Abstract
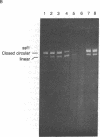
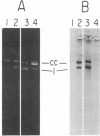
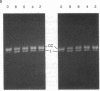
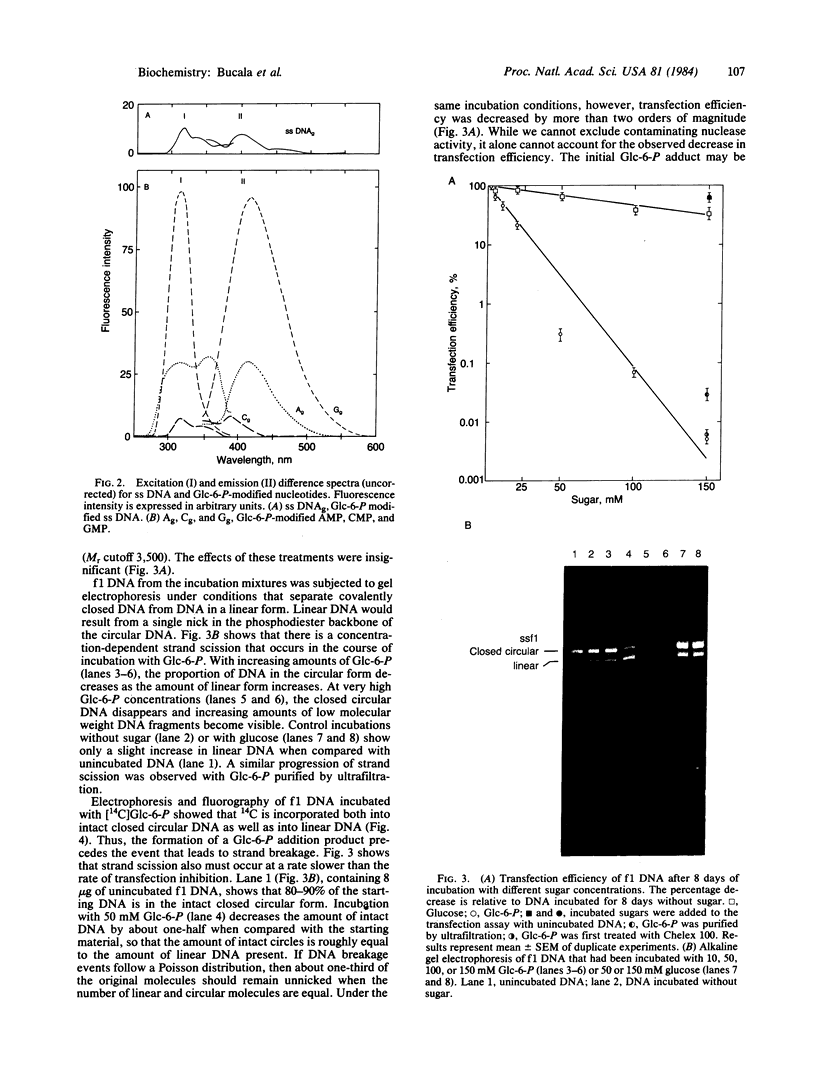
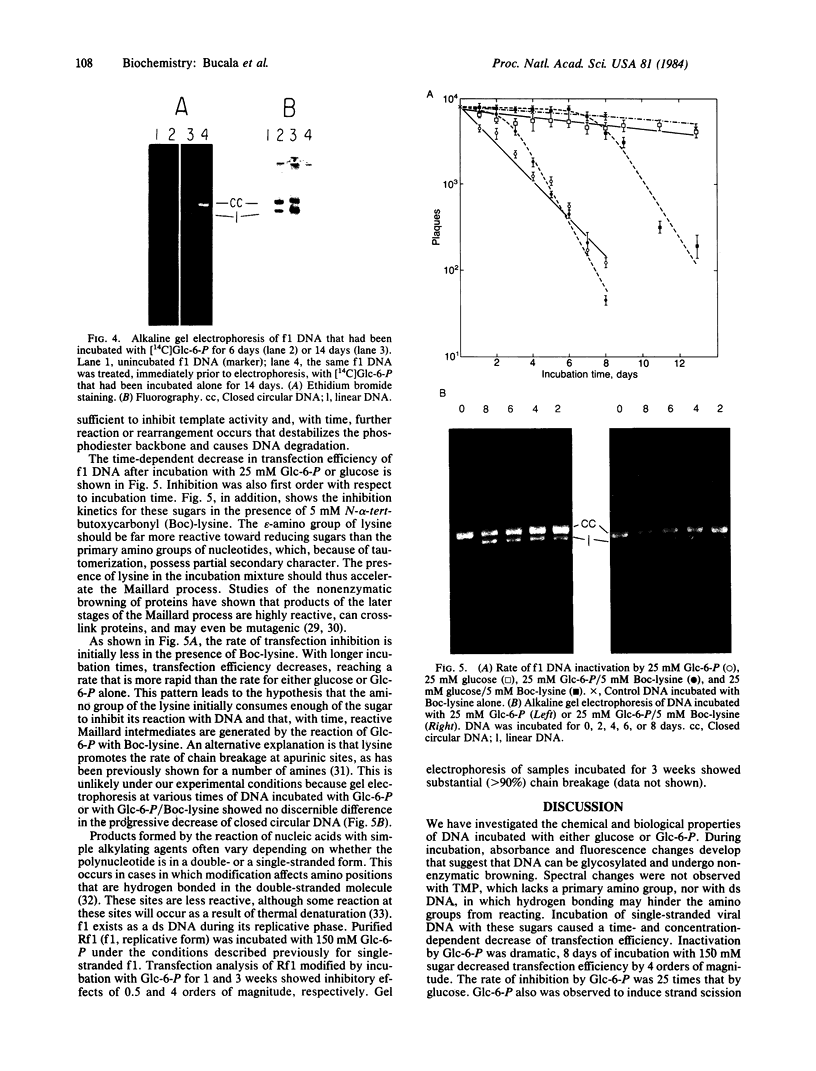
Reducing sugars react nonenzymatically with protein amino groups to initiate a process called nonenzymatic browning. Long-lived proteins, such as collagen and the lens crystallins, accumulate sufficient modification in vivo that they acquire many of the chemical properties characteristic of aged proteins. We have obtained evidence that nucleic acids also can undergo nonenzymatic modification by sugars. Incubation of DNA or nucleotides with glucose 6-phosphate (Glc-6-P) produces spectral changes similar to those described for nonenzymatic browning proteins. The occurrence of chemical modification was verified by measuring the transfection efficiency of viral DNA after incubation with glucose and Glc-6-P. A loss of transfection potential occurred that was first order with respect to time and sugar concentration. The rate of inactivation by Glc-6-P was 25 times that of glucose; 8 days of incubation with 150 mM Glc-6-P decreased transfection by 4 orders of magnitude. Glc-6-P also produced strand scission in a time- and concentration-dependent manner. We conclude that glucose, Glc-6-P, and possibly other sugars can react with DNA to produce significant structural and biological alterations. Since nucleic acids are long-lived molecules in the resting cell, the accumulation of these addition products might be a mechanism for the decreased genetic viability characteristic of the aged organism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMITAGE P., DOLL R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954 Mar;8(1):1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschbacher H. U., Chappuis C., Manganel M., Aeschbach R. Investigation of Maillard products in bacterial mutagenicity test systems. Prog Food Nutr Sci. 1981;5(1-6):279–293. [PubMed] [Google Scholar]
- Berdyshev G. D., Zhelabovskaya S. M. Composition, template properties and thermostability of liver chromatin from rats of various age at deproteinization by NaC1 solutions. Exp Gerontol. 1972 Oct;7(5):321–330. doi: 10.1016/0531-5565(72)90040-x. [DOI] [PubMed] [Google Scholar]
- Bodell W. J., Singer B. Influence of hydrogen bonding in DNA and polynucleotides on reaction of nitrogens and oxygens toward ethylnitrosourea. Biochemistry. 1979 Jun 26;18(13):2860–2863. doi: 10.1021/bi00580a029. [DOI] [PubMed] [Google Scholar]
- Bojanović J. J., Jevtović A. D., Pantić V. S., Dugandzić S. M., Jovanović D. S. Thymus nucleoproteins. Thymus histones in young and adult rats. Gerontologia. 1970;16(5):304–312. doi: 10.1159/000211792. [DOI] [PubMed] [Google Scholar]
