Abstract
Background
McCune-Albright syndrome (MAS) is a rare non-inherited disorder characterized by the clinical triad of precocious puberty, cafe-au-lait skin lesions, and fibrous dysplasia of bone.
Case Presentation
We report a girl with MAS, presenting initially with vaginal bleeding at the age of 17 months. Ultrasonography revealed unilateral ovarian cysts and ureteral and ovarian enlargement. Considering the clinical and paraclinical findings, the patient diagnosed as a case of gonadotropin-independent precocious puberty was treated with medroxy-progestrone acetate (MPA) for three months. During the follow up, recurrent episodes of bleeding, ovarian activation and cyst formation, as well as breast size development were reported. At the age of 5.5 years, fibrous dysplasia was detected, which in coexistence with precocious puberty confirmed the diagnosis of MAS. The patient had no cafe-au-lait skin macles during follow up.
Conclusion
Considering that clinical manifestations of MAS appear later in the course of recurrent periods of ovarian activation and cyst formation, a careful clinical observation and follow up of patients is necessary and the diagnosis of MAS must be kept in mind in cases with gonadotropin-independent precocious puberty.
Keywords: McCune-Albright Syndrome, Bleeding, Fibrous Dysplasia of Bone, Precocious Puberty
Introduction
McCune-Albright syndrome (MAS) is a rare non-inherited disorder characterized by the clinical triad of precocious puberty, cafe-au-lait skin spots, and fibrous dysplasia (FD) of bone[1].
The atypical form consists of only two of these conditions. The diagnosis is considered confirmed when at least two of the cardinal features are present[1]. MAS develops from activation of a post-zygotic, sporadic or somatic mutation of the GNAS1 gene with different clinical manifestations, depending on the tissue carrying the mentioned mutations[2]. The prevalence of the syndrome is reported to be between 1/100,000-1/1,000,000 and has been observed more commonly in females[3].
The syndrome was first described by Fuller Albright at Massachusetts and by Donovan McCune at the Columbia University College of Physicians and Surgeons in 1936[4]. During the time, different reports from MAS have led to better understanding of the syndrome and its pathophysiology.
MAS is also associated with other forms of endocrine dysfunction including hyper-thyroidism, pituitary adenomas (excessive secretion of growth hormone, hyperprolactin-emia), adrenal primary hyperplasia (Cushing syndrome), ovarian cysts and hypophosphat-emia[5]. Gonadal hyperfunction is considered to be the most common endocrine dysfunction in females, which manifests by precocious puberty, vaginal bleeding from the early phase of the syndrome to ovarian cysts. It usually presents prior to the age of four.
It is a gonadotropin-independent phenomen-on and vaginal bleeding in these patients occurs as early as 3 months of age[6].
Herein, we report a girl with MAS, presenting initially with vaginal bleeding at the age of 17 months. Ovarian cysts were detected during the first evaluation and fibrous dysplasia was detected during follow up, which confirmed the diagnosis MAS.
Case Presentation
This patient, a 17-month-old girl, was referred to our pediatric endocrinology clinic because of vaginal bleeding from three days ago. She had normal growth and development and had no history of disease.
In physical examination, the height was 78.5cm (25–50 percentiles) and the weight was 10 kg (25 percentile). The pubertal stage was B3, according to Tanner score. There were no café-au-lait spots, and examinations of other organs were normal as well. Pelvic ultrasonography revealed enlargement of uterus (45×12×20 mm) and ovaries (Lt. 16×23 mm and Rt. 32×14 mm) and a unilateral ovarian cyst of 15×33 mm of the left ovary. Pelvic Magnetic Resonance Imaging (MRI) did not reveal any abnormalities and confirmed the ultrasonographic results. Routine laboratory tests and serum level of LH, FSH, DHEAS, TSH, T4, and prolactin gave normal results. Serum estradiol (140pg/ml; NL range <10pg/ml) was elevated.
Considering the clinical and paraclinical findings, the patient diagnosed as a case of gonadotropin-independent precocious puberty received medroxy-progestrone acetate (MPA) for three months.
Follow-up investigations revealed regression of ovarian cyst and breast size (B2).
After 19 months, at age 3, the patient was referred again because of vaginal bleeding. Height 100 cm (95 percentile), weight 15.2 kg (75 percentile). Bone age was consistent with 5 years. Patient had a Tanner score of B3. Pelvic ultrasonography revealed enlargement of uterus (17×34×54 mm) and ovaries (Lt.11×12 mm and Rt.37×35 mm).
