Abstract
Objective
Infections are the major cause of morbidity and mortality in febrile neutropenic patients with malignancy. Rapid diagnostic tests are needed for prompt diagnosis and early treatment which is crucial for optimal management. We assessed the utility of soluble triggering receptor expressed on myeloid cells (sTREM-1) in the diagnosis of bacteremia and fungemia in febrile neutropenic patients.
Methods
Sixty-five febrile neutropenic children with malignancy hospitalized in Mofid Children's Hospital during a period of one year from January 2007 were recruited for this cross sectional study (mean age 66.2± 37 months; 35 females and 30 males). Thirty patients (46.2%) had acute lymphoblastic leukemia, 2 (3.1%) acute myeloid leukemia, one (1.5%) lymphoma and 32 (49.2%) were under treatment for solid tumors. Simultaneous blood samples were collected for measurement of serum sTREM-1 levels and for blood cultures which were grown in BACTEC media. Gold standard for the presence of infection was a positive BACTEC culture as a more sensitive method compared to current blood culture techniques.
Findings
Blood cultures with BACTEC system were positive in 13(20%) patients (12 bacterial and one fungal culture). The mean serum sTREM-1 level in BACTEC positive patients was 948.2±592.9 pg/ml but in BACTEC negative cases it was 76.3±118.8 pg/ml (P<0.001). The optimal cut-off point of sTREM-1 for detecting patients with positive result of BACTEC was 525 pg/ml with sensitivity and specificity of 84.6% and 100%, respectively.
Conclusion
Our study revealed a significant association between serum sTREM-1 level and bacteremia and fungemia in febrile neutropenic patients suffering malignancy with acceptable sensitivity and specificity.
Keywords: Neutropenia, Fever, sTREM-1, BACTEC, Malignancy
Introduction
Infections are the leading cause of morbidity and mortality in febrile patients with malignancy who become neutropenic due to chemotherapy [1]. For the last fifty years, fever has been recognized as a major sign of infection in neutropenic patients; one of the great successes in oncology, i.e., reduction in the mortality of cancer patients, has been achieved through the routine use of empiric antibiotics in febrile patients with neutropenia since 1970 [2, 3].
Sever neutropenia, defined as an absolute neutrophil count, of less than 500/mm3 is the most important risk factor in children under chemotherapy. In neutropenic patients, fever is defined as an oral temperature of ≥38.3°C, or two consecutive readings of ≥38 °C during an hour [5–7]. In these patients fever is a medical emergency, because about half of them may have a serious bacterial infection [1]. The etiology of fever in most neutropenic children is unknown, but studies have indicated the presence of local infections or bacteremia in 10–40% of these patients [8]. Bacteremia has been reported in 13% of febrile neutropenic patients and the gram positive cocci were found to be the most common organisms [9].
The primary empirical treatment of febrile neutropenic patients varies according to the type of risk [10, 11]. In high risk patients, the traditional therapy of two or three antibiotics and in low risk patients, monotherapy is recommended [12, 13].
Due to high incidence of infections in febrile neutropenic patients, it is necessary to devise new rapid diagnostic tests. These tests help us in early diagnosis of infections and therefore may prevent unnecessary usage of wide spectrum antibiotics. One of the new methods for detecting bacteremia and fungemia is Soluble Triggering Receptor Expressed on Myeloid Cells (sTREM-1) test. sTREM-1 is a superfamily of immunoglobulins produced by phagocytes, especially macrophages that are stimulated by microbial products and therefore its level will be increased when and where macrophages accumulate [14].
The predictive value of sTREM-1 for the presence of bacterial infections is questionable [15–18]. In previous studies, the sensitivity and specificity of level of sTREM-1 was found to cover a wide range with many cut-off points [19–23].
In this study, we tried to evaluate the utility of sTREM-1 in the diagnosis of bacteremia and fungemia in febrile neutropenic patients with malignancy, and define a cut-off point for sTREM-1 with an acceptable sensitivity and specificity.
Subjects and Methods
Children, aged 15 months to 15 years, admitted in the Pediatric Hematology ward from January 2007 to January 2008 with malignancy were recruited for this cross sectional study. Inclusion criteria consisted of children with oral temperature ≥38 °C lasting for at least one hour or ≥38.3°C recorded once plus an absolute neutrophil count of less than 500/mm3. We excluded children under treatment with G-CSF or those already on antibiotics before the beginning of fever and neutropenia except for prophylaxis. Ethic committee in Shahid Beheshti University of Medical Sciences approved this study.