- Brown P. R., Krstulovic A. M. Practical aspects of reversed-phase liquid chromatography applied to biochemical and biomedical research. Anal Biochem. 1979 Oct 15;99(1):1–21. doi: 10.1016/0003-2697(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Littlefield J. W., Soeldner J. S. Diabetes mellitus and aging: diminished planting efficiency of cultured human fibroblasts. Proc Natl Acad Sci U S A. 1969 Sep;64(1):155–160. doi: 10.1073/pnas.64.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Karran P., Ormerod M. G. Is the ability to repair damage to DNA related to the proliferative capacity of a cell? The rejoining of X-ray-produced strand breaks. Biochim Biophys Acta. 1973 Feb 23;299(1):54–64. doi: 10.1016/0005-2787(73)90397-3. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- MOHAMMAD A., FRAENKEL-CONRAT H., OLCOTT H. S. The browning reaction of proteins with glucose. Arch Biochem. 1949 Nov;24(1):157–178. [PubMed] [Google Scholar]
- MURPHY D. P. The birth of congenitally malformed children in relation to maternal age. Ann N Y Acad Sci. 1954 Jan 15;57(5):503–506. doi: 10.1111/j.1749-6632.1954.tb36425.x. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mauron J. The Maillard reaction in food; a critical review from the nutritional standpoint. Prog Food Nutr Sci. 1981;5(1-6):5–35. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mills J. L. Malformations in infants of diabetic mothers. Teratology. 1982 Jun;25(3):385–394. doi: 10.1002/tera.1420250316. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Orgel L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing: a correction. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1476–1476. doi: 10.1073/pnas.67.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Farber R. A., Tarrant G. M., Holliday R. Altered rate of DNA replication in ageing human fibroblast cultures. Nature. 1974 Oct 4;251(5474):434–436. doi: 10.1038/251434a0. [DOI] [PubMed] [Google Scholar]
- Price G. B., Modak S. P., Makinodan T. Age-associated changes in the DNA of mouse tissue. Science. 1971 Mar 5;171(3974):917–920. doi: 10.1126/science.171.3974.917. [DOI] [PubMed] [Google Scholar]
- Reynolds T. M. Chemistry of nonenzymic browning. II. Adv Food Res. 1965;14:167–283. doi: 10.1016/s0065-2628(08)60149-4. [DOI] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKSELA E., MOORHEAD P. S. ANEUPLOIDY IN THE DEGENERATIVE PHASE OF SERIAL CULTIVATION OF HUMAN CELL STRAINS. Proc Natl Acad Sci U S A. 1963 Aug;50:390–395. doi: 10.1073/pnas.50.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980 Nov;66(5):1179–1181. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Stevens V. J., Vlassara H., Abati A., Cerami A. Nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1977 May 10;252(9):2998–3002. [PubMed] [Google Scholar]
- Szilard L. ON THE NATURE OF THE AGING PROCESS. Proc Natl Acad Sci U S A. 1959 Jan;45(1):30–45. doi: 10.1073/pnas.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]