The results of GnRh test at 30, 60, and 120 min were as follow;
LH= 3 → 4.8 → 5.7 → 7.9 μu/ml FSH=2.9 → 16 → 16.5 → 18 μu/ml.
In order to evaluate the gonadotropin-dependent precocious puberty, brain MRI was performed, which did not reveal any abnormalities. The patient received LHRH-agonist therapy for two months. The treatment was discontinued after that period because of spotting, increased breast size, and inappro-priate response to LHRH-agonist.
During follow-up, the patient had recurrent periods of vaginal bleeding and bilateral ovarian cysts. In this period she was treated with medroxy-progestrone acetate, LHRH-agonist, and ciproteron acetate. In order to confirm presence of MAS, when she was 5.5 years old, serum levels of Ca, P, cortisol, TSH, and T4 were measured in outpatient clinic, which gave normal results.
In physical examination, height was 127 cm (>95 percentile), weight was 28 kg (>95 percentile), and the bone age was 8.5–9 years. The patient had a Tanner score of B3. In face, there was an asymmetry on right and left maxillary bones.
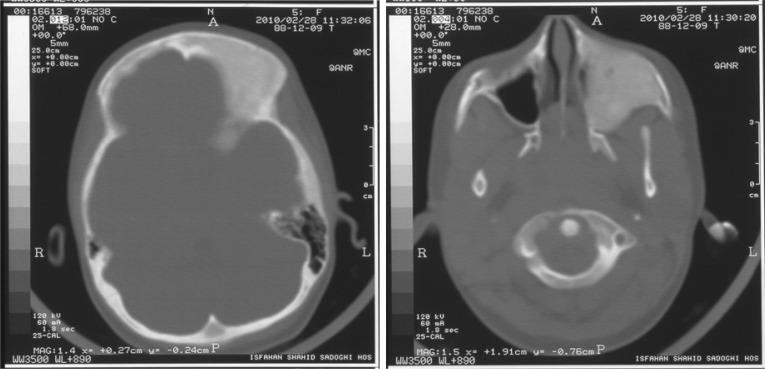
Radiography of femoral bone was normal but CT scan of maxillary bone revealed a fibrous dysplasia of the right maxillary bone (Fig. 1 and 2).
Fig. 1.
Brain CT scan of the patient with gonadotropin-independent precocious puberty and maxillary fibrous dysplasia
By coexistence of pseudoprecocious puberty and fibrous dysplasia the diagnosis McCune–Albright syndrome was confirmed.
Discussion
The diagnosis of McCune-Albright syndrome classically consists of the triad precocious puberty, cafe-au-lait skin spots, and fibrous dysplasia of bone[1]. The mutation and activation of Gs protein alpha subunit is the underlying genetic abnormality responsible for the syndrome. MAS may have different heterogeneous clinical manifestations regarding the number of mutated cells and the affected organs[2].
Literature review of the MAS cases indicates that most of the previously reported cases of MAS were presented as atypical or incomplete forms of the syndrome and there were rare cases with the classical symptoms. It seems that more practical definition of MAS could be any possible combination of FD and at least one of the typical precocious puberty and cafe-au-lait skin spots as reported by other authors[7].
In the current study, we report an atypical case of MAS missing cafe-au-lait skin spots. The patient was referred with vaginal bleeding and precocious puberty. Occurrence of fibrous dysplasia of maxillary bone at age 5.5 years confirmed the diagnosis MAS.
In a case series of MAS in India, there was a two-year-and-eight-month-old female with vaginal bleeding, cafe-au-lait skin macules, and FD of hip and right femur [1]. Our patient had no cafe-au-lait skin spots.
Café au lait spots are large melanotic macules without any definitive underlying abnormality. Previous studies have indicated that cafe-au-lait skin spots occur in approximately 60% of MAS cases [8]. In a study, 25% of children aged 6–15 years had at least one cafe-au-lait skin spot, whereas in another study 95% of cases showed these lesions [9, 10]. It seems that the lesions become apparent in future during follow-up period. However, prior studies indicated that precocious puberty and its related sexual dysfunctions occur before the development of bone lesions and skin café-au-lait spots [3].
