Abstract
Objective
There are several problems associated to the management of patients with phenylketonuria (PKU). Social status could be one of the affecting factors on dietary adherence in these patients. The aim of this study was to evaluate family social status and dietary adherence of PKU patients in Iranian population.
Methods
In a cross-sectional study, we studied 105 Iranian PKU patients (born 1984 to 2010), treated and followed at Mofid Children's Hospital, Tehran. Social status was defined by number of children in family, number of affected children in family, maternal and paternal education, marital and employment status of the parents. Age at diagnosis and duration of treatment were also recorded. Mean plasma phenylalanine level was considered as a sign of dietary adherence in PKU patients and was calculated considering the phenylalanine measurements throughout at least one year.
Findings
Mean plasma phenylalanine concentration was 5.9±3.6 mg/dl in patients <12 years old and 13.1±3.9 mg/dl in patients >12 years old. Blood phenylalanine concentrations in 47.6% of patients were in normal age-related reference range. There was a significant association between divorced and unemployed parents, and higher levels of blood phenylalanine concentration (P=0.02 and P=0.03 respectively). There was a significant positive correlation between number of affected children in the family (r=0.43, P<0.001), age at diagnosis (r=0.2, P=0.03), treatment duration (r=0.7, P=<0.001) and blood phenylalanine concentrations. There was no significant relation between parental education, family size and dietary adherence.
Conclusion
Social status affects dietary adherence to some extent. We suggest exploring care-givers dietary knowledge as the next step to improve dietary compliance in these patients.
Keywords: Phenylketonuria, Mental Retardation, Marital status, Hyperphenylalaninaemia, PKU
Introduction
The goal of treatment in phenylketonuria (PKU) is to control hyperphenylalaninemia by phenylalanine dietary restriction[1]. Patients with persistent plasma levels of phenylalanine >6 mg/dL (360µmole/L) should be treated [2]. Continuation of this dietary restriction during the whole life is necessary for optimal outcomes [3, 4] and families should be trained, as most of the treatment is done by themselves [5]. There are severe problems associated to the management of PKU cases. Mothers of the PKU children have the most important role in helping children take responsibility for their own. A study recommended that illiteracy of the mothers decreased the rate of compliance with the rigid and long-term dietary treatment [6]. Socioeconomic, psychosocial and emotional factors and health care system can be barriers to dietary compliance in PKU patients too [7].
There are a lot of differences in health services for PKU patients between Iran and other countries such as providing free supplements for patients. As there are not accurate data on family social status of these patients, this study is performed to evaluate family social status and dietary adherence of Iranian PKU patients. We hope by this research, we were able to realize some barriers of dietary compliance in Iran.
Subjects and Methods
In this cross-sectional study, we studied all 105 treated PKU patients (46 males, 59 females, born between 1984 to 2010) diagnosed by newborn screening, or later, and followed at the metabolism clinic of Mofid Children's Hospital, which is a referral children's hospital in Tehran, Iran. We considered a patient to have PKU if plasma phenylalanine concentrations were more than 6 mg/dl in the untreated newborn infant/ child [2].
Social status was defined by number of children in family, number of affected children in family, maternal and paternal education, marital status and employment status of the parents. Age at diagnosis and duration of treatment were also recorded.
The educational level of parents was classified into four groups of illiterate, primary school, high school, and higher education (which includes university education or passing any courses after graduating from high school). The marital status was classified into divorced or non divorced, and employment status into employed or unemployed.
Dietary Phenylalanine intake, was adjusted to achieve blood phenylalanine levels between 2-6 mg/dl in children less than 12 years old and between 2-10 mg/dl in patients more than 12 years old[2]. This level of control was considered as appropriate control, and blood phenylalanine less than 2 mg/dl was considered as overtreatment.
Mean plasma phenylalanine level was considered as a sign of dietary adherence in PKU patients and understanding the factors that might have an effect on it. Therefore, it could help us in promoting the treatment and supporting the families.
Plasma phenylalanine was measured by fluorometeric method which is a quantitative and precise method [2]. The mean plasma phenylalanine for each patient was calculated, considering the phenylalanine measurements throughout at least one year.All the information was collected via interviewing by the same blinded interviewer after informed parents consented orally with the aim of this study.
