Abstract
Schizophrenia has been increasingly conceptualized as a disorder of brain connectivity, in large part due to findings emerging from white matter and functional connectivity (FC) studies. This work has focused primarily on within-hemispheric connectivity, however some evidence has suggested abnormalities in callosal structure and interhemispheric interaction. Here we examined functional connectivity between homotopic points in the brain using a technique called voxel-mirrored homotopic connectivity (VMHC). We performed VMHC analyses on resting state fMRI data from 23 healthy controls and 25 patients with schizophrenia or schizoaffective disorder. We found highly significant reductions in VMHC in patients for a number of regions, particularly the occipital lobe, the thalamus, and the cerebellum. No regions of increased VMHC were detected in patients. VMHC in the postcentral gyrus extending into the precentral gyrus was correlated with PANSS Total scores. These results show substantial impairment of interhemispheric coordination in schizophrenia.
Keywords: Schizophrenia, resting state, interhemispheric, MRI
1. Introduction
Interhemisphereric interaction is primarily mediated by the brain’s commisural system, including the corpus callosum, anterior commissure, posterior commissure, interthalamic adhesions, and cerebellar commissures (Hoptman and Davidson, 1994). Most callosal fibers connect homotopic regions across the two hemispheres (LaMantia and Rakic, 1990). Resting state fMRI (rsfMRI) is a technique that permits assessment of inter- as well as intrahemispheric functional connectivity (FC). The present study uses rsfMRI to assess the integrity of interhemispheric interaction in schizophrenia.
Since the early 1960s, it has been known that humans who have had forebrain commissures sectioned, including the corpus callosum, show disconnection phenonemena on divided sensory spatial field tasks (Gazzaniga et al., 1962; Geschwind and Kaplan, 1962; Nebes, 1972). Deficits in sustained attention (Dimond, 1979a; Dimond, 1979b) and other cognitive processes have also been observed in split brain patients. The bulk of studies on laterally presented cognitive tasks in healthy individuals suggest that there is advantage to bihemispheric processing (Belger and Banich, 1992; Banich and Belger, 1990), provided that each hemisphere has competence at the given task. Moreover, interhemispheric interaction may be particularly important in the strategic deployment of attentional resources in response to task demands (Levy and Trevarthen, 1976).
Within sensory regions, Sergent and Bindra (Sergent and Bindra, 1981) proposed that the left visual hemisphere is biased towards processing of high spatial frequencies, whereas the right hemisphere is more specialized for low spatial frequencies. This distinction has been mapped onto a local/global dimension (Ivry and Robertson, 1998; Robertson and Ivry, 2000). The right hemisphere also appears to be specialized for line orientation (Umilta et al., 1974), line bisection (Bowers and Heilman, 1980; Foxe et al., 2003), and mental rotation (Robertson et al., 1987). Given the advantages for interhemispheric interaction and the dysconnectivity processes implicated in schizophrenia, it would not be surprising if interhemispheric interaction deficits played an important role in the cognitive deficits, psychiatric symptoms, and sensory abnormalities seen in the disorder. Thus, deficits in interhemispheric interaction may contribute to sensory/cognitive impairments in schizophrenia.
The evidence for interhemispheric interaction deficits in schizophrenia predates recent conceptualizations of schizophrenia as a disorder of dysconnectivity (Friston and Frith, 1995; Bullmore et al., 1997; Stephan et al., 2009) and is fairly consistent, despite the fact that it has been underemphasized. Early postmortem studies found increased callosal thickness (Bigelow and Rosenthal, 1972), but more recent postmortem studies (Casanova et al., 1990), and the vast majority of in vivo imaging studies have found reduced callosal thickness or length, and/or altered shape in schizophrenia (Woodruff et al., 1995; Arnone et al., 2008). White matter density abnormalities in the corpus callosum also have been observed (Hulshoff Pol et al., 2004). Moreover, Highley et al. found reduced fiber density in the anterior commissure in women, but not men, with schizophrenia (Highley et al., 1999).
A number of diffusion tensor imaging (DTI) studies have found reduced fractional anisotropy (FA) in the corpus callosum in schizophrenia (Ardekani et al., 2003; Kubicki et al., 2008). It is likely that DTI studies understate the extent of callosal deficits in schizophrenia, in part because they are typically plagued by low image resolution. Many DTI sequences are obtained in the axial plane, which compounds this problem because it maximizes partial volume effects in the callosum. Indeed, in voxelwise studies, Dougherty noted that FA effect sizes are smallest in the corpus callosum (Dougherty et al., 2005), most likely because its thin dorsoventral extent is particularly susceptible to intersubject registration errors and partial volume effects. Nonetheless, recent DTI work has suggested interhemispheric hypoconnectivity in patients with schizophrenia and their relatives (Knochel et al., 2012; Whitford et al., 2010), which in turn predict interhemispheric transfer time (Whitford et al., 2011) and psychotic symptoms (Whitford et al., 2010).