Gonadotropin-independent precocious puberty is considered as the most common endocrine presentation of the syndrome, which is characterized by vaginal bleeding, development of ovarian cysts, and increased breast size. It is presented by the sporadic development of functional ovarian cysts, which consequently result in transient elevations of serum estradiol, independent of gonadotropin secretion[11].
Though there are reports of precocious puberty in 2- or 9-day-old neonates, it mainly occurs at the age of 1–6 years[12–14]. Benedict et al indicated that one third of MAS syndrome patients had precocious puberty[14], whereas the rate of this presentation as the initial clinical sign was 71% in the study of Risign et al[15]. Our patient was referred because of vaginal bleeding and breast enlargement.
Fibrous dysplasia, which accounts for 7% of total benign bone tumors, is one of the components of this syndrome[3]. It may be monostotic or polyostotic. It can involve any bone, but most commonly affects the long bones, the ribs, and the skull. FD in the craniofacial bones accounts for 25–35% of all FD cases and usually presents as a painless facial-skull asymmetry[16, 17]. Risign et al and Lumbroso et al, have reported the rate of FD to be 98% and 46%, respectively[15, 18]. Hart et al have investigated the onset and presentation of different FD lesions according to their location and accordingly have reported that FD onset has a region-specific pattern, 90% of craniofacial, extremities, and axial skeleton lesions were present by 3.4 years, 13.7 years, and 15.5 years, respectively[19]. But, overall, the median age for onset of FD lesions is 6–10 years[19]. In the current case report, the age of maxillary FD onset was 5.5 years.
In a 27-year-old female with MAS, FD was presented 24 years after precocious puberty[20].
Computed tomography (CT) scanning considered as the gold-standard imaging tool for FD, should be repeated annually before the stability of the FD lesions[3].
MAS is accompanied by different endocrino-pathies such as hyperthyroidism, occult thyrotoxicosis, hypercortisolism, GH excess and hyperprolactinemia. Thyroid disorder is the second commonest endocrine disorder. The rate of thyroid involvement with and without hyperthyroidism is reported to be 19–38% and 63%, respectively in MAS cases[14, 21, 22]. The rate of Cushing disease, hyperprolactinoma, and GH excess in MAS are reported to be 6%, 30–40%, and 21%, respectively. GH excess is accompanied by progression of FD in MAS[2, 18, 23]. Other endocrine disorders associated with MAS are primary hyperparathyroidism and hypophos-phateemic rickets[2]. None of the above-mentioned endocrine disorders were detected in our reported case.
Non-endocrine disorders associated with MAS are cholestasis, nephrocalcinosis, and cardiac involvement[2], which did not exist in the current case of MAS.
Conclusion
Considering that the clinical manifestations of MAS appear later in the course of recurrent periods of ovarian activation and cyst formation, a careful clinical observation and follow up of patients with recurrent episodes of mentioned clinical presentations is necessary. Also, the diagnosis of MAS must be kept in mind in cases with gonadotropin-independent precocious puberty, even in the absence of cafe-au-lait skin spots. Moreover, different radiologic and laboratory assessments should be performed in these cases during the follow-up period in order to investigate the presence of accompanying endocrinological and non-endocrinological disorders.