We took simultaneous samples of peripheral blood for measurement of serum sTREM-1 and for inoculation into BACTEC media for blood culture. In this study the gold standard for bacterial and fungal infections was a positive BACTEC culture. BACTEC culture system has special culture media with fluorescent alarm that determine the growth of bacterias and funguses. This alarm system informs the growth of bacterias and funguses several hours before detection of their kinds of species at special culture media. Our BACTEC system was model 1920 production of Beckton Dikenson (BD) Company.
Serum sTREM-1 levels were compared in culture positive and negative cases. Enzyme-linked immunosorbent assay (ELISA) kits with 96 well plates made by BD Company with research and development model DY1278 were used for measurement of sTREM-1 serum levels.
For ELISA, 3cc blood was taken from patients and centrifuged for 10 minutes; the serum collected in sterile microtubes and frozen at −70°C. After collection of 65 serum samples, before defreezing them, 100λ capture antibody was added to the plates and incubated for one night at room temperature. Then the wells were washed with 400λ wash buffer and thereafter incubated with 30λ diluted solution for an hour at room temperature. After washing, 100λ from samples and standards were added to the reagent diluent and incubated for 2 hours at room temperature. After repeated washing, 100λ streptavidin [Horse Radish Peroxidase (HRP)] was added and incubated for 20 minutes and washed again, then, 100λ substrate solution was added and samples were incubated for 20 minutes. Next, 50λ stop solution was added and at 450nm[Optical Density (OD)], serum level of sTREM-1 was determined.
Relevant demographic, clinical and para-clinical data, the type of malignancy, the interval from the last chemotherapy, days of fever, the status of the malignancy, i.e. remission or relapse, chest x-ray, complete blood count and C-reactive protein (CRP) were obtained from the patient's files.
Categorical variables expressed as counts and percents and quantitative variables summarized as mean±standard deviation. For comparison of mean sTREM-1 values between two groups, Student t-test was preformed. Receiver operating characteristic (ROC) curve was used for evaluating the diagnostic value of sTREM-1 for predicting BACTEC results.
Also sensitivity, specificity, positive predictive value, negative predictive value, positive and negative likelihood ratio (LR+ and LR-) and diagnostic accuracy (percentage of all true results) were calculated for different cut-off points according to the conventional formula. An optimal cut-off point was chosen as the sensitivity plus specificity were maximized. Statistical significance level was defined less than 0.05.
Findings
Sixty-five patients with malignancy were enrolled in this study. 35 (53.8%) were female and 30(46.2%) were male. The mean age was 66.2±37 months. 30 (46.2%) patients had acute lymphoblastic leukemia (ALL), 2 (3.1%) acute myeloid leukemia (AML), 1 (1.5%) lymphoma and 32 (49.2%) solid tumors. 57 (87.7%) patients were in remission and 8 (12.3%) in relapse. The mean interval between the beginning of fever and the last chemotherapy was 14.3±12.0 days. The mean duration of fever before admission was 3.63±2.2 days. The mean temperature was 39.2±0.3°C, mean systolic blood pressure was 102.0±40.0 mmHg and the mean diastolic blood pressure was 63.4±8.7 mmHg.
The mean sTREM-1 serum level was 250.68± 447.98 pg/ml (range 3 to 2000pg/ml). CRP was negative in 21 (32.3%), 1+ in 19 (29.2%), 2+ in 18 (27.7%) and 3+ in 7 (10.8%) patients.
Blood cultures with BACTEC method were positive in 13 (20%) children, and negative in 52 (80%). From these 13 patients, 12 (92.2%) patients had positive bacterial cultures and one culture was positive for fungal infection. The prevalence and species of microorganisms in BACTEC culture medium included: 6 (46.7%) Staphylococcus epidermidis, 3 (23.1%) E coli, 1 (7.8%) Enterobacter aerogenes, 1 (7.8%) Proteus mirabilis, 1 (7.8%) Yersinia enterocolitica, 1 (7.8%) Candida albicans.
Three of our patients died. Their BACTEC and sTREM-1 results were as follows:
One case with negative BACTEC and sTREM-1=500pg/ml. One case with positive BACTEC (S epidermidis) and sTREM-1=800pg/ml and another case with positive BACTEC (S. epidermidis) and sTREM-1=600pg/ml.