Relatively few behavioral studies have investigated functional interhemispheric interaction in schizophrenia. The Poffenberger (Poffenberger, 1912) paradigm has long been used to measure interhemispheric dynamics. In this task, simple stimuli are presented either ipsilateral or contralateral to the response hand. The contralateral – ipsilateral difference in reaction time is taken as an estimate of interhemispheric transfer time, and is typically greater than 0. Patients with schizophrenia showed a prolongation of the ipsilateral advantage (Shelton and Knight, 1984), suggesting impaired interhemispheric transfer efficiency. Another early study showed that patients with schizophrenia are impaired on cross-localization tasks on which patients with callosal agenesis are also impaired (Craft et al., 1987). Patients also show deficits consistent with the callosum’s role in optimizing processing under computationally complex situations. Under such conditions, healthy individuals show an advantage to processing information presented bilaterally compared to the same amount of information presented initially to one hemisphere alone (Belger and Banich, 1992). This bilateral advantage suggests that it can be more efficient for the two hemispheres to interact than for one hemisphere to perform all of the processing. The bilateral advantage is absent in patients with schizophrenia (Barnett et al., 2007), suggesting a deficit in interhemispheric interaction in schizophrenia.
Psychophysiological measures of interhemispheric functional interaction also have been observed to be abnormal in schizophrenia. For example, in a word task, healthy controls show increased event related potential (ERP) amplitudes to bilateral (and identical) stimulation compared to unilateral stimulation. Patients showed a reduction in this “bilateral redundancy gain” (Mohr et al., 2008). In addition, deficits in electroencephalographic (EEG) measures of interhemispheric alpha band coherence have been found in patients with schizophrenia (Morrison-Stewart et al., 1996) and in individuals at genetic risk for schizophrenia (Winterer et al., 2001). Another longitudinal study found that reduced symptom severity in schizophrenia was associated with higher EEG beta coherence (Higashima et al., 2006), although some studies have found higher interhemispheric coherence in both never-medicated patients with schizophrenia (Wada et al., 1998) and in siblings of patients with schizophrenia (Mann et al., 1997).
Despite the evidence of interhemispheric interaction deficits in schizophrenia, there are no studies examining FC between homotopic brain sites, which are the gray matter regions that are connected by commissural fibers. Here we evaluated interhemispheric resting state FC in patients with schizophrenia. We used a measure, voxel-mirrored homotopic connectivity (VMHC; (Zuo et al., 2010), in which the time series for each voxel in one hemisphere was correlated with that of its homotopic voxel (i.e., from the other hemisphere). Similar methodologies have shown deficits in interhemispheric functional connectivity in autism (Anderson et al., 2011), showing that the method is sensitive to abnormal interhemispheric FC in psychopathology. Given the extensive evidence of both structural and functional disconnections in schizophrenia, we expected to observe reductions in homotopic connectivity in schizophrenia.
Methods
1.1. Participants
Participants were 25 healthy controls and 28 patients who met DSM-IV-TR (American Psychiatric Association, 2000) criteria for schizophrenia or schizoaffective disorder (n=4) after a Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient version (SCID-I/P (First et al., 2002)). Controls had no major Axis I disorders as determined with the SCID-I/NP (First et al., 2001). Additional details are reported in Hoptman et al. (Hoptman et al., 2010b; Hoptman et al., 2010a). Patients with head injuries with a loss of consciousness of greater than 20 minutes were excluded, as were patients with neurological disorders noted. In addition, participants with substance abuse diagnoses in the last month or dependence in the last six months were excluded. Demographic data are given in Table 1. Controls and patients did not differ in age or parental socioeconomic status as measured by the Hollingshead scale. All participants signed informed consent as approved by the local Institutional Review Boards.
Table 1.
Demographics of study participants
| Variable | Controls (N=23) |
Patients (N=25) | t | p |
|---|---|---|---|---|
| Age (years) | 41.6 ± 11.4 | 35.5 ± 10.8 | −1.88 | .07 |
| Education (years) | 15.4 ± 2.9 | 12.4 ± 2.1 | −4.08 | 1.7 × 10−4 |
| Handedness score (right)1 | 16.7 ± 3.9 | 15.5 ± 5.7 | −0.86 | .39 |
| Parental SES2 | 39.8 ± 12.1 | 39.9 ± 14.4 | 0.17 | .99 |
| Illness Duration (years)3 | -- | 11.5 ± 6.6 | -- | -- |
| PANSS Total4 | -- | 76.8 ± 17.0 | -- | -- |
| PANSS Positive4 | -- | 18.6 ± 6.3 | -- | -- |
| PANSS Negative4 | -- | 20.8 ± 6.2 | -- | -- |
| CPZ equivalents (mg) | -- | 819.9 ± 621.8 | -- | -- |
| Χ2 | p | |||
| Gender5 | 16/7 | 22/3 | 2.51 | .16 |
Note: PANSS = Positive and Negative Syndrome Scale, CPZ = chlorpromazine,
From Edinburgh handedness questionnaire (Oldfield, 1971),
From Hollingshead (Hollingshead, 1957),
Defined as age at first psychiatric hospitalization,
missing for 1 patient,
by Fisher’s exact test.
1.2. MRI Acquisition
Scanning took place on the 1.5T Siemens Vision Scanner (Erlangen, Germany) at the Nathan Kline Institute Center for Advanced Brain Imaging. Participants received a magnetization prepared rapidly acquired gradient echo (MPRAGE) T1-weighted scan (TR =11.6 ms, TE = 4.9 ms, TI = 1122 ms, flip angle = 8, matrix = 256 × 256, FOV = 256 mm, slice thickness = 1 mm, 190 slices, no gap, 1 acquisition), and a six minute resting state fMRI scan (TR = 2000 ms, TE = 50 ms, flip angle = 85, matrix = 64 × 64, FOV = 224 mm, 5 mm slice thickness, 22 slices, no gap, 180 acquisitions). For the resting state scan, participants were instructed to close their eyes and remain awake.
