09 |
| PF protein levels | −0.54 | 0.004 | 0.46 | 0.003 | 0.22 | 0.06 | 0.16 | 0.08 |
| PF/serum protein ratio | −0.65 | 0.006 | 0.55 | 0.004 | 0.15 | 0.08 | 0.12 | 0.1 |
| PF glucose levels | 0.11 | 0.1 | −0.64 | 0.006 | 0.22 | 0.06 | −0.21 | 0.06 |
| PF/serum glucose ratio | 0.15 | 0.08 | −0.59 | 0.005 | 0.15 | 0.08 | −0.20 | 0.06 |
| PF RBC count | 0.10 | 0.09 | 0.56 | 0.004 | 0.22 | 0.06 | 0.13 | 0.1 |
| Nucleated cell count (cells/µL) | 0.11 | 0.1 | 0.60 | 0.005 | 0.13 | 0.1 | 0.14 | 0.09 |
| pH | 0.11 | 0.1 | −0.56 | 0.004 | 0.13 | 0.1 | −0.13 | 0.1 |
| PF LDH levels | 0.12 | 0.1 | 0.69 | 0.007 | 0.12 | 0.1 | 0.21 | 0.06 |
| PF/serum LDH ratio | 0.14 | 0.09 | 0.57 | 0.005 | 0.16 | 0.08 | 0.17 | 0.07 |
Ang: Angiopeptin; PF: Pleural fluid; RBC: Red Blood Cell; LDH: Lactate dehydrogenase
Discussion
Ang-1 and Ang-2 have been shown [27, 28] to be involved in the pathogenesis of a variety of human diseases. Their role in pleural diseases has not been examined sufficiently [29].
Our study revealed that PF Ang-2 showed significant elevation in pleural exudates than in transudates. That coincided with Kalomenidis et al [29] study who found that PF Ang-2 levels were significantly higher in pleural exudates than in transudates. Pleural inflammation exists in a mutually dependent association with hyperpermeability of the pleural vasculature and constitutes the pathogenetic basis of the vast majority of exudative Pes [29]. On the other hand, transudative PEs are formed as a result of fluid extravasation that is caused by a disruption of the equilibrium of hydrostatic and/or osmotic pressures across an intact endothelial membrane[30].
In our study PF Ang-2 levels were significantly higher than serum Ang-2 levels in patients with pleural exudates and patients with transudates. That agree with Kalomenidis et al [29] who suggested that Ang-2 may be locally produced in the pleural cavity in patients with inflammatory pleural diseases and may play a role in the promotion of pleural inflammation and hyperpermeability and participate in the formation of exudative PEs. Further, it is probable that Ang-2 is mainly produced by the endothelial and perivascular cells of the pleural microvasculature, since neither mesothelial nor inflammatory cells have been reported to express Ang-2 [27].
In our study, PF Ang-1 levels were significantly lower than serum Ang-1 levels both in patients with exudates and those with transudates. Ang-1 is not produced in the pleural cavity in patients with either heart failure or pleural diseases characterized by inflammation and vascular hyperpermeability. Probably, the presence of Ang-1 in the pleural cavity is the result of diffusion from the blood[29].
In this study, Ang-2 levels were significantly higher in tuberculous than in non-tuberculous pneumonic PEs and empyema exudates. Higher levels of Ang-2 in tuberculous PEs than in pneumonic PEs may suggest that Ang-2 plays a primary role in the pathogenesis of pleural exudates of different etiologies [29]. Inspite of significantly higher serum Ang-2 in patients with pleural exudates than in those with transudates, we did not reveal significant differences between different etiologies of transudates or different etiologies of exudates as regards serum Ang-2 levels.
In our study, PF Ang-2 levels showed significant positive correlation with PF RBC count, PF nucleated cell count, PF protein levels, PF/serum protein ratio, PF LDH levels and PF/serum LDH ratio. By contrast, there was significant negative correlation with PF pH, PF glucose levels and PF/serum glucose ratio. That coincides with Kalomenidis et al [29] who demonstrated that PF levels of Ang-2 correlated with markers of vascular hyperpermeability and pleural inflammation. Both low PF pH and glucose levels are mainly due to increased metabolism in the pleural space, occurring in patients with pleural disease characterized by intense inflammation[29].
In our study, PF Ang-1 levels showed highly significant negative correlations with PF protein and PF/serum protein ratio. Both PF protein and PF/serum protein ratio are indexes of pleural vascular permeability and inversely correlated with PF Ang-1, that is in line with the previously reported data showing that Ang-1 is an antipermeability factor [16, 17].
In our study there was highly significant positive correlation between serum and pleural levels of Ang-2 in patients presented with PE.
One new and important finding in this study was the detection of two cutoff levels for both serum and PFs Ang-2 with valuable discriminative properties, differentiating between transudative and exudative PEs, which were 3ng/ml and 8ng/ml respectively. Predictive potentials of serum and PF levels of Ang-2 cutoff levels were as the following: Sensitivity 90% and 95% respectively, specificity 92.5% and 97.5% respectively, positive predictive value 92.3% and 97.4% respectively and negative predictive value 90.2% and 95.1% respectively.
Mandriota and Pepper [30] detected a significant correlation between Ang-2 levels and PF VEGF levels which may suggest a functional association between these two growth factors in the pathogenesis of exudative PEs. Kalomenidis et al[29] speculated that Ang-2 may amplify the hyperpermeability and proinflammatory signal produced by VEGF.
One of our limitations in this study was that we did not follow serum and PFs levels of Ang-2 to detect its prognostic value. To our knowledge, this was the 1st trial to detect Ang-2 cutoff points for categorization of PE to exudates or transudates.