References
- 1.Rao S, Colaco MP, Desai MP. McCune Albright Syndrome (MCAS): a case series. Indian Pediatr. 2003;40(1):29–35. [PubMed] [Google Scholar]
- 2.Diaz A, Danon M, Crawford J. McCune-Albright syndrome and disorders due to activating mutations of GNAS1. J Pediatr Endocrinol Metab. 2007;20(8):853–80. doi: 10.1515/jpem.2007.20.8.853. [DOI] [PubMed] [Google Scholar]
- 3.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:1–12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivkees SA. McCune-Albright syndrome: 70 years of fascination and discovery. J Pediatr Endocrinol Metab. 2007;20(8):849–51. doi: 10.1515/jpem.2007.20.8.849. [DOI] [PubMed] [Google Scholar]
- 5.Rubio NI, Nader Sh, Brosnan PG. Mccune Albright syndrome: case report and review of literature. Int J Endocrinol Metab. 2006;4(3):167–75. [Google Scholar]
- 6.de Sanctis C, Lala R, Matarazzo P, et al. Pubertal development in patients with McCune-Albright syndrome or pseudo-hypoparathyroidism. J Pediatr Endocrinol Metab. 2003;16(Suppl 2):293–6. [PubMed] [Google Scholar]
- 7.Medina YN, Rapaport R. Evolving diagnosis of McCune-Albright syndrome. Atypical presentation and follow up. J Pediatr Endocrinol Metab. 2009;22(4):373–7. doi: 10.1515/jpem.2009.22.4.373. [DOI] [PubMed] [Google Scholar]
- 8.Landau M, Krafchik BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999;40(6 Pt 1):877–90. doi: 10.1016/s0190-9622(99)70075-7. [DOI] [PubMed] [Google Scholar]
- 9.Tekin M, Bodurtha JN, Riccardi VM. Café au lait spots: the pediatrician's perspective. Pediatr Rev. 2001;22:82–90. doi: 10.1542/pir.22-3-82. [DOI] [PubMed] [Google Scholar]
- 10.De Sanctis C, Lala R, Matarazzo P, et al. McCune-Albright syndrome: a longitudinal clinical study of 32 patients. J Pediatr Endocrinol Metab. 1999;12:817–26. doi: 10.1515/jpem.1999.12.6.817. [DOI] [PubMed] [Google Scholar]
- 11.Eugster E. Peripheral precocious puberty: causes and current management. Horm Res. 2009;71(Suppl 1):64–7. doi: 10.1159/000178041. [DOI] [PubMed] [Google Scholar]
- 12.Hackett LJ, Christopherson WM. Polyostotic fibrous dysplasia. J Pediatr. 1949;35(6):767–71. doi: 10.1016/s0022-3476(49)80121-1. [DOI] [PubMed] [Google Scholar]
- 13.Arlien-Soberg U, Iversen T. Albright's syndrome. A brief survey and report of a case in a seven-year-old girl. Acta Paediatr. 1956;45(5):558–68. doi: 10.1111/j.1651-2227.1956.tb06916.x. [DOI] [PubMed] [Google Scholar]
- 14.Benedict PH. Endocrine features of Albright's syndrome. Metabolism. 1962;11:30–45. [PubMed] [Google Scholar]
- 15.Ringel MD, Schwindinger WF, Levine MA. Clinical implications of genetic defects in G proteins. The molecular basis of McCune-Albright and Albright hereditary osteodystrophy. Medicine (Baltimore) 1996;75(4):171–84. doi: 10.1097/00005792-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Boston BA. McCune-Albright Syndrome. e medicine updated. Available at: http://emedicine.medscape.com/article/923026 Access date: Apr, 2010.
- 17.Hart ES, Kelly MH, Brillante B, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22(9):1468–74. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- 18.Lumbroso S, Paris F, Sultan C. Activating Gs( mutations: analysis of 113 patients with signs of McCune-Albright syndrome –a European Collaboration study. J Clin Endocrinol Metab. 2004;89(5):2107–13. doi: 10.1210/jc.2003-031225. [DOI] [PubMed] [Google Scholar]
- 19.Leet AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res. 2004;19(4):571–7. doi: 10.1359/JBMR.0301262. [DOI] [PubMed] [Google Scholar]
- 20.Malchoff CD, Reardon G, MacGillivray DC, et al. An unusual presentation of McCune-Albright syndrome confirmed by an activating mutation of the Gs alpha-subunit from a bone lesion. J Clin Endocrinol Metab. 1994;78(3):803–6. doi: 10.1210/jcem.78.3.8126161. [DOI] [PubMed] [Google Scholar]
- 21.Mastorakos G, Mitsiades NS, Doufas AG, Koutras DA. Hyperthyroidism in McCune-Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid. 1997;7(3):433–9. doi: 10.1089/thy.1997.7.433. [DOI] [PubMed] [Google Scholar]
- 22.Feuillan PP, Shawker T, Rose SR, et al. Thyroid abnormalities in the McCune-Albright syndrome: ultrasonography and hormonal studies. J Clin Endocrinol Metab. 1990;71(6):1596–601. doi: 10.1210/jcem-71-6-1596. [DOI] [PubMed] [Google Scholar]
- 23.Akintoye SO, Chebli C, Booher S, et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. J Clin Endocrinol Metab. 2002;87(11):5104–12. doi: 10.1210/jc.2001-012022. [DOI] [PubMed] [Google Scholar]