1.3. Data Analysis
Resting state data were preprocessed as described elsewhere in detail (Margulies et al., 2007; Hoptman et al., 2010b). Briefly, the first 10 volumes were discarded to eliminate T1 relaxation effects. Thereafter, images were motion-corrected using AFNI (Cox, 1996). Time series were smoothed using a 6 mm FWHM Gaussian kernel using FSL, and grand mean scaled to a value of 10000 using FSL. Subsequently, the data were bandpass filtered (0.005 Hz–0.1 Hz) and linear and quadratic trends were removed using AFNI. They were then spatially normalized to MNI space (2×2×2 mm3 resolution) using FSL (www.fmrib.ox.ac.uk/fsl). The MPRAGE image was segmented using FSL’s FAST software to obtain the masks for white matter (WM) and cerebrospinal fluid (CSF). The time series for CSF and WM were then averaged across voxels for each tissue type. These time series, as well as the global signal time series and the time series for the six motion parameters were used as covariates of no interest in a general linear model (GLM). These were regressed out from the MNI-space EPI time series. The above steps were carried out using the FCONN scripts (version 1.1), which are part of the 1000 functional Connectomes project and are available at http://www.nitrc.org/projects/fcon_1000/ (Biswal et al., 2010). The residual time series for each subject was registered to a symmetric MNI template and was used to compute the homotopic functional connectivity.
Methods for computing VMHC are described in (Zuo et al., 2010). For each participant, homotopic functional connectivity was computed as the resting state functional connectivity (RSFC) between any pair of symmetric inter-hemispheric voxels. Specifically, in symmetrical MNI brain space, we calculated the Pearson’s correlation coefficient between each voxel’s residual time series and that of its symmetrical interhemispheric counterpart. The resultant values were referred to as the VMHC and were used for subsequent group-level analyses. The analyses were masked by a gray matter segmentation of the symmetric MNI template (thresholded at 40%). Global VMHC was extracted as average of voxel-wise VMHC within the gray matter mask for each subject and was used as a covariate in the analyses described below. In some cases, whole brain coverage was not possible, so computations were limited to voxels for which all subjects had data.
Group-level analyses were conducted using FSL’s ordinary least squares (OLS) model implemented in FLAME. Two-sample t-tests on VMHC maps between patients and controls were performed to examine the differences in VMHC between the two groups. This statistical procedure produced thresholded z-statistic maps of clusters defined by a threshold of Z = 2.3 and a corrected cluster threshold of p = .05 using Gaussian Random Field theory (Worsley, 2001). The resultant maps revealed brain regions showing significantly different VMHC between patients and healthy controls. Local maxima were identified using the AFNI program 3dmaxima, using a search radius of 15 voxels.
Because small amounts of movement from volume to volume can influence RSFC results (Power et al., 2012a; Power et al., 2012b), we computed framewise displacement (FD) for our data, which corresponds to the temporal derivative of the movement parameters. These were used as covariates in the group level analyses. Three patients (one with schizoaffective disorder) and three controls had FD > 0.5 mm on greater than 35 volumes (i.e., less than 4.8 min of useable data) and were eliminated from final analyses. Mean FD did not differ between patients (M ± SD = 0.22 ± 0.08 mm) and controls (0.20 ± 0.09 mm) included in the final sample (t[46] = 0.50, p = .62).
2. Results
2.1. Group differences
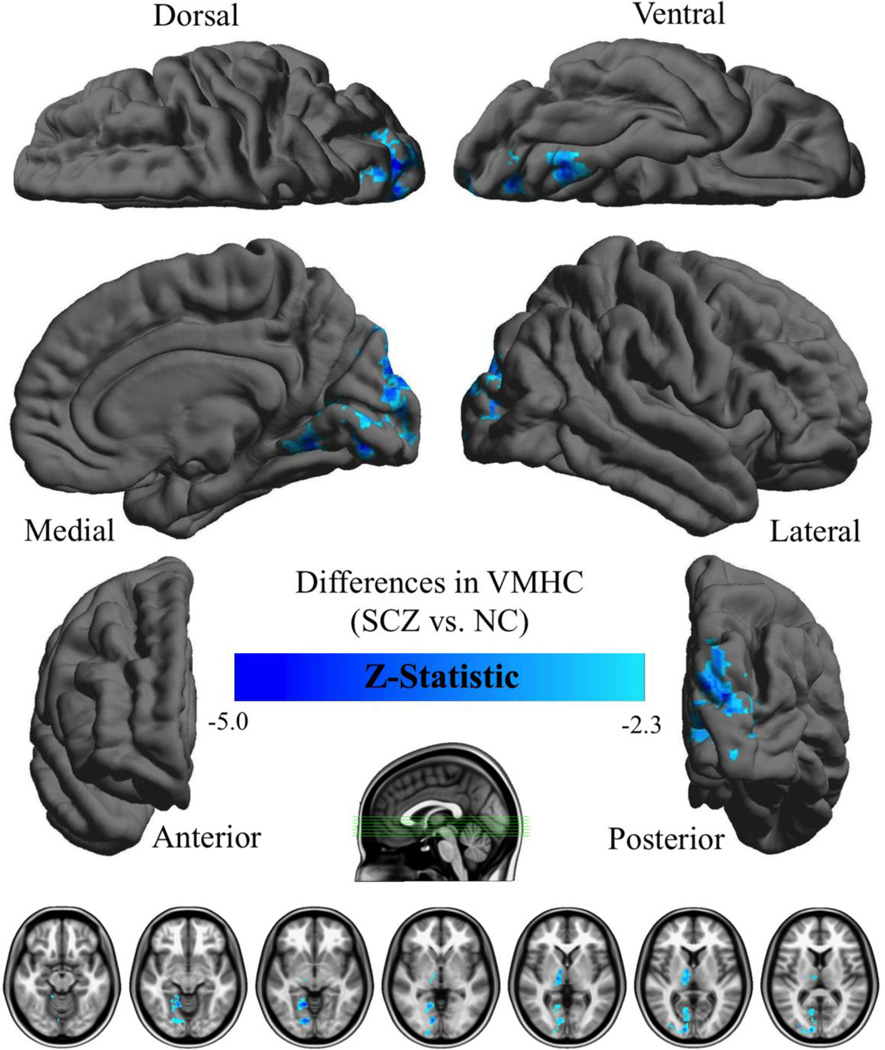
Results are shown in Table 2 and Figure 1. Patients showed deficits in VMHC in a large spatial extent, primarily between left and right lingual gyri, cuneus, thalamus, and the declive of the cerebellum. No areas showed increased VMHC in patients. We also examined the results excluding patients with schizoaffective disorder, where results were essentially the same as in the larger analysis (see Supplementary Figure 1).