Anova t-test was done to understand the association between educational levels of parents and blood phenylalanine levels. Independent t-test was done to assess the association between employment status of parents and phenylalanine levels. Mann-Whitney U test was done to understand the association between marital status of parents and phenylalanine levels. Pearson correlation test was performed to assess the correlation between number of children in family, affected children in family, age at diagnosis, treatment duration and Phenylalanine levels. Statistical significance was based on P-value <0.05.
Findings
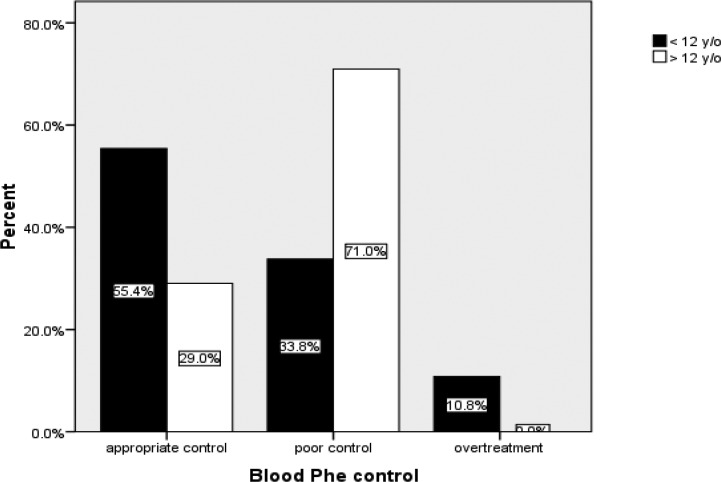
The mean plasma Phenylalanine concentration was 8.06±4.9 mg/dl (range 1.13 to 23.60 mg/dl). It was 5.9±3.6 mg/dl in patients <12 y/o and, 13.1±3.9 mg/dl in patients >12 y/o. 50 (47.6%) patients kept their mean plasma phenylalanine concentrations in normal range. The rest of the patients had mean plasma phenylalanine concentrations above age related reference range (44.8%), or under the normal range (<2 mg/dl, 8%) as overtreatment. Fig. 1 shows the percentage of patients classified in two age groups.
Fig. 1.
Comparison of dietary control between two age groups
We couldn't find any significant association between four levels of maternal or paternal education and mean blood phenylalanine levels (P=0.34 and P=0.33 respectively). Table 1 displays mean phenylalanine concentrations in each group of parental educational level.We had only 3 divorced parents. The mean rank of phenylalanine in their children was 92 and it was 51.8 in the rest of cases (mean plasma phenylalanine concentration in patients with divorced and not divorced parents was 14.56 mg/dl and 7.86 mg/dl respectively). There was a significant increase in mean phenylalanine rank of patients whose parents were divorced (P=0.02). 17 of 105 patients had at least one unemployed parent (the one who was responsible for the family income). The mean phenylalanine in the unemployed and employed group was 10.36mg/dl and 7.61mg/dl respectively. This increase in the mean blood phenylalanine concentration of unemployed group was significant (P=0.03).
Table 1.
Parental educational level and mean phenylalanine concentrations in each group
| Education level | Maternal Education | Paternal Education | ||
|---|---|---|---|---|
| Frequency | Phe Means mg/dl | Frequency | Phe Means mg/dl | |
| illiterate | 5 (4.8%) | 5.4 | 1 (1%) | 8.6 |
| primary school | 18 (17.1%) | 9.6 | 19 (18.1%) | 7.5 |
| high school | 72 (68.6%) | 7.9 | 69 (65.7%) | 8.6 |
| higher education | 10 (9.5%) | 7.8 | 16 (15.2%) | 6.2 |
| Total | 105 (100) | 105 (100) | ||
Phe: phenylalanine
There was a significant positive correlation between the age at diagnosis and the mean blood phenylalanine (r=0.2, P=0.03) of the patients. Also, there was a significant positive correlation between treatment duration and mean blood
phenylalanine (r=0.7, P<0.001). There was a positive correlation between number of children in the family and patients' mean blood phenylalanine, although not significant (r=0.1, P=0.08).
There was a significant positive correlation between the number of affected patients in a family and mean blood phenylalanine (r=0.43, P<0.001). Table 2 shows the central tendency and dispersion of the above variables.
Table 2.