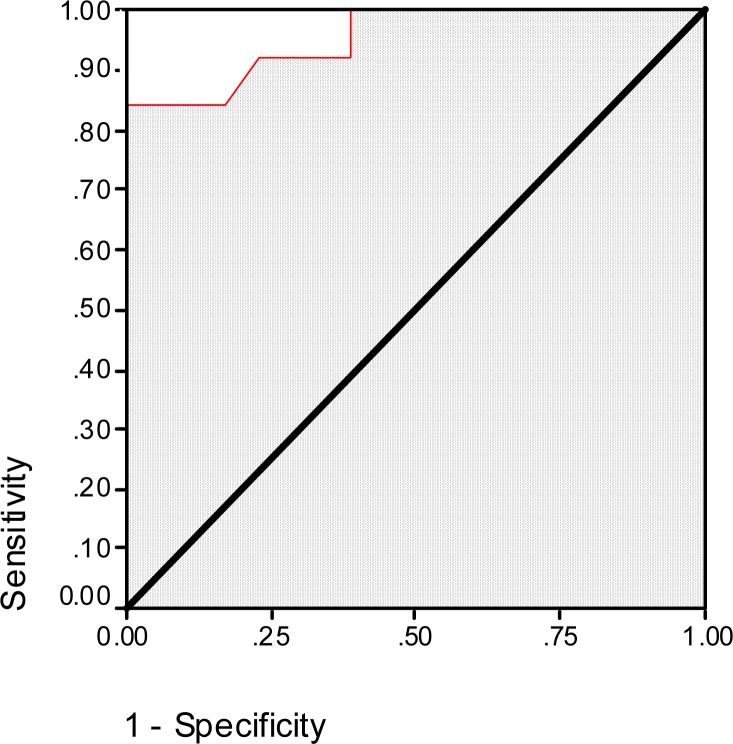
The mean serum sTREM-1 level in BACTEC positive patients was 948.2±592.9 pg/ml but in BACTEC negative cases it was 76.3±118.8 pg/ml (P<0.001). ROC curve of sTREM-1 level for the prediction of BACTEC results is presented as Fig. 1. Based on this curve, the area under curve is 0.96 (SE=0.03, P<0.001). As the data in Table 1 shows, the best cut-off point of sTREM-1 for the prediction of BACTEC result is 525 pg/ml with sensitivity and specificity of 84.6% and 100%, respectively.
Fig. 1.
Receiver operating characteristic curve of soluble triggering receptor expressed on myeloid cells (sTREM-1) for the prediction of BACTEC results in febrile neutropenic patients with malignancy
Table 1.
Sensitivity, specificity, positive predictive value, negative predictive value, LR+, LR- and accuracy for some cut-off points of sTREM-1 for the prediction of BACTEC results in febrile neutropenic patients with malignancy
| Cut-off point | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | Accuracy |
|---|---|---|---|---|---|---|---|
| 25.5 | 100% | 61.54% | 39.39% | 100% | 2.60 | 0.00 | 69.2% |
| 38 | 92.31% | 61.54% | 37.50% | 96.97% | 2.40 | 0.13 | 67.6% |
| 75 | 92.31% | 71.15% | 44.44% | 97.37% | 3.20 | 0.11 | 75.3% |
| 125 | 92.31% | 76.92% | 50.00% | 97.56% | 4.00 | 0.10 | 80% |
| 160 | 84.62% | 82.69% | 55.00% | 95.56% | 4.89 | 0.19 | 83.1% |
| 185 | 84.62% | 86.54% | 61.11% | 95.74% | 6.29 | 0.18 | 86.1% |
| 300 | 84.62% | 92.31% | 73.33% | 96.00% | 11.00 | 0.17 | 90.8% |
| 450 | 84.62% | 98.08% | 91.67% | 96.23% | 44.00 | 0.16 | 95.4% |
| 525 | 84.62% | 100% | 100% | 96.30% | ∞ | 0.15 | 96.9% |
| 575 | 76.92% | 100% | 100% | 94.55% | ∞ | 0.23 | 95.4% |
sTREM: Soluble Triggering Receptor Expressed on Myeloid Cells / PPV: Positive Predictive Value / NPV: Negative Predictive Value / LR+: Positive Likelihood Ratio / LR-: Negative Likelihood Ratio
Discussion
There was no difference between the two sexes in culture-positive and culture-negative patients.
Most patients in our study had ALL. The sTREM-1 cut-off point was 525pg/ml. This level is more than what was shown in Gibot's study in France, serum sTREM-1 level for sepsis which was 60pg/ml [14] and Determann in Netherlands (cerebro-spinal fluid sTREM-1 level for meningitis) who reported levels of 20pg/ml [24]. Also, it is different from Jin Won Huh's research in Seoul, who reported a mean level of 184pg/ml in his study of bronchoalveolar lavage fluid sTREM-1 level in patients with pneumonia [25]. These differences may be due to different sampling sites and different types of infections.