Table 2.
Regions showing group differences in voxel-mirrored homotopic connectivity
| Talairach Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Voxels | Z1 | BA2 | X | Y | Z |
| Lingual gyrus/culmen | 1335 | 4.97 | 19 | ±14 | −53 | −4 |
| Cuneus | -- | -- | 18 | ±18 | −80 | 24 |
| Thalamus | 226 | 4.07 | -- | ±8 | −6 | 4 |
| Declive of cerebellum | 459 | 4.65 | -- | ±36 | −67 | −20 |
Note.
Maximum z-statistic
BA = Brodmann area
Figure 1.
Surface renderings of group differences in homotopic voxel-mirrored connectivity (VMHC). Depicted are regions in which VMHC was greater in healthy controls than patients. Group differences were based on clusters defined by Z > 2.3 and a corrected cluster threshold of p = .05 (Gaussian Random Field Theory corrected). The final Z-statistic maps are visualized as six hemispheric surfaces (cortical regions) and seven symmetric axial slices (subcortical regions).
Interhemispheric interaction can vary with strength and consistency of handedness (Hoptman and Davidson, 1994). To examine this issue, we repeated the group analyses limited to only right-handers. Results were essentially identical to those reported above.
The role of medication dosage in interhemispheric interaction deficits in schizophrenia is unknown. We thus examined the correlation between chlorpromazine (CPZ) equivalents and VMHC within the mask containing all voxels found to have reduced connectivity. This correlation was not significant, r = .01, p = .95, suggesting that it is unlikely that our results can be attributed to a neuroleptic dosage effect. To examine effects of medication effects on VMHC, we computed a multiple regression analysis in patients only with global mean VMHC and FD as covariates of no interest and medication dosage as a covariate of interest. Medication dosage did not correlate with VMHC in that analysis. Moreover, VMHC remained significantly greater than 0 throughout the entire brain after controlling for that variable. Lastly, illness duration correlated negatively with VMHC within the mask containing all voxels found to have reduced connectivity, r = −.54, p = .012. Interestingly, despite this region-level relationship, voxel-wise multiple regression analyses found a positive relationship between illness duration and VMHC in a medial dorsal nucleus region (Talairach coordinate [6,-10, 4]; see supplementary Figure 2) that slightly overlapped (3%) with the thalamic cluster found to have reduced VMHC in patients. There were voxels with significant negative correlations within the deficit region, as would be expected, though they did not survive statistical correction with Gaussian Random Fields. Nonetheless, VMHC remained significantly greater than 0 throughout the brain despite controlling for illness duration.
We also examined the relationship between PANSS scores and VMHC. To do so, we computed separate multiple regression analyses in the patients group with PANSS Positive, Negative, General, and Total scores as covariates. For the PANSS Total score, we found a negative correlation between VMHC and symptomatology in the postcentral gyrus extending into precentral gyrus (Talairach coordinate [48,-14, 32]). The other scales did not show significant positive or negative correlations with VMHC. An examination of correlations at subthreshold levels of significance for the other scales suggested that those with the General subscale were closest to significance.
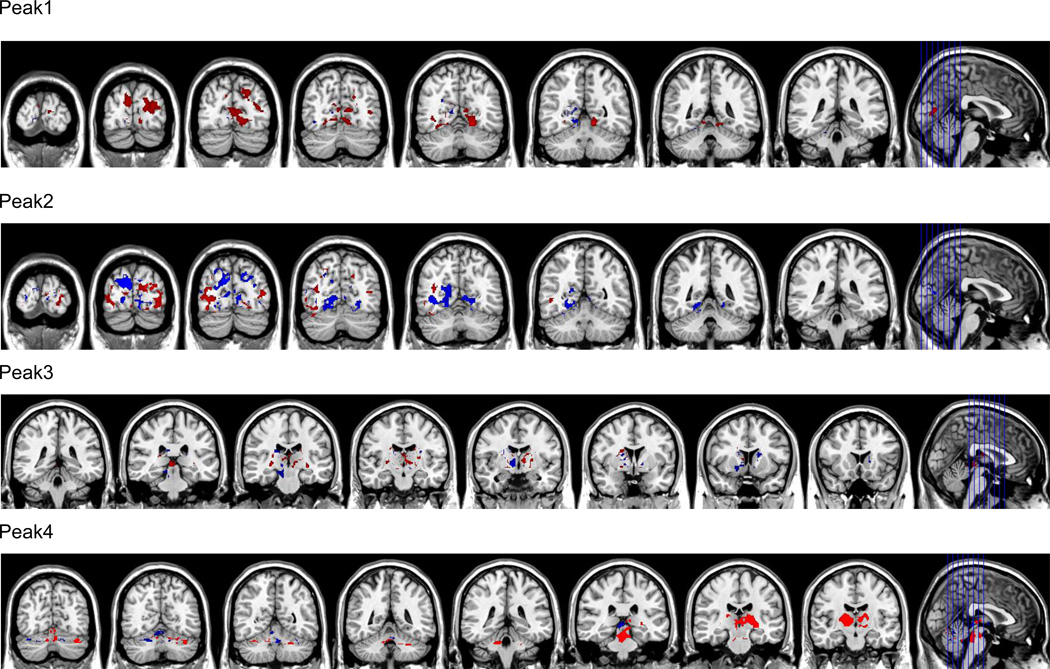
In order to determine whether deficits in VMHC could be attributed to abnormalities in RSFC in one hemisphere or the other, we performed separate GLMs on the residualized time series data in nonsymmetric MNI space, with global VMHC and FD as covariates. Seeds (spheres with 4 voxel radius) for these analyses were generated based on each local maximum and its homotopic homolog. These were thresholded at p = .05 (corrected for Gaussian Random Fields). To visualize these results, for each seed pair, we computed the right-left asymmetry in positive FC in the group difference region (see Figure 2). These analyses showed that both hemispheres were involved in the observed effects.
Figure 2.
Asymmetries in functional connectivity as seeded for local maxima (left and right) of group differences shown in Figure 1. Seeds were placed in left and right hemispheres based on maxima shown in Table 1. Asymmetries were computed as the right – left difference in positive FC in the group difference region for each of the peak pairs. Peak 1 = lingual gyrus, Peak 2 cuneus, Peak 3 = thalamus, Peak 4 = declive of cerebellum. Data are shown in radiological convention, with every 8th slice depicted (red = right > left, blue = left > right).
3. Discussion
The primary finding of this work is that the correlation between homologous brain regions was reduced in patients with schizophrenia and schizoaffective disorder. These reductions encompassed large areas, primarily including occipital regions, and the thalamus, as well as the cerebellum. These findings are consistent with literature reviewed above showing abnormalities in interhemispheric interaction in schizophrenia using a variety of methods including behavioral, psychophysiological, and structural MRI investigations. We also found that higher PANSS Total scores were correlated with lower VMHC in postcentral gyrus extending into the precentral gyrus, suggesting that this measure might have implications for psychopathology.
The lack of frontal results is interesting given that anterior white matter abnormalities have been emphasized in the schizophrenia literature (Ellison-Wright et al., 2008). It may be that these will be detected at higher field strength as discussed below. Because the corpus callosum is organized topographically (Hoptman and Davidson, 1994), the findings may have implications for anatomical differences in the callosum, such that more posterior regions might be differentially affected, although this idea awaits confirmation from concurrent structural imaging studies.
On the other hand, many of the regions in which we showed reduced VMHC were in posterior sensory areas. Patients with schizophrenia also show deficits in relatively low level visual sensory functions, including contrast sensitivity (Butler et al., 2008) and P1 event related potential componentry (Foxe et al., 2001). These low-level deficits appear to upwardly generalize to other cognitive deficits seen in schizophrenia, such as perceptual closure (Doniger et al., 2001; Sehatpour et al., 2010), visual attention (Martinez et al., 2007; Martinez et al., 2011), and controlled visual processing (Dias et al., 2011). It may be that deficits in VMHC in higher-level areas are in part due to feed-forward unilateral projections from lower-level sensory areas in addition to direct interhemispherically mediated influences. Thus, although the role of impaired interhemispheric interaction has not been investigated in these paradigms, it could contribute at both sensory and anterior levels. Future studies should address VMHC deficits relative to specific aspects of cognitive dysfunction.
At a more general level, behavioral studies of interhemispheric interaction suggest that it can be more efficient for the two hemispheres to interact than for one hemisphere to perform all of the processing (Belger and Banich, 1992; Banich and Karol, 1992). Deficits in interhemispheric interaction in schizophrenia therefore present a cognitive processing bottleneck in the disorder. A related issue is that interhemispheric interaction appears to be particularly important in the strategic deployment of attentional resources in response to task demands (Levy and Trevarthen, 1976) and in sustained attention (Dimond, 1979b). Thus, a deficit in interhemispheric interaction might be related to some of the attentional problems shown in schizophrenia (Nuechterlein et al., 2009).
In the present study, we also found VMHC deficits involving the thalamus, a region that has also been implicated in schizoprenia based upon structural imaging (Andreasen et al., 1994) and postmortem studies (Byne et al., 2002); see (Dwork et al., 2008) for a review). However, because deficits in this region partially overlapped with a region that correlated with illness duration, the potential role of chonicity needs to be clarified.
We found a negative correlation between PANSS Total scores and VMHC in the postcentral gyrus extending into the precentral gyrus. Although this correlation was not within the region in which patients showed deficits in VMHC, it may suggest that reductions in this measure have implications for psychiatric symptomatology. The scale with the clearest relationship to VMHC was the General Psychopathology subscale, which has many items, including tension, posturing, motor retardation, and impulse control that relate to motor functions. Top down interactions in post/precentral regions may contribute to symptoms such as hallucinations (Whitford et al., 2012a). Moreover, motor cortex may contribute to "active sensing" of the environment, which contributes to social engagement (Schroeder et al., 2010), which also is problematic in the disorder. Clearly, however, the relationship between VMHC and symptomatology awaits further explication with larger samples.
The mechanism underlying these deficits in VMHC is unknown. They could be related to widespread white matter integrity abnormalities observed in schizophrenia (White et al., 2009). Deficits in white matter connectivity in the corpus callosum could disrupt the synchrony between homotopically connected regions because neural signals are not transmitted with fidelity. Another, not mutually exclusive, explanation is that dysfunctions in local gray matter structure could account for the deficits. Reductions in cortical thickness (Goghari et al., 2007) and gray matter volume (Shenton et al., 2001) have been observed in many of the regions in which we observed abnormal VMHC. Thus, reduced neuropil or aberrant local oscillatory firing within these regions may disrupt coherent low frequency oscillatory activity and/or its generation in one region, and thereby, impair its functional connectivity with other regions. Finally, when considering the structural underpinnings of VMHC, it is important to note that although the callosum is the largest conduit for information transfer and coordination between the hemispheres, alternative pathways (e.g., subcortical) exist. In support of this notion, a recent examination of RSFC in a split brain patient (Uddin et al., 2008) found correlated spontaneous activity in homotopic regions within the visual system and default mode network, despite the lack of any intact commissural fibers. Which of these, or other, explanations accounts for the present findings awaits combined functional/structural studies.
It is unlikely that the results presented here can be accounted for by differences in hand preference or medication dosage, as VMHC in deficit regions was not correlated with these variables. It also appears that both hemispheres contributed to the observed effects, because when we examined the asymmetry of RSFC for local maxima in our data (on both sides of the midline), both leftward and rightward asymmetries were observed.
The study has several limitations. All patients were chronic and were taking antipsychotic medication. Indeed, there were correlations between illness duration and VMHC in this sample. It is possible that drug-naïve, first episode patients would show different results. It should be noted in this regard that abnormal callosal thickness in the genu (Walterfang et al., 2008) and white matter integrity in the splenium (Cheung et al., 2008) have been found in first-episode patients, including in never-medicated subjects. Negative findings also have been reported, although as noted in the Introduction, DTI scans have typically not been optimized to study the corpus callosum, and the more recent literature is more consistent (Knochel et al., 2012; Whitford et al., 2010; Whitford et al., 2012b). Abnormalities have also been shown in the genu of ultra high risk individuals who go on to express the disease (Walterfang et al., 2008). Thus, structural abnormalities in the callosum appear to be present at least at disease onset. We only examined linear relationships in this study. It is possible that slope or nonlinear effects are also present in the data. Also, the structural basis of these findings is unclear, as noted above. Studies using resting state fMRI along with higher resolution DTI and ERP methods would provide critical information on structural and temporal aspects of interhemispheric interaction in the same subjects. Another limitation is that there are existing asymmetries in cortical structure. We attempted to mitigate these issues by using a symmetric template. We should note that in previous (unreported) analyses we had used the standard MNI template available from FSL with essentially identical results. We used a 1.5T scanner for the current study. It is possible that more widespread results might be discovered at higher field, which offers higher signal-to-noise ratios. Finally, whole brain coverage was not always possible in our scans due to technical limitations. It is possible that differences in VMHC might be present in the regions not covered in our scans, which should be studied in further work.
The current results show that interhemispheric resting state fMRI measures of VMHC are reduced in schizophrenia. Moreover, reduced VMHC is associated with higher total PANSS scores in pre/postcentral gyrus. These data are consistent with structural and functional deficits in interhemispheric interaction in schizophrenia. They also suggest an important new avenue to explore in order to better understand the nature of the deficits that are so disabling in patients with schizophrenia.
Supplementary Material
Acknowledgement
We thank Clare Kelly, PhD, for providing the script to compute framewise displacement, and Raj Sangoi (RT)(R)(MR) for his assistance in scanning the participants.
Role of Funding Source
Supported by NIH grants R01MH64783 and R21 MH084031 to MJH, R01 MH066374 to PDB, and R37 MH049334 and P50 MH086385 to DJC, and by grants provided by the National Alliance for Research on Schizophrenia and Depression and gifts from Linda and Richard Schaps, Jill and Bob Smith, and the Taubman Foundation to Francisco Xavier Castellanos. Dr. Xi-Nian Zuo acknowledges support from Startup Foundation for Distinguished Research Professor of Institute for Psychology (Y0CX492S03), the Natural Science Foundation of China (81171409).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report the following conflicts of interest: Dr. Daniel C. Javitt does consulting for Sanofi, Solvay, Pfizer, Lundbeck, AstraZeneca, NPS Pharmaceuticals, Takeda, Sepracor, Schering Plough, Cypress Bio, and Merck. He has research support from Pfizer, and he has equity in Glytech, and AASI. He also serves on the advisory board of Pfizer. Matthew J. Hoptman, Pamela D. Butler, Debra D’Angelo, Cristina J. Mauro, Xi-Nian Zuo, and Michael P. Milham have declared that there are no conflicts of interest in relation to the subject of this study.
Contributors
Matthew J. Hoptman designed the study, undertook the statistical analyses, and wrote the manuscript. Xi-Nian Zuo and Michael P. Milham developed the analytic method. Ms. Mauro assisted in the literature review. All authors contributed to and have approved the final manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, Dubray MB, Lange N, Alexander AL, Abildskov T, Nielsen JA, Cariello AN, Cooperrider JR, Bigler ED, Lainhart JE. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O'Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2008;101:124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Banich MT, Belger A. Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex. 1990;26:77–94. doi: 10.1016/s0010-9452(13)80076-7. [DOI] [PubMed] [Google Scholar]
- Banich MT, Karol DL. The sum of the parts does not equal the whole: evidence from bihemispheric processing. J Exp Psychol Hum Percept Perform. 1992;18:763–784. doi: 10.1037//0096-1523.18.3.763. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Kirk IJ, Corballis MC. Bilateral disadvantage: lack of interhemispheric cooperation in schizophrenia. Consciousness & Cognition. 2007;16:436–444. doi: 10.1016/j.concog.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Belger A, Banich MT. Interhemispheric interaction affected by computational complexity. Neuropsychologia. 1992;30:923–929. doi: 10.1016/0028-3932(92)90036-l. [DOI] [PubMed] [Google Scholar]
- Bigelow L, Rosenthal R. Schizophrenia and the corpus callosum. Lancet. 1972;1:694. doi: 10.1016/s0140-6736(72)90503-x. [DOI] [PubMed] [Google Scholar]
- Biswal B, Mennes M, Zuo XN, Gohel S, Kelly C, Smith S, Beckmann C, Bucker R, Colcombe S, Dogonowski A-M, Ernst M, Hyde JS, Kotter R, McMahon K, Maddon D, Madsen K, Butler PD, Hampson M, Hoptman MJ, Kiviniemi V, Li S-J, Lin C-P, Lowe M, Mayberg H, Peltier S, Petersen S, Raichle M, Rombouts S, Rypma B, Schlagger B, Schmidt S, Siegle GJ, Sorg C, Teng G-J, Villringer A, Walter M, Wang L-H, Whitfield-Gabrieli S, Windishchberger C, Zhang H-Y, Zang Y-F, Castellanos FX, Milham MP. Towards Discovery Science of Human Brain Function: The '1000 Connectomes' Project. Proceedings of the National Academy of Sciences, USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Sanders RD, Goldberg TE, Bigelow LB, Christison G, Torrey EF, Weinberger DR. Morphometry of the corpus callosum in monozygotic twins discordant for schizophrenia: a magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 1990;53:416–421. doi: 10.1136/jnnp.53.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craft S, Willerman L, Bigler ED. Callosal dysfunction in schizophrenia and schizo-affective disorder. J Abnorm Psychol. 1987;96:205–213. doi: 10.1037//0021-843x.96.3.205. [DOI] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiat. 2011;68:654–664. doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimond SJ. Performance by split-brain humans on lateralized vigilance tasks. Cortex. 1979a;15:43–50. doi: 10.1016/s0010-9452(79)80005-2. [DOI] [PubMed] [Google Scholar]
- Dimond SJ. Tactual and auditory vigilance in split-brain man. J Neurol Neurosurg Psychiatry. 1979b;42:70–74. doi: 10.1136/jnnp.42.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC. Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. Am J Psychiatry. 2001;158:1818–1826. doi: 10.1176/appi.ajp.158.11.1818. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch G, Potanina P, Bammer R, Wandell BA. Occipital-callosal pathways in children: Validation and atlas development. Ann N Y Acad Sci. 2005;1064:98–112. doi: 10.1196/annals.1340.017. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Smiley JF, Colibazzi T, Hoptman MJ. Postmortem and in vivo structural pathology in schizophrenia. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2008. pp. 281–302. [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Biometrics Division. New York: New York State Psychiatric Institute; 2001. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders-patient edition (SCID-I/P, 11/2002 revision) Biometrics Research Department. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. NeuroReport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Right hemisphere control of visuospatial attention: line-bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003;19:710–726. doi: 10.1016/s1053-8119(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Gazzaniga MS, Bogen JE, Sperry RW. Some functional effects of sectioning the cerebral commissures in man. Proc Natl Acad Sci U S A. 1962;48:1765–1769. doi: 10.1073/pnas.48.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Kaplan E. A human cerebral deconnection syndrome. A preliminary report. Neurology. 1962;12:675–685. doi: 10.1212/wnl.12.10.675. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- Higashima M, Takeda T, Kikuchi M, Nagasawa T, Koshino Y. Functional connectivity between hemispheres and schizophrenic symptoms: a longitudinal study of interhemispheric EEG coherence in patients with acute exacerbations of schizophrenia. Clinical EEG & Neuroscience: Official Journal of the EEG & Clinical Neuroscience Society (ENCS) 2006;37:10–15. doi: 10.1177/155005940603700104. [DOI] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Roberts HC, Walker MA, Crow TJ. The size and fiber composition of the anterior commissure with respect to gender and schizophrenia. Biol Psychiatry. 1999;45:1120–1127. doi: 10.1016/s0006-3223(98)00323-0. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. 1957 [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AMC, Castellanos FX, Javitt DC, Milham MP. Amygdalofrontal functional connectivity and aggression in schizophrenia. Schizophrenia Bulletin. 2010a;36:1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Davidson RJ. How and why do the two cerebral hemispheres interact? . Psychol Bull. 1994;116:195–219. doi: 10.1037/0033-2909.116.2.195. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, Milham MP. Amplitude of low frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr Res. 2010b;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, Kahn RS. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Robertson LC. The two sides of perception. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, van dV, V, Prvulovic D, Haenschel C, Uhlhaas P, Pantel J, Hampel H, Linden DE. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. NeuroImage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, Kikinis R, McCarley RW, Shenton ME. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophrenia Research. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol. 1990;291:520–537. doi: 10.1002/cne.902910404. [DOI] [PubMed] [Google Scholar]
- Levy J, Trevarthen C. Metacontrol of hemispheric function in human split-brain patients. J Exp Psychol Hum Percept Perform. 1976;2:299–312. doi: 10.1037//0096-1523.2.3.299. [DOI] [PubMed] [Google Scholar]
- Mann K, Maier W, Franke P, Roschke J, Gansicke M. Intra- and interhemispheric electroencephalogram coherence in siblings discordant for schizophrenia and healthy volunteers. Biological Psychiatry. 1997;42:655–663. doi: 10.1016/s0006-3223(96)00497-0. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of Magnocellular Dysfunction on Processing Attended Information in Schizophrenia. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Ramanathan DS, Foxe JJ, Javitt DC, Hillyard SA. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27:7963–7973. doi: 10.1523/JNEUROSCI.0031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr B, Pulvermuller F, Rockstroh B, Endrass T. Hemispheric cooperation--a crucial factor in schizophrenia? Neurophysiological evidence. Neuroimage. 2008;41:1102–1110. doi: 10.1016/j.neuroimage.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Morrison-Stewart SL, Velikonja D, Corning WC, Williamson P. Aberrant interhemispheric alpha coherence on electroencephalography in schizophrenic patients during activation tasks. Psychological Medicine. 1996;26:605–612. doi: 10.1017/s0033291700035674. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Dominance of the minor hemisphere in commissurotomized man on a test of figural unification. Brain. 1972;95:633–638. doi: 10.1093/brain/95.3.633. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophr Bull. 2009;35:182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Poffenberger AT. Reaction time to retinal stimulation with special reference to time lost in conduction through nerve centers. Archives of Psychology. 1912;21:1–73. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012a;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage. 2012b Mar 13; doi: 10.1016/j.neuroimage.2012.03.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Ivry R. Hemispheric asymmetries: attention to visual and auditory primatives. Current Directions in Psychological Science. 2000;9:59–63. [Google Scholar]
- Robertson LC, Palmer SE, Gomez LM. Reference frames in mental rotation. J Exp Psychol Learn Mem Cogn. 1987;13:368–379. doi: 10.1037//0278-7393.13.3.368. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67:772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Bindra D. Differential hemispheric processing of faces: methodological considerations and reinterpretation. Psychol Bull. 1981;89:541–554. [PubMed] [Google Scholar]
- Shelton EJ, Knight RG. Inter-hemispheric transmission times in schizophrenics. Br J Clin Psychol. 1984;23(Pt 3):227–228. doi: 10.1111/j.2044-8260.1984.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Mooshagian E, Zaidel E, Scheres A, Margulies DS, Kelly AM, Shehzad Z, Adelstein JS, Castellanos FX, Biswal BB, Milham MP. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. NeuroReport. 2008;19:703–709. doi: 10.1097/WNR.0b013e3282fb8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta C, Rizzolatti G, Marzi CA, Zamboni G, Franzini C, Camarda R, Berlucchi G. Hemispheric differences in the discrimination of line orientation. Neuropsychologia. 1974;12:165–174. doi: 10.1016/0028-3932(74)90001-3. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nanbu Y, Jiang ZY, Koshino Y, Hashimoto T. Interhemispheric EEG coherence in never-medicated patients with paranoid schizophrenia: analysis at rest and during photic stimulation. Clinical Electroencephalography. 1998;29:170–176. doi: 10.1177/155005949802900408. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Yung A, Wood AG, Reutens DC, Phillips L, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophrenia Research. 2008;103:1–10. doi: 10.1016/j.schres.2008.04.042. [DOI] [PubMed] [Google Scholar]
- White T, Magnotta VA, Bockholt HJ, Williams S, Wallace S, Ehrlich S, Mueller BA, Ho BC, Jung RE, Clark VP, Lauriello J, Bustillo JR, Schulz SC, Gollub RL, Andreasen NC, Calhoun VD, Lim KO. Global White Matter Abnormalities in Schizophrenia: A Multisite Diffusion Tensor Imaging Study. Schizophr.Bull. 2009 Oct 29; doi: 10.1093/schbul/sbp088. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME. Schizophrenia, Myelination, and Delayed Corollary Discharges: A Hypothesis. Schizophr Bull. 2012a;38:486–494. doi: 10.1093/schbul/sbq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Ghorashi S, Schneiderman JS, Hawley KJ, McCarley RW, Shenton ME, Spencer KM. Predicting inter-hemispheric transfer time from the diffusion properties of the corpus callosum in healthy individuals and schizophrenia patients: a combined ERP and DTI study. NeuroImage. 2011;54:2318–2329. doi: 10.1016/j.neuroimage.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Wood SJ, Yung A, Cocchi L, Berger G, Shenton ME, Kubicki M, Phillips L, Velakoulis D, Yolken RH, Pantelis C, McGorry P, Amminger GP. Structural abnormalities in the cuneus associated with Herpes Simplex Virus (type 1) infection in people at ultra high risk of developing psychosis. Schizophr Res. 2012b;135:175–180. doi: 10.1016/j.schres.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Radler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr Res. 2001;49:129–143. doi: 10.1016/s0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zhang Y-F, Castellanos FX, Milham MP. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. Journal of Neuroscience. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.