Mean (SD), Median and range of age of diagnosis, treatment duration, Number of children in family and affected children in family of 105 cases with phenylketonuria
| Parameters | Mean (SD) | Median | Range |
|---|---|---|---|
| Age of Diagnosis / Month | 18.84 (26.56) | 11.00 | 0.5–168 |
| Treatment duration / Year | 7.07 (6.60) | 4.75 | 0.08–23.50 |
| Number of children in family | 1.99 (0.97) | 2.00 | 1–4 |
| Affected children in family | 1.27 (0.59) | 1.00 | 1–3 |
Discussion
It seems that younger patients (<12 y/o) in our study, had a better dietary control. They have kept the mean phenylalanine concentrations below 6 mg/dl. In contrast, averagely 12 year-old patients did not control their plasma phenylalanine concentrations properly. Gokmen Ozel et al also found the blood phenylalanine concentration higher in school-age children than in preschool children [8]. In a study of Crone et al, mean Phenylalanine was kept on constant concentrations until 13 years old, but increased after 13 [9]. In another study during the first year of life, average blood phenylalanine levels were <5 mg/dl in 45% of cases. However, some of children with good metabolic control moved to poor control section during the mentioned study [6].
Of societal barriers in dietary adherence, especially in adolescents, we could mention to "peer pressure" as stated in Nevins' study[10]. Therefore it is much easier to control children, in the preschool ages than in elder ages, when the children are in open area of school and are going to be autonomous.
Caregivers' low educational level could be one of the factors affecting dietary adherence [11]. There was an association between higher knowledge in parents and lower phenylalanine concentrations in a study of Bekhof et al. Also, patient's age and parental educational level were effectively related to blood phenylalanine concentrations [12]. Mothers' illiteracy was a negative factor on dietary compliance in Ozalp et al study [6]. MacDonald et al, believe that dietary knowledge is an essential factor on dietary compliance [13]. In contrast, Gokmen Ozel et al found that there was a non significant negative correlation between maternal total knowledge score (r=−0.140, P=0.093), and median blood phenylalanine concentration [8]. Olsson et al found parents' educational level was not statistically significant related to blood phenylalanine concentrations [14]. MacDonald et al found was a positive but not significant correlation between higher knowledge of mothers and lower blood phenylalanine levels in their children (r= 0.27, P=0.06) [11]. We also, found no relation between the four groups of maternal or paternal educational level and patients' plasma phenylalanine concentrations. This may be due to nutrition specialists who train families and increase parents' knowledge independently from their education. We suppose, in comparison with educational level, having precise dietary knowledge is a more vital demand of families and patients.
In spite of very slight number of divorced parents (3 of 105) in our study, dietary adherence in these cases was significantly poorer than non divorced ones. Also, Olsson et al found divorced parents had more difficulty in controlling their children's phenylalanine level [14]. This result apparently leads to the consequence that child neglect happens more common in divorced families.
Phenylalanine levels were not affected by parental employment status in a study of MacDonald et al. In contrary, Gokme Ozel et al realized that currently employed mothers had more knowledge than unemployed ones. In our study unemployment of the parents, who were responsible for family income, significantly impressed the patients' phenylalanine levels. Although the specific "Phenylalanine free" formulas are given at no cost to the families in our referral hospitals, these unemployed families are under pressure to afford many other requirements of their children.
We found, blood phenylalanine concentrations did not raise significantly by increase in family members. As well, a study found weakening in children's care giving in thickly populated families, also they found maternal knowledge was lower in large families [8]. But MacDonald et al found that phenylalanine levels were not affected by family size. Quite the opposite, a study accepted that large families could improve the dietary compliance by food shopping, cooking and supporting each other [15]. By increasing number of affected children in the family we had an increase in blood phenylalanine concentrations. It seems that by increasing affected children in the family there will be more financial pressure on them, because in contrast with many other countries, secondary services like special foods or supplements are not available free of charge for them.
Since there was a positive correlation between age at diagnosis and phenylalanine levels in our study, it looks that by a late diagnosis there is an increase in plasma phenylalanine levels. Besides, late diagnosis causes more complications including physical and mental retardation that could be another barrier for dietary compliance.
The mean Family income reported by parents was below the poverty line. Therefore, we did not insert the data concerning family income to the study, because most of the families have various sources of income and their report might not be valid.
The interesting finding in our study was the positive correlation between duration of treatment and phenylalanine levels. It looks as if growing up does not lead to better dietary compliance, but it may be due to some kind of refractory behavior in these children.