Conclusion
We can conclude that Ang-2 levels were elevated in exudative PEs and correlated with levels of markers of pleural inflammation and pleural vascular hyperpermeability. It could categorize PE to exudates and transudates with valuable discriminative properties. That was detected more obviously in pleural fluids than in serum.
Further in vivo studies to detect the role of Angiopoietin in the pathogenesis of pleural diseases and its relation to different causative agents will provide a basis for the development of novel therapeutic strategies in which Ang-2 inhibitors may be used to treat patients with persistent exudative PEs.
Acknowledgment
The authors would like to thank Dr. Mohammed Osman for his statistical assistance. We also give our special thanks to Dr Fatima M Mahmoud for her comments on editing this manuscript. The authors also thank the staff of chest Unit in Zagazig University Children's Hospital and the staff of Outpatient Clinics in the same Hospital for their collaboration in sampling as well as our patients who participated in the study. Our institutional review committee of ethical research approved the study.
Conflict of Interest
None
References
- 1.Jay ST. Diagnostic procedures for pleural diseases. Symposium on pleural diseases. Clin Chest Med. 1985;6(3):33–48. [PubMed] [Google Scholar]
- 2.Maskel NA, Butland RJA. BTS guidelines for the investigation of unilateral pleural effusion in adults. Thorax. 2003;58(supp2):118–7. doi: 10.1136/thorax.58.suppl_2.ii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vavetsi R, Bonovas S, Polizou P, et al. The diagnostic role of glycosaminoglycans in pleural effusions. BMC Pulm Med. 2009;18(9):9. doi: 10.1186/1471-2466-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 5.Burgess LJ, Maritz FJ, Taljaard JJF. Comparative analysis of the biochemical parameters used to distinguish between pleural transudates and exudates. Chest. 1995;107(6):1604–09. doi: 10.1378/chest.107.6.1604. [DOI] [PubMed] [Google Scholar]
- 6.Valdes L, Pose A, Suarez J. Cholesterol: a useful parameter for distinguishing between pleural exudates and transudates. Chest. 1991;99(5):1097–102. doi: 10.1378/chest.99.5.1097. [DOI] [PubMed] [Google Scholar]
- 7.Meisel S, Shamis A, Tjaler M. Pleural fluid to serum bilirubin concentration ratio for the separation of transudates from exudates. Chest. 1990;98(1):141–4. doi: 10.1378/chest.98.1.141. [DOI] [PubMed] [Google Scholar]
- 8.Tahaoglu K, Kizkin O. Alkaline phosphatase: distinguishing between pleural exudates and transudates [letter] Chest. 1994;105(6):1912–3. doi: 10.1378/chest.105.6.1912b. [DOI] [PubMed] [Google Scholar]
- 9.Pachon EG, Navas IP, Sanchez JF. Pleural fluid to serum cholinesterase ratio for the separation of transudates and exudates. Chest. 1996;110(1):97–101. doi: 10.1378/chest.110.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Roth BJ, O'mear TF, Gragun WH. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest. 1990;98(3):546–9. doi: 10.1378/chest.98.3.546. [DOI] [PubMed] [Google Scholar]
- 11.Hamed EA, El-Noweihi AM, Mohamed AZ, et al. Vasoactive mediators (VEGF and TNF-α) in patients with malignant and tuberculous pleural effusions. Respirology. 2004;9(1):81–6. doi: 10.1111/j.1440-1843.2003.00529.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12(6):933–94. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 13.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 14.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Park KJ, Kim YS, et al. Serum Angiopoietin-2 as a clinical marker for lung cancer. Chest. 2007;132(1):200–6. doi: 10.1378/chest.06-2915. [DOI] [PubMed] [Google Scholar]
- 16.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6(4):460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 17.Segura RM. Useful clinical biological markers in diagnosis of pleural effusions in children. Paediatr Respir Rev ; (Suppl A) 2004;5:S205–12. doi: 10.1016/s1526-0542(04)90039-5. [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Moon SO, Park SK, et al. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89(6):477–9. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 19.Gamble JR, Drew J, Trezise L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87(7):603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 20.Pizurki L, Zhou Z, Glynos K, et al. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139(2):329–36. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 22.Scharpfenecker M, Fiedler U, Reiss Y, et al. The tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118(4):771–80. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 23.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314(2):738–44. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 24.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112(12):154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 25.Davies CWH, Gleeson FV, Davies RJO, et al. British Thoracic Society (BTS) guidelines for the management of pleural infection. 2003;58(Suppl. II):ii18–28. doi: 10.1136/thorax.58.suppl_2.ii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Candeira S, Hernandez L. The separation of transudates and exudates with particular reference to the protein gradient. Curr Opin Pulm Med. 2004;10(4):294–8. doi: 10.1097/01.mcp.0000128430.34150.80. [DOI] [PubMed] [Google Scholar]
- 27.Jones FP. Not just angiogenesis-wider roles for the angiopoietins. J Pathol. 2003;201(4):515–27. doi: 10.1002/path.1452. [DOI] [PubMed] [Google Scholar]
- 28.Medina J, Sanz-Cameno P, Garcia-Buey L, et al. Evidence of angiogenesis in primary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42(1):7–11. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Kalomenidis I, Kollintza A, Sigala I, et al. Angiopoietin-2 levels are elevated in exudative pleural effusions. Chest. 2006;129(5):1259–66. doi: 10.1378/chest.129.5.1259. [DOI] [PubMed] [Google Scholar]
- 30.Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83(8):852–9. doi: 10.1161/01.res.83.8.852. [DOI] [PubMed] [Google Scholar]