In a more recent study, Miedema et al, 2010, who determined CRP, interleukin (IL)-8, procalcitonin (PCT), and sTREM-1 as predictors for bacterial infection in febrile neutropenia, sTREM-1 levels were below the detection limit, 7 pg/ml, in majority cases and therefore excluded from their analysis [26]. Their results are in contrast with our findings and we must clear, whether neutropenic children can produce a sufficient release of sTREM-1 in response to infection.
Although sTREM-1 has recently been suggested as a marker for bacterial infection [16, 18], only a few studies have been published before in neutropenic patients. We studied sTREM-1 levels during bacterial infections in neutropenic patients, who might have enough monocytes to produce measurable sTREM-1 levels. Our study has illustrated acceptable sensitivity and specificity of sTREM-1 with a cut-off point 525pg/ml for detection of blood infections in this patient population. It is obvious that until more studies are carried out, usage of this diagnostic method instead of blood culture cannot be accepted.
In our study, there was no significant association between serum sTREM-1 level and duration of fever before admission, interval between fever with the last chemotherapy, severity of neutropenia and mortality rate.
As mentioned above, in Gibot's study, fungemia was found in 3% of febrile neutropenic patients with an increased serum sTREM-1 level [14]. In our study there was one case of fungemia with serum sTREM-1=2000pg/ml that similar to other studies, the percentage of positive fungal blood cultures (7.8%) was small; therefore due to high cost of BACTEC blood culture, fungal blood culture with BACTEC may be recommended in unresponsive cases to empirical antibacterial treatment.
Limitation of our study was that cut-off point in sTREM-1 level in neutropenic patients with bacterial infections was not studied.
Conclusion
Our study shows a significant association between serum sTREM-1 level and presence of bacteremia and fungemia in febrile neutropenic patients with cancer. The cut-off point of 525pg/ml has a high sensitivity and specificity in detection of blood infections. We recommend starting immediate empiric antibiotic therapy at sTREM-1 level of ≥525pg/ml while waiting for the result of BACTEC culture. However, withholding empiric antibiotic therapy with lower sTREM-1 levels cannot be recommended at present and needs further study.
Acknowledgment
We appreciate all supports of Pediatric Infectious Research Center and personnel of infection and hematologic wards of Mofid Children's Hospital.
Conflict of Interest
All authors have no conflicts of interest to declare and did not any financial or non-financial conflict of interest.
References
- 1.Auletta JJ, Oriordan MA, Nieder ML. Infections in children with cancer: A continued need for the comprehensive physical examination. J Pediatr Hematol Oncol. 1999;21(6):501–8. [PubMed] [Google Scholar]
- 2.Bodey GP, whitecar JP, Middleman E, Rodriguez V. Carbenicillin therapy for pseudomonas infections. JAMA. 1971;218(1):62–6. [PubMed] [Google Scholar]
- 3.Schimpff S, Satterlee W, Young VM, Serpick A. Empiric therapy with carbenicillin and gentamycin for febrile patients with cancer and granulocytopenia. N Engl Med J. 1971;284(19):1061–5. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- 4.Bodey GP, Buckley M, Sathe YS, et al. Infections complicating chronic diseases. In: Moffet HL, Fisher RG, Boyce TG, editors. Moffet's Pediatric Infectious Diseases: A Problem-Oriented Approach. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 705–37. [Google Scholar]
- 5.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730–51. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 6.Wolff LJ, Ablin AR, Altamn AJ, Johnson FL. The management of fever. In: Ablin AR, editor. Supportive care of children with cancer: Current therapy and guidelines from children's cancer group. Baltimore: John Hopkins University Press; 1997. pp. 25–38. [Google Scholar]
- 7.Klaassen RJ, Goodman TR, Pham B, Doyle JJ. Low risk prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol. 2000;18(5):1012–9. doi: 10.1200/JCO.2000.18.5.1012. [DOI] [PubMed] [Google Scholar]
- 8.Mustafa M, Carlson L, Tkzewski I, et al. Comparative study of cefepime versus ceftazidim in empiric treatment of pediatric cancer patients with fever and neutropenia. Ped Inf Dis J. 2001;20(3):362–9. doi: 10.1097/00006454-200103000-00036. [DOI] [PubMed] [Google Scholar]
- 9.Paqanini H, Boloqnu R, Debbqq R, et al. Fever and neutropenia in children with cancer in one pediatric hospital in Argentina. Pediatr Hematol Oncol. 1998;15(5):405–13. doi: 10.3109/08880019809016568. [DOI] [PubMed] [Google Scholar]
- 10.Alexander SW, Walsh TJ, Freifeld AG, Pizzo PA. Infectious complications in pediatric cancer patients. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 1239–83. [Google Scholar]
- 11.De Pauw BE, Deresinski SC, Feld R, et al. Ceftazidime compared with piperacillin and tobramycin for the empiric treatment of fever in neutropenic patients with cancer. Ann Intern Med. 1994;120(10):834–44. doi: 10.7326/0003-4819-120-10-199405150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hathorn JW, Rubin M, Pizzo PA. Empiric antibiotic therapy in the febrile neutropenic cancer patient: Clinical efficacy and impact of monotherapy. Antimicrob Agents Ehemother. 1987;31(7):971–7. doi: 10.1128/aac.31.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzo PA, Hathorn JW, Hiemenz J, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986;315(9):552–8. doi: 10.1056/NEJM198608283150905. [DOI] [PubMed] [Google Scholar]
- 14.Gibot S, Cravoisy A, Kolopp-Sarda MN, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells-1) plasma concentrations during sepsis. Crit Care Med. 2005;33(4):792–6. doi: 10.1097/01.ccm.0000159089.16462.4a. [DOI] [PubMed] [Google Scholar]
- 15.Gibot S, Le Renard PE, Bollaert PE, et al. Surface triggering receptor expressed on myeloid cells 1 expression patterns in septic shock. Intensive Care Med. 2005;31(4):594–7. doi: 10.1007/s00134-005-2572-x. [DOI] [PubMed] [Google Scholar]
- 16.Rivera-Chavez FA, Minei JP. Soluble triggering receptor expressed on myeloid cells-1 is an early marker of infection in the surgical intensive care unit. Surg Infect (Larchmt) 2009;10(5):435–9. doi: 10.1089/sur.2009.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bopp C, Hofer S, Bouchon A, et al. Soluble TREM-1 is not suitable for distinguishing between systemic inflammatory response syndrome and sepsis survivors and nonsurvivors in the early stage of acute inflammation. Eur J Anaesthesiol. 2009;26(6):504–7. doi: 10.1097/EJA.0b013e328329afca. [DOI] [PubMed] [Google Scholar]
- 18.Phua J, Koay ES, Zhang D, Lee KH. How well do serum sTREM-1 measurements prognosticate in septic shock. Anaesth Intensive Care. 2008;36(5):654–8. doi: 10.1177/0310057X0803600504. [DOI] [PubMed] [Google Scholar]
- 19.Wessman SJ, Scoop FJ, Johnson GM, et al. Septicemia in pediatric oncology patients: the significance of viridans streptococcal infections. J Clin Oncol. 1990;8(3):453–9. doi: 10.1200/JCO.1990.8.3.453. [DOI] [PubMed] [Google Scholar]
- 20.Gassas A, Grant R, Richardson S, et al. Predicators of viridans streptococcal shock: Syndrome in bacteremic children with cancer and stem-cell transplant recipients. J Clin Oncol. 2004;22(7):1222–7. doi: 10.1200/JCO.2004.09.108. [DOI] [PubMed] [Google Scholar]
- 21.Gibot S, Cravoisy A. Soluble form of the Triggering Receptor Expressed on Myeloid cells-1 as a marker of microbial infection. Clin Med Res. 2004;2(3):181–7. doi: 10.3121/cmr.2.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 23.Routsi C, Giamarellos-Bourboulis EJ, Antonepoalon A, Kollias S. Does soluble triggering receptor expressed on myeloid cells-1 play any role in the pathogenesis of septic shock. Clin exr Immunol. 2005;142(1):62–7. doi: 10.1111/j.1365-2249.2005.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Determann RM, Weisfelt M, Gans J, et al. Soluble triggering receptor expressed on myeloid cells 1: a biomarker for bacterial meningitis. Intensive Care Med. 2006;32(8):1243–7. doi: 10.1007/s00134-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 25.Miedema KG, de Bont ES, Elferink RF. Support Care Cancer. 2010. The diagnostic value of CRP, IL-8, PCT, and sTREM-1 in the detection of bacterial infections in pediatric oncology patients with febrile neutropenia. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huh JW, Lim CM, Koh Y, et al. Diagnostic utility of the soluble triggering receptor expressed on myeloid cells-1 in bronchoalveolar lavage fluid from patients with bilateral lung infiltrates. Crit Care. 2008;12(1):R6. doi: 10.1186/cc6770. [DOI] [PMC free article] [PubMed] [Google Scholar]