Conclusion
Social status affects dietary adherence by some means. Although, increasing in blood phenylalanine levels in elder patients could have many explanations, spending more time on training families and patients themselves, might lead to understanding the importance of dietary control and its continuation during whole life. Consulting the families to have better contraception and have less affected children in family, financial and emotional support especially for divorced parents can help them to have a better dietary adherence. Finally sending children to special PKU schools could help them to avoid peer pressure.Consequently, finding the level of dietary knowledge in patients and their caregivers could be the next step.
Acknowledgment
We gratefully thank Maryam Beheshti and Maryam Azizi for their valuable support as dieticians.
Conflict of Interest
None
References
- 1.Blau N, Duran M, Blaskovics ME, Gibson KM, editors. Berlin Heidelberg New York: Springer-Verlag; 2003. Physician's Guide to the Laboratory Diagnosis of Metabolic Diseases. [Google Scholar]
- 2.Rezvani I. Defects in metabolism of amino acids. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson Textbook of Pediatrics. 18th edn. Philadelphia: Saunders-Elsevier; 2007. pp. 529–567. [Google Scholar]
- 3.National Institutes of Health Consensus Development Conference Statement: Phenylketonuria: screening and management, October 16-18, 2000. Pediatrics. 2001;108(4):972–82. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- 4.Bosch AM, Tybout W, van Spronsen FJ, et al. The course of life and quality of life of early and continuously treated Dutch patients with phenylketonuria. J Inherit Metab Dis. 2007;30(1):29–34. doi: 10.1007/s10545-006-0433-6. [DOI] [PubMed] [Google Scholar]
- 5.Blau N, Hoffmann GF, Leonard J, Clarke JTR. Physician's Guide to the Treatment and Follow-Up of Metabolic Diseases: Springer-Verlag Berlin Heidelberg New York. 2006;18:416–17. [Google Scholar]
- 6.Ozalp L, Coskun T, Okatli AT, et al. The influence of socioeconomic and cultural factors on compliance with dietary treatment, and growth and development in PKU children. J Inher Metab Dis [Abstract] 1998;21(Suppl 3):7–8. [Google Scholar]
- 7.MacDonald A. Diet and compliance in phenylketonuria. Eur J Pediatr. 2000;159(Suppl 2):S136–41. doi: 10.1007/pl00014375. [DOI] [PubMed] [Google Scholar]
- 8.Gokmen Ozel H, Kucukkasap T, Koksal G. Does maternal knowledge impact blood phenylalanine concentration in Turkish children with phenylketonuria? J Inherit Metab Dis. doi: 10.1007/s10545-008-0775-3. (In Press) [DOI] [PubMed] [Google Scholar]
- 9.Crone MR, van Spronsen FJ, Oudshoorn K, et al. Behavioural factors related to metabolic control in patients with phenylketonuria. J Inherit Metab Dis. 2005;28(5):627–37. doi: 10.1007/s10545-005-0014-0. [DOI] [PubMed] [Google Scholar]
- 10.Nevins TE. Why do they do that?” The compliance conundrum. Pediatr Nephrol. 2005;20(7):845–8. doi: 10.1007/s00467-005-1926-5. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald A, Davies P, Daly A, et al. Does maternal knowledge and parent education affect blood phenylalanine control in phenylketonuria? J Hum Nutr Diet. 2008;21(4):351–8. doi: 10.1111/j.1365-277X.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- 12.Bekhof J, van Spronsen FJ, Crone MR, et al. Influence of knowledge of the disease on metabolic control in phenylketonuria. Eur J Pediatr. 2003;162(6):440–2. doi: 10.1007/s00431-003-1197-8. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald A, Gokmen-Ozel H, van Rijn M, et al. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis. 2010;33(6):665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- 14.Olsson GM, Montgomery SM, Alm J. Family conditions and dietary control in phenylketonuria. J Inherit Metab Dis. 2007;30(5):708–15. doi: 10.1007/s10545-007-0493-2. [DOI] [PubMed] [Google Scholar]
- 15.Ipsiroglu OS, Herle M, Spoula E, et al. Transcultural pediatrics: compliance and outcome of phenylketonuria patients from families with an immigration background. Wien Klin Wochenschr. 2005;117(15-16):541–7. doi: 10.1007/s00508-005-0327-x. [DOI] [PubMed] [Google